
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 04 October 2022
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.910222
This article is part of the Research Topic Diagnostics and Treatment for Bone and Joint Infections View all 10 articles
Purpose: This study aims to evaluate the potential of C-reactive protein to lymphocyte count ratio (CLR) for the prediction of surgical site infection (SSI) following posterior lumbar interbody fusion (PLIF) and the instrumentation of lumbar degenerative diseases.
Methods: In this retrospective study, we considered patients with a lumbar degenerative disease diagnosis surgically treated by the instrumented PLIF procedure from 2015 to 2021. Patient data, including postoperative early SSI and other perioperative variables, were collected from their respective hospitalization electronic medical records. The receiver operator characteristic curve was constructed to determine the optimal cut-off value for CLR, and the ability to predict SSI was evaluated by the area under the curve (AUC). According to the cut-off value, patients were dichotomized with high- or low-CLR, and between-group differences were compared using univariate analysis. The independent impact of CLR on predicting SSI was investigated by multivariate logistics regression analysis.
Results: A total of 773 patients were included, with 26 (3.4%) developing an early SSI post-operation. The preoperative CLR was 11.1 ± 26.1 (interquartile range, 0.4–7.5), and the optimal cut-off was 2.1, corresponding to a sensitivity of 0.856, a specificity of 0.643, and an AUC of 0.768 (95% CI, 0.737–0.797). CLR demonstrated a significantly improved prediction ability than did lymphocyte count (P = 0.021) and a similar ability to predict an infection as C-response protein (P = 0.444). Patients with a high CLR had a significantly higher SSI incidence than those with a low CLR (7.6% vs. 0.8%, P < 0.001). After adjustment for numerous confounding factors, CLR ≥ 2.1 was associated with an 11.16-fold increased risk of SSI, along with other significant variables, i.e., diabetes, preoperative waiting time, and surgical duration.
Conclusion: A high CLR exhibited an improved ability to predict incident SSI and was associated with a substantially increased risk of SSI following instrumented PLIF. After better-design studies verified this finding, CLR could potentially be a beneficial tool in surgical management.
Postoperative surgical site infection (SSI) remains a major issue after spinal surgeries, despite adequate prophylactic antibiotics being routinely administered before and after surgery (1). Compared to other approaches, the instrumented posterior lumbar interbody fusion (PLIF) procedure is more likely to be affected by postoperative SSI, and the incidence rate was reported to vary from 1.5% to 7.2% (2–6). SSI is an intractable issue that is resistant to antibiotics in half of the cases, whereby 30% necessitated revision surgery or implant removal (3). Furthermore, even if managed promptly and appropriately, patients with SSI would have greater long-term back pain and less than half of the probability (27% vs. 60%) of achieving a minimum clinically important difference compared to those without (7). Besides, the substantial economic burden from prolonged hospitalization stays, readmission for revision procedures, and nursing care significantly impacted patients and their families (8, 9).
The preoperative identification of patient and clinical factors or biomarkers that effectively predict the postoperative SSI can inform risk evaluation and stratification, facilitating the implementation of targeted prevention measures, which should thus aid in the avoidance of excessive medical resource consumption and the chance of resultant antibiotic resistance. In contrast with patient and clinical factors that are sometimes subjective and obtuse in showing body status (e.g., body's response to tissue injury, inflammatory, and immune status), and SSI, serum biomarkers are more sensitive, objective, and prompt (10). For example, C-response protein (CRP) is a biomarker and is not only a typical acute phase reactant protein in response to inflammation but also an indicator of injury duration in the face of repeated tissue injury (11). The ability of elevated serum CRP concentration to predict SSI after spinal surgeries has been extensively demonstrated in recent studies (12, 13), which was appraised as “more predictive than prehistoric” (13). However, false negatives were often encountered for various reasons, including the low sensitivity in low-virulence-bacterial infections where serum CRP concentration was low (14, 15). Similar observations have also been shown for another biomarker, lymphocyte count (12, 16, 17), which is also particularly important for the immune response state. However, a previous study determined the optimal cut-off of both CRP and lymphocyte to be within the normal reference range, e.g., 4.4 mg/L (reference range, <8 mg/L) for CRP, and 1.2 × 109/L (reference range, 1.1–3.2 × 109/L) for lymphocyte count, respectively (12), thus limiting their clinical viability. In other words, the “seemingly normal” value for a biomarker is underpowered to alert the treating surgeons to the increased risk of SSI following surgery.
