- 1Department of Gynecology, The First Affiliated Hospital of Hebei North University, Zhangjiakou, China
- 2Department of Emergency, The First Affiliated Hospital of Hebei North University, Zhangjiakou, China
Background: To investigate the efficacy and safety of bevacizumab combined with pemetrexed in the treatment of recurrent and metastatic cervical cancer.
Methods: Clinical data of 65 patients with recurrent and metastatic cervical cancer who were admitted to our hospital were collected for retrospective analysis. All patients were administered with bevacizumab combined with pemetrexed for 4–6 cycles (21 days as 1 cycle). The short-term clinical efficacy and adverse reactions were compared between the two groups. In addition, the survival status of patients was followed up and recorded.
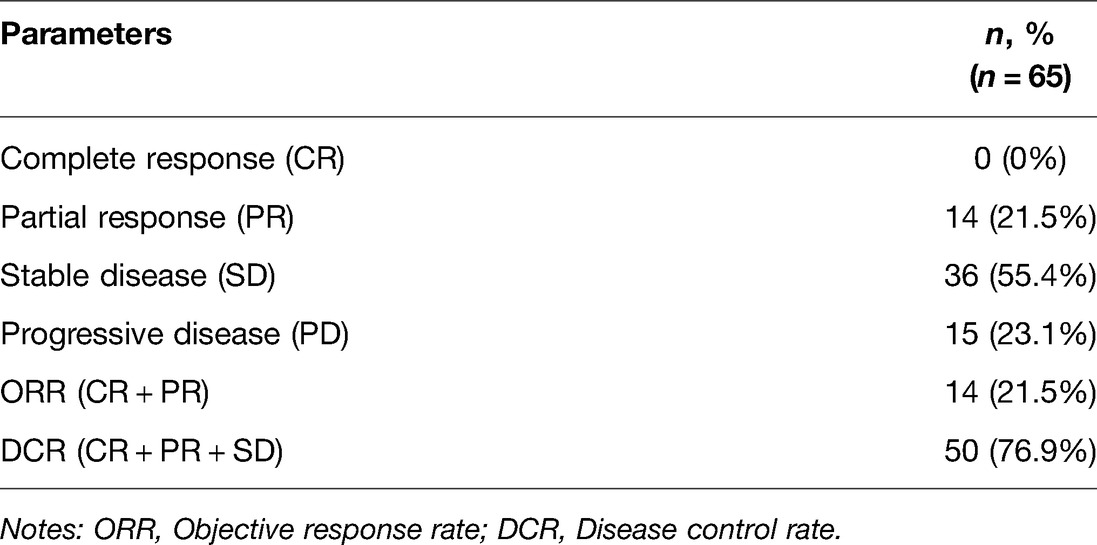
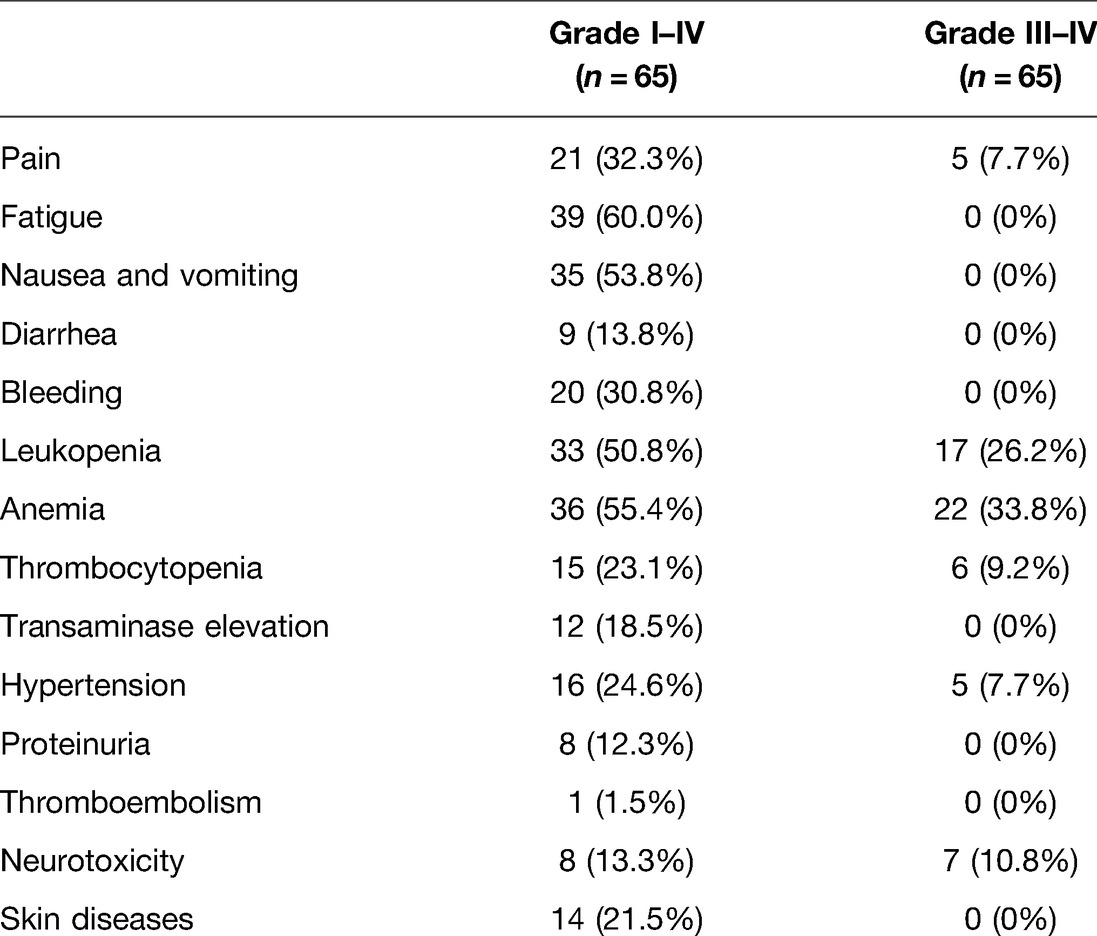
Results: At least 4 cycles of chemotherapy were given to the 65 patients. There were 0 cases of complete response (CR), 14 cases of partial response (PR), 36 cases of stable disease (SD) and 15 cases of progressive disease (PD). The objective response rate (ORR) and the disease control rate (DCR) were 21.5% (14/65) and 76.9% (50/65), respectively. DCR was superior in patients with squamous cell carcinoma to that in those with adenocarcinoma (p = 0.039), but no statistically significant difference was found in ORR. Patients with extra-pelvic metastatic lesions had a better efficacy than those with intra-pelvic metastatic lesions, but the difference was not statistically significant (p > 0.05). The post-treatment adverse reactions mainly involved fatigue, nausea and vomiting, bleeding, leukopenia, anemia, thrombocytopenia, transaminase elevation, hypertension, proteinuria and neurotoxicity, most of which were grade I–II