We conducted this study by considering their indicative value of inflammatory/immune status, the demonstrated ability to predict SSI, and their inherent limitations. We hypothesize that CRP to lymphocyte ratio (CLR), derived from both biomarkers, is a better index than predicting SSI after instrumented PLIF. We also hypothesize that high CLR is independently associated with an increased risk of SSI.
This retrospective study was performed following the Helsinki Declaration. The study protocol was approved by the ethics committee of the local institution, which waived the need for informed consent because of the identification anonymity.
Patient electronic medical records were retrieved to identify those who underwent an instrumented PLIF procedure for a lumbar degenerative disease, i.e., degenerative disc disease, spondylolisthesis, spinal stenosis, or a combination of the above, in our hospital, between January 2015 and December 2021. The inclusion criteria were age ≥18 years and complete medical records. The exclusion criteria were procedures other than an instrumented PLIF, obvious symptoms, signs, or preexisting conditions directly affecting the preoperative CRP concentration or lymphocyte count (e.g., respiratory or urinary tract infection, autoimmune hepatitis, liver cirrhosis, rheumatoid arthritis, tumor, etc.), past surgery at lumbar vertebra, primary or metastatic lumbar tumor, or incomplete medical records.
The instrumented PLIF procedure was performed with total facetectomy and subtotal intervertebral discectomy for adequate posterior decompression, cages with local or allergenic bone graft inserted into the intervertebral space, and fixation of a fused segment with a screw-rod system. The operations were performed by six orthopedic or spinal surgeons. As per the standard guidelines, prophylactic intravenous single-dose cephalosporins (e.g., cefazolin and cefamandole nafate) were routinely administered 30 min prior to skin incision. For operative procedures exceeding 3 h, an additional dose would be given. After the operation, prophylactic antibiotics were routinely administered. However, the duration relied on the perceived individualized risk of infection, often one to three days and occasionally up to one week, which was at the discretion of their treating surgeons.
Reviewing the electronic medical records, we identified early SSIs during hospitalization. The US Center for Disease Control and Prevention 2017 was used to diagnose and classify SSI (18). A superficial SSI refers to an infection involving skin and subcutaneous tissues with possible symptoms or signs (i.e., redness, tenderness, heat, and pain over the wound site) and can be resolved by local wound care and antibiotics treatment without the need for surgical intervention. Deep SSI refers to an infection involving the deep issue (i.e., fascia, muscle tissues, or vertebra space), with resultant marked serious symptoms/signs (e.g., fever, pain, tenderness, persistent wound discharge or dehiscence, abscess or gangrenosis), often requiring surgical intervention.
Blood sampling and testing were performed following the manufacturer's instructions. CLR was calculated by dividing the serum CRP concentration in mg/L by the lymphocyte count in 109/L. A preoperative blood sample was extracted to obtain the measurements. For patients with multiple measurements for biomarkers of interest (including CRP, lymphocyte count, and the below-mentioned ones), the one closest to the operation was chosen to minimize the time-dependent effect. Using the manufacturer's recommended cut-offs, these biomarkers were interpreted, and the normal range was <8 mg/L or 1.10–3.20 × 109/L for CRP and lymphocytes.
Two researchers (XW and XM) extracted the variables of interest from the medical records. These included socioeconomic features (age, gender, type of insurance), lifestyle (current smoking, alcohol drinking), comorbidities [body mass index (BMI) calculated by dividing body weight in kilograms by square of height in meters, diabetes, hypertension, heart disease, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), renal insufficiency, peripheral vascular disease, past any operation in the lumbar spine], surgery-related variables [preoperative waiting time, American Society of Anesthesiologists (ASA) score, operated levels, surgical duration, intraoperative bleeding, allogeneic blood transfusion, allograft bone use, postoperative prophylactic use of antibiotics], and laboratory test results [albumin, white blood cell (WBC), neutrophil, lymphocyte, red blood cell (RBC) and platelet count, hematocrit, hemoglobin, and fasting blood glucose (FBG)].
Continuous data were presented with a mean ± standard deviation (SD), and their normality status was detected employing a Kolmogorov–Smirnov test. A Student t-test or Mann–Whitney-U test was performed based on the normality status, as appropriate. Categorical data were presented with figures and percentage values, and a between-group comparison was performed by a Chi-square test or Fisher's exact test.
The optimal cut-off value of CLR to predict SSI was determined by the receiver operating characteristic (ROC) curve when the Youden index (specificity plus sensitivity −1) was maximized. The corresponding sensitivity, specificity, and area under the ROC curve (AUC) with a 95% confidence interval (95% CI) were calculated. Additionally, a similar method was used for CRP and lymphocyte count for comparison purposes. The AUCs for three biomarkers were pairwise compared by a Z-test (19), using the MedCalc software version 14.8.1 (MedCalc Software Ltd, Ostend, Belgium).
Based on the above-determined optimal cut-off value of CLR, patients were dichotomized into the high- or low-CLR groups, and the differences were detected by univariate analysis. Variables tested with P < 0.10 during univariate analysis were further selected for adjustment in the multivariate logistic regression model, using the “enter” method to minimize the confounding effects. The magnitude of CLR associated with SSI was indicated by odds ratios (ORs) and 95% CI. The goodness-of-fit of the multivariate model was evaluated by the Hosmer–Lemeshow (H–L) test, with P > 0.05 indicating an acceptable result and a higher Nagelkerke R2 value (normal range, <1.0) suggesting a better result. P < 0.05 was set as the statistical significance level. The analysis was performed using SPSS 26.0 (IBM, Armonk, New York, USA).
There were 773 patients (348 males and 425 females), with an average age of 51.8 ± 12.8 years. The mean preoperative stay was 3.3 ± 2.4 days, and the operating level was 2.1 ± 1.8. Of the patients, 13.8% (107/773) received an allogeneic bone or bone substitute graft, and 32.7% (253/773) received an intraoperative allogeneic transfusion. The surgical time for the procedure was 175.6 ± 51.1 min, and approximately half (45.8%, 354/773) had a procedure lasting above 3 h. Postoperatively, prophylactic antibiotics use ≥3 days was administered in 21.1% (163/773) of the patients. In total, 26 (3.4%) patients had an early SSI postoperatively, including 12 (1.6%) deep and 14 (1.8%) superficial SSIs.
The preoperative CLR was 11.1 ± 26.1, with a range of 0–215.8 (interquartile range, 0.4–7.5). The ROC curve determined the optimal cut-off as 2.1; the corresponding sensitivity and specificity were 0.856 and 0.643, respectively; the AUC was 0.768 (95%CI, 0.737–0.797). Patients with a high CLR had a significantly higher SSI incidence rate than those with a low CLR (7.6%, 22/289 vs. 0.8%, 4/484; crude OR = 9.2; P < 0.001). The optimal value of CRP was 4.0 mg/L, corresponding to the sensitivity, specificity, and AUC of 0.808, 0.651, and 0.759 (95% CI, 0.727–0.789), respectively. Meanwhile, the cut-off value for lymphocyte count was 1.5, and the sensitivity, specificity, and AUC were 0.681, 0.692, and 0.660 (95% CI, 0.555–0.765), respectively (Figure 1). The Z-test demonstrated a significantly improved prediction ability of CLR compared to that of lymphocyte count (Z value, 2.309; P = 0.021), but was nonsignificant compared to CRP (Z value, 0.765; P = 0.444). It was nonsignificantly different from CRP with lymphocyte count (Z value, 1.723; P = 0.085).