that ameliorated after symptomatic therapy. Grade III adverse reactions mainly included pain in 5 cases (7.7%), leukopenia in 17 cases (26.2%), anemia in 22 cases (33.8%), thrombocytopenia in 6 cases (9.2%), hypertension in 5 cases (7.7%) and neurotoxicity in 7 cases (10.8%). The follow-up results manifested that median overall survival (OS) and median progression-free survival (PFS) were 10.6 months and 6.6 months, respectively.
Conclusion: Bevacizumab combined with pemetrexed exhibits certain efficacy in the treatment of recurrent and metastatic cervical cancer, with tolerable adverse reactions. Therefore, this therapeutic option deserves clinical popularization and application.
Introduction
Patients with recurrent and metastatic cervical cancer are usually treated by means of chemotherapy, surgery and/or radiotherapy, but they have a poor prognosis. Generally, replacement of chemotherapy agents or optimal supportive therapy is available for those with failure of first-line chemotherapy. However, the therapeutic methods are reported to be limited (1). Bevacizumab can prolong the survival time of patients with advanced cervical cancer, as previously discussed, and has been approved by the European Union (EU) and U.S. Food and Drug Administration for the treatment of advanced cervical cancer (2, 3). According to National Comprehensive Cancer Network (NCCN) guidelines and expert consensus of the Chinese Society of Clinical Oncology (CSCO), pemetrexed, with favorable efficacy in the treatment of multiple tumors, is recommended for the second-line and above chemotherapy of cervical cancer (4, 5). This study aimed to investigate the efficacy and safety of bevacizumab combined with pemetrexed as a second-line chemotherapy regimen for patients with recurrent and metastatic cervical cancer.