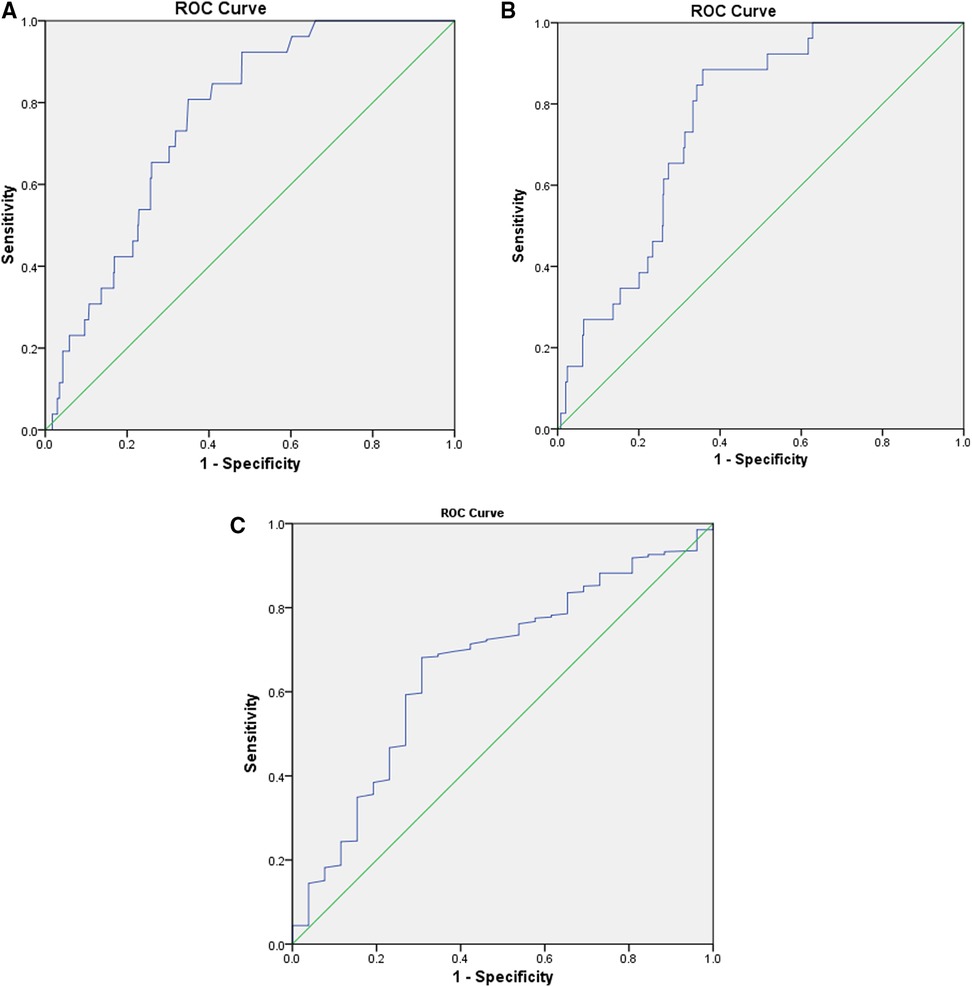
Figure 1. The ROC curves were constructed to determine the optimal cut-off values for CLR, CRP, and lymphocyte count. The optimal cut-off of CLR was 2.1, corresponding to a sensitivity of 0.856, specificity of 0.643, and an AUC of 0.768 (95% CI, 0.737–0.797) (A). The optimal value of CRP was 4.0 mg/L, corresponding to a sensitivity of 0.808, specificity of 0.651, and AUC of 0.759 (95% CI, 0.727–0.789) (B); while for lymphocyte count, the cut-off value was 1.5, and the sensitivity, specificity, and AUC was 0.681, 0.692, and 0.660 (95% CI, 0.555–0.765) (C). AUC, area under the curve; CLR, C-reactive protein to lymphocyte ratio; CRP, C-response protein; ROC, receiver operator characteristic.
Patients with a high CLR value were significantly different from those with a low CLR value in terms of age in the form of either continuous (P = 0.007) or categorical variables (P = 0.006), prevalence of obesity (P = 0.031), hypertension (P = 0.031), diabetes (P = 0.036), peripheral vascular disease (P < 0.001), preoperative waiting time (P < 0.001), allograft bone (P < 0.001), intraoperative bleeding (P < 0.001), allogenic blood transfusion (P < 0.001), surgical duration (P = 0.010), WBC count (P < 0.001), albumin <35 g/L (P < 0.001), FBG > 6.1 mmol/L (P < 0.001), neutrophil count >6.3 × 109/L (P < 0.001), lymphocyte count (P < 0.001), RBC count (P < 0.001), hemoglobin (P < 0.001), and hematocrit (P < 0.001) (Table 1).
The multivariate logistic regression analysis, adjusted for the above significant variables and those with P < 0.10 (BMI in continuous form, ASA score, and history of any operation), displayed that CLR ≥ 2.1 was associated with an 11.16-fold increased risk of SSI. The other significant variables included diabetes (OR, 3.31; 95% CI, 1.04–9.55), preoperative waiting time in a day (OR, 1.16; 95% CI, 1.01–1.35), and surgical duration in each 30-min increment (OR, 1.24; 95% CI, 1.06–1.59) (Table 2). The H–L test showed an acceptable goodness-of-fit of the multivariate model (P = 0.537, Chi-square = 6.993; Nagelkerke R2 = 0.273).
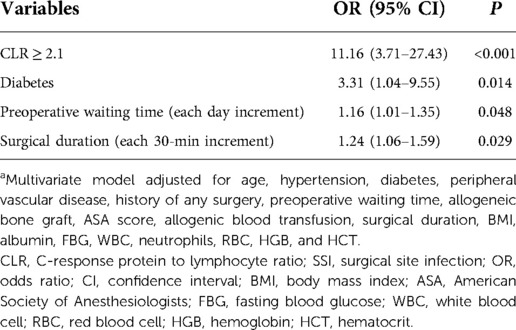
Table 2. Multivariate analysis of CLR in association with SSI after adjustment for numerous variablesa.
We verified our previous study reported that preoperative high CLR value (≥2.1) was significantly associated with an 11.16-fold risk of SSI following instrumented PLIF for lumbar degenerative disease. We also found that CLR indicated a better predicting ability, with an AUC of 0.768, a significant difference for lymphocyte count (AUC, 0.660; P = 0.021), but nonsignificant for CRP (AUC, 0.759; P = 0.444). CLR revealed a higher sensitivity than the original index (CLR, 0.856; CRP, 0.808; and lymphocyte count, 0.681).
SSI is a disastrous complication after spinal orthopedics or other surgeries, and exploring the potential new indexes has been a primary task in clinical research. However, existing risk prediction models based on identified clinical risk factors demonstrated less robustness in predicting postoperative SSI (2, 20–22). The underlying reasons are related to the heterogeneous population and the time-dependent confounding effects of biomarkers. On the other hand, patient self-reported comorbidities as a component of a risk prediction model were a contributor since these self-reported medical conditions may not mirror the true pathophysiological basis. An ideal prediction tool should be readily accessible, easy to use, and rely upon preoperatively routinely measured laboratory parameters. Inflammation/immune biomarkers fit these characteristics well, and more importantly, they are often highly sensitive to the body's pathophysiologic response and have been presenting notable changes before clinical signs or manifestations emerge (23).
During the past decade, numerous derived novel biomarkers have been employed in research and in clinical practice, demonstrating good prognostication for clinical outcomes or complications, including Modified Glasgow Prognostic Score (mGPS), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), CRP to albumin ratio (CAR), systemic immune-inflammation index (SII), fibrinogen to albumin ratio (FAR), lymphocyte to monocyte ratio (LMR), and monocyte to high-density lipoprotein ratio, among others (24–28). As for CLR or lymphocyte to CRP ratio (LCR), the previous studies on surgical tumors (osteosarcoma, gastric cancer, lung cancer, or pancreatic cancer) (29–31) and on infectious events following surgeries (32–34) have demonstrated its effectiveness in providing prognostic information. To the best of our knowledge, this study was the first to apply CLR in spinal orthopedic surgeries to predict the incidence of postoperative SSI.