Materials and Methods
General Data
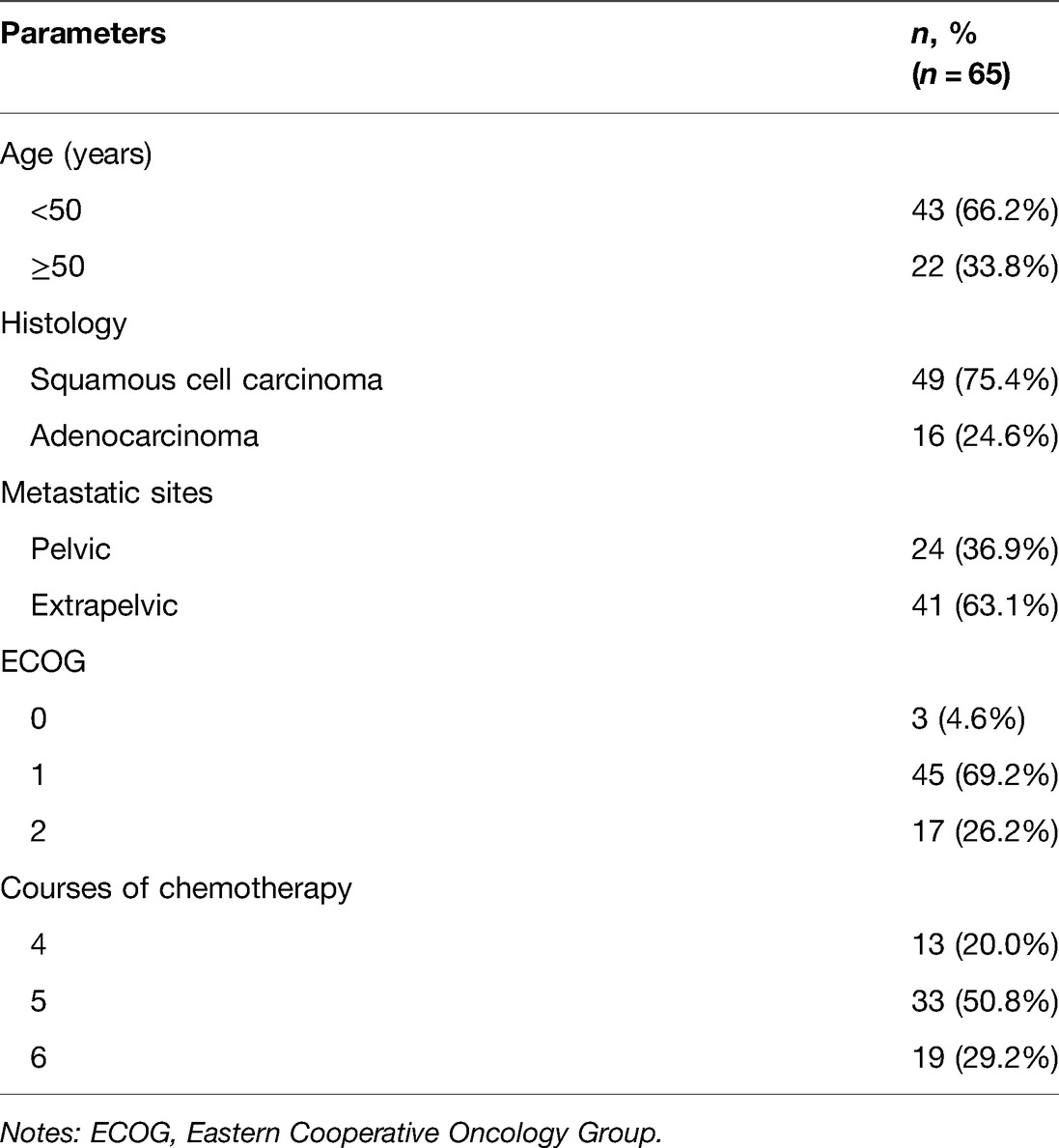
Clinical data of 65 patients with recurrent and metastatic cervical cancer who were admitted to our hospital were collected. The patients were aged 36.4–71.1 years old, averagely (60.4 ± 9.4) years old. The inclusion criteria were as follows: (a) patients histopathologically diagnosed with cervical cancer, (b) those with disease progression or relapse after first-line combination chemotherapy or concurrent chemotherapy and radiotherapy, (c) those with a measurable target lesion on CT, MRI or ultrasound images, (d) those with basically normal routine blood tests and no obvious hepatic and renal dysfunction, (e) those with an Eastern Cooperative Oncology Group (ECOG) score of 0–2 points, and (f) those with an expected survival time ≥3 months. The exclusion criteria involved: (a) patients with allergy to pemetrexed or bevacizumab, (b) those with severe cardiac, pulmonary, hepatic or renal impairment, (c) those with coagulation disorder within 2 weeks prior to treatment, (d) those with hepatitis B or HIV infection, (e) those with requirement of anticoagulant therapy for relevant diseases, (f) those with other malignancies, or g) those with mental disorders or unable to cooperate in treatment for other reasons. The general information and clinical baseline data of patients are displayed in Table 1. This study complied with the Declaration of Helsinki, and all patients signed written informed consent.