In this study, CLR demonstrated better predictive ability than the original index, lymphocyte count, or CRP, with AUC increasing from 0.660 and 0.808 to 0.868 (P value, 0.021 and 0.444). This suggests that the predictive effect of this new biomarker was remarkably strengthened after the division calculation, which was related to the simultaneous uptrend of CRP and downtrend of the lymphocyte count. Most importantly, the predictive effect via this cut-off (≥2.1) is, albeit related to CRP and lymphocyte count, incompletely dependent on either one taken individually. In other words, CLR could still exceed the cut-off value even if both biomarkers are simultaneously in reference intervals. The identified optimal cut-off value of CRP and lymphocyte count was exactly within the range of the manufacturer's reference interval (CRP: cut-off, 4.0 mg/L; reference interval, <8 mg/L; lymphocyte count, cut-off, 1.5 × 109/L; reference interval, 1.1 to 3.2 × 109/L). In clinical practice, applying this seemingly normal value as a cut-off for either CRP or lymphocyte count is hardly possible to alert healthcare providers of the substantially increased risk of postoperative SSI. Therefore, CLR can be considered a pragmatic and independent predictive tool.
The other clinical importance of using CLR is guiding postoperative administration. In this study, CLR ≥ 2.1 corresponds to a sensitivity of 0.856, suggesting that patients with a CLR < 2.1 are at low risk (0.8%, 4/484) of postoperative SSI and can thus be considered to execute “no antibiotic strategy” or “less use strategy” postoperatively, to reduce the possibility of multiple drug-resistant bacteria. It is worth noting that CLR's specificity is only 0.643, suggesting a high probability of false positive results. Therefore, a positive CLR result is a determiner of active preventive interventions; combined systemic medical conditions and local operative conditions (i.e., lumbar disease per se) should be evaluated for an informed decision.
The results show that preoperative CLR, derived from CRP and lymphocyte count, is a feasible and predictive biomarker for the early incidence of SSI following instrumented PLIF procedures for degenerative lumbar diseases. An elevated CLR ≥ 2.1 was independently associated with an 11.15-fold risk of SSI. This value may alert surgeons of the high risk of postoperative SSI, better facilitating the implementation of feasible targeted preventive measures.
The data analyzed in this study is subject to the following licenses/restrictions: In accordance with the institutional policy, data used in this study are not available publicly but can be obtained from the corresponding author upon justified request for scientific research purposes. Requests to access these datasets should be directed to Xun Ma,eHVubWFkb2MxNzc2QDEyNi5jb20=.
This study was approved by the Ethics Committee of the Third Hospital of Shanxi Medical University (No. 2022-095), which waived the need for informed consent because of the identification anonymity.
XM conceived the idea and designed the study. XW and XM inquired about the medical records and collected the data. JZ prepared the figures and tables and CC performed the statistical analyses. All the authors interpreted the data and contributed to the preparation of the manuscript. All authors contributed to the article and approved the submitted version.
We are grateful to Kuo Zhao and Junzhe Zhang of the Department of Orthopedics and to Xin Li of the Department of statistics and applications for their kind assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CLR, C-reactive protein to lymphocyte count ratio; PLIF, posterior lumbar interbody fusion; SSI, surgical site infection; CRP, C-response protein; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists; WBC, white blood cell; FBG, fasting blood glucose; SD, standard deviation; ROC, receiver operator characteristic; AUC, area under the curve; OR, odds ratio; H–L, Hosmer–Lemeshow
1. Maria S, Deyanira C, Francesca S, Lucia M, Alessandro R, Silvia T, et al. Spinal fusion surgery and local antibiotic administration: a systematic review on key points from preclinical and clinical data. Spine (Phila Pa 1976). (2020) 45(5):339–48. doi: 10.1097/BRS.0000000000003255
2. Pei H, Wang H, Chen M, Ma L, Liu G, Ding W. Surgical site infection after posterior lumbar interbody fusion and instrumentation in patients with lumbar degenerative disease. Int Wound J. (2021) 18(5):608–15. doi: 10.1111/iwj.13562
3. Lee JS, Ahn DK, Chang BK, Lee JI. Treatment of surgical site infection in posterior lumbar interbody fusion. Asian Spine J. (2015) 9(6):841–8. doi: 10.4184/asj.2015.9.6.841
4. Kim JH, Ahn DK, Kim JW, Kim GW. Particular features of surgical site infection in posterior lumbar interbody fusion. Clin Orthop Surg. (2015) 7(3):337–43. doi: 10.4055/cios.2015.7.3.337
5. Mirovsky Y, Floman Y, Smorgick Y, Ashkenazi E, Anekstein Y, Millgram MA, et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech. (2007) 20(2):127–31. doi: 10.1097/01.bsd.0000211266.66615.e5
6. Yu L, Xu R, Ma W, Liu G. Meta-analysis of outcomes of transforaminal lumbar interbody fusion versus posterior lumbar interbody fusion for degenerative lumbar diseases. Chin J Spine Spinal Cord. (2013) 23(10):886–90. doi: 10.3969/j.issn.1004-406X.2013.10.04
7. Petilon JM, Glassman SD, Dimar JR, Carreon LY. Clinical outcomes after lumbar fusion complicated by deep wound infection: a case-control study. Spine (Phila Pa 1976). (2012) 37(16):1370–4. doi: 10.1097/BRS.0b013e31824a4d93
8. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. (2017) 96(1):1–15. doi: 10.1016/j.jhin.2017.03.004
9. Wenzel RP. Minimizing surgical-site infections. N Engl J Med. (2010) 362(1):75–7. doi: 10.1056/NEJMe0908753
10. Ziegler MA, Bauman JC, Welsh RJ, Wasvary HJ. Can the American College of Surgeons National Surgical Quality Improvement Program Risk Calculator predict outcomes for urgent colectomies? Am Surg. (2022) 88(1):65–9. doi: 10.1177/0003134820973392
11. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. (1993) 91(4):1351–7. doi: 10.1172/JCI116336
12. Iwata E, Shigematsu H, Koizumi M, Nakajima H, Okuda A, Morimoto Y, et al. Lymphocyte count at 4 days postoperatively and CRP level at 7 days postoperatively: reliable and useful markers for surgical site infection following instrumented spinal fusion. Spine (Phila Pa 1976). (2016) 41(14):1173–8. doi: 10.1097/BRS.0000000000001501
13. Hoeller S, Roch PJ, Weiser L, Hubert J, Lehmann W, Saul D. C-reactive protein in spinal surgery: more predictive than prehistoric. Eur Spine J. (2021) 30(5):1261–9. doi: 10.1007/s00586-021-06782-8
14. Akgün D, Müller M, Perka C, Winkler T. The serum level of C-reactive protein alone cannot be used for the diagnosis of prosthetic joint infections, especially in those caused by organisms of low virulence. Bone Joint J. (2018) 100-b(11):1482–6. doi: 10.1302/0301-620X.100B11.BJJ-2018-0514.R1
15. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. (2012) 65(2):158–68. doi: 10.1111/j.1574-695X.2012.00938.x
16. Imabayashi H, Miyake A, Chiba K. A novel approach for identifying serological markers indicative of surgical-site infection following spine surgery: postoperative lymphopenia is a risk factor. J Orthop Sci. (2021) 27(3):588–93. doi: 10.1016/j.jos.2021.03.003
17. Iwata E, Shigematsu H, Yamamoto Y, Tanaka M, Okuda A, Morimoto Y, et al. Lymphocyte count at 4 days postoperatively: a reliable screening marker for surgical site infection after posterior lumbar decompression surgery. Spine (Phila Pa 1976). (2018) 43(18):E1096–e1101. doi: 10.1097/BRS.0000000000002617
18. Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. (2017) 152(8):784–91. doi: 10.1001/jamasurg.2017.0904
19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44(3):837–45. doi: 10.2307/2531595
20. Klemencsics I, Lazary A, Szoverfi Z, Bozsodi A, Eltes P, Varga PP. Risk factors for surgical site infection in elective routine degenerative lumbar surgeries. Spine J. (2016) 16(11):1377–83. doi: 10.1016/j.spinee.2016.08.018
21. Broda A, Sanford Z, Turcotte J, Patton C. Development of a risk prediction model with improved clinical utility in elective cervical and lumbar spine surgery. Spine (Phila Pa 1976). (2020) 45(9):E542–51. doi: 10.1097/BRS.0000000000003317
22. Rushton AB, Verra ML, Emms A, Heneghan NR, Falla D, Reddington M, et al. Development and validation of two clinical prediction models to inform clinical decision-making for lumbar spinal fusion surgery for degenerative disorders and rehabilitation following surgery: protocol for a prospective observational study. BMJ Open. (2018) 8(5):e021078. doi: 10.1136/bmjopen-2017-021078
23. Goulart A, Ferreira C, Estrada A, Nogueira F, Martins S, Mesquita-Rodrigues A, et al. Early inflammatory biomarkers as predictive factors for freedom from infection after colorectal cancer surgery: a prospective cohort study. Surg Infect (Larchmt). (2018) 19(4):446–50. doi: 10.1089/sur.2017.294
24. Zhu X, Yao Y, Yao C, Jiang Q. Predictive value of lymphocyte to monocyte ratio and monocyte to high-density lipoprotein ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. (2018) 13(1):211. doi: 10.1186/s13018-018-0910-2
25. Yombi JC, Schwab PE, Thienpont E. Neutrophil-to-lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C-reactive protein distribution for the follow-up of early inflammation after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2016) 24(10):3287–92. doi: 10.1007/s00167-015-3921-0
26. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442
27. Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, Tanaka T, et al. Evaluation of modified Glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas. (2016) 45(2):211–7. doi: 10.1097/MPA.0000000000000446
28. Guo S, He X, Chen Q, Yang G, Yao K, Dong P, et al. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC Cancer. (2017) 17(1):171. doi: 10.1186/s12885-017-3119-6
29. Fan Z, Luo G, Gong Y, Xu H, Qian Y, Deng S, et al. Prognostic value of the C-reactive protein/lymphocyte ratio in pancreatic cancer. Ann Surg Oncol. (2020) 27(10):4017–25. doi: 10.1245/s10434-020-08301-3
30. He Y, Gong R, Peng KW, Liu LZ, Sun LY, Wang HY. Lymphocyte-to-C-reactive protein ratio is a potential new prognostic biomarker for patients with lung cancer. Biomark Med. (2020) 14(9):717–26. doi: 10.2217/bmm-2019-0452
31. Hu H, Deng X, Song Q, Lv H, Chen W, Xing X, et al. Prognostic value of the preoperative lymphocyte-to-C-reactive protein ratio and albumin-to-globulin ratio in patients with osteosarcoma. Onco Targets Ther. (2020) 13:12673–81. doi: 10.2147/OTT.S287192
32. Shi W, Wang Y, Zhao X, Yu T, Li T. CRP/albumin has a promising prospect as a new biomarker for the diagnosis of periprosthetic joint infection. Infect Drug Resist. (2021) 14:5145–51. doi: 10.2147/IDR.S342652
33. Güneş H, Yurttutan S, Çobanuşağı M, Doğaner A. CRP/albumin ratio: a promising marker of gram-negative bacteremia in late-onset neonatal sepsis. Turk Arch Pediatr. (2021) 56(1):32–6. doi: 10.14744/TurkPediatriArs.2020.99076
Keywords: posterior lumbar interbody fusion, perioperative management, risk prediction tool, creative protein to lymphocyte count ratio (CLR), surgical site infection
Citation: Wu X, Ma X, Zhu J and Chen C (2022) C-reactive protein to lymphocyte ratio as a new biomarker in predicting surgical site infection after posterior lumbar interbody fusion and instrumentation. Front. Surg. 9:910222. doi: 10.3389/fsurg.2022.910222
Received: 1 April 2022; Accepted: 12 September 2022;
Published: 4 October 2022.
Edited by:
Markus Rupp, University Medical Center Regensburg, GermanyReviewed by:
Ziya Levent Gokaslan, Brown University, United States© 2022 Wu, Ma, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Ma eHVubWFkb2MxNzc2QDEyNi5jb20=
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.