Therapeutic Methods
Bevacizumab (Shanghai Roche Pharmaceutical Co., Ltd., Shanghai, China) was administered by intravenous drip, 15 mg/kg, on the 1st day. The dosage would be adjusted in case of body mass change more or less than 10%. Pemetrexed (Shandong Qilu Pharmaceutical Co., Ltd) 500 mg/m2 was given by intravenous drip (>10 min), on the 2nd day. The treatment lasted for 4–6 cycles (21 days as 1 cycle). One week prior to administration with pemetrexed, patients took orally 400 μg of folic acid daily, and were intramuscularly injected with 1,000 μg of vitamin B12. Dexamethasone (4 mg) was given orally, twice daily, 1 day before, on the day of and 1 day after treatment with pemetrexed. Folic acid was discontinued 21 days after last administration with pemetrexed. Vitamin B12 was injected once every 3 cycles.
Observational Indicators
Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 was utilized to evaluate the clinical efficacy with following indicators: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Objective response rate (ORR) = (CR + PR)/(total cases) × 100%, and disease control rate (DCR) = (CR + PR + SD)/(total cases) × 100%.
During the follow-up period, adverse reactions were recorded and evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.0.
Through follow-up visits, progression-free survival (PFS) and overall survival (OS) were recorded. The period from enrollment to disease progression or death was regarded as PFS, while that from enrollment to death for any reason was defined as OS. The follow-up period ended in May 2021.
Statistical Analysis
Statistical Product and Service Solutions (SPSS) 22.0 software (IBM, Armonk, NY, USA) was adopted for statistical analysis. Measurement data were expressed by mean ± standard deviation and compared by two-sample t-test between two groups. Enumeration data were expressed by percentage (%), and compared by chi-square test between two groups. Kaplan-Meier method was employed to plot the survival curve. P < 0.05 was considered statistically significant.
Results
Comparison of Short-Term Clinical Efficacy
At least 4 cycles of chemotherapy were given to the 65 patients. There were 0 cases of CR, 14 cases of PR, 36 cases of SD and 15 cases of PD. ORR and DCR were 21.5% (14/65) and 76.9% (50/65), respectively (Table 2).
There were 0 cases of CR, 12 cases of PR, 29 cases of SD and 8 cases of PD among 49 patients with squamous cell carcinoma. ORR and DCR were 24.5% (12/49) and 83.7% (41/49), respectively. Among 16 patients with adenocarcinoma, there were 0 cases of CR, 2 cases of PR, 7 cases of SD and 7 cases of PD. ORR and DCR were 12.5% (2/16) and 56.3% (9/16), respectively. Among 24 patients with intra-pelvic metastasis, there were 0 cases of CR, 4 cases of PR, 12 cases of SD and 8 cases of PD. ORR and DCR were 16.7% (4/24) and 66.7% (16/24), respectively. Among 41 patients with extra-pelvic metastasis, there were 0 cases of CR, 10 cases of PR, 24 cases of SD and 7 cases of PD. ORR and DCR were 24.4% (10/41) and 82.9% (34/41), respectively. After combination treatment with bevacizumab and pemetrexed, DCR was superior in patients with squamous cell carcinoma to that in those with adenocarcinoma (p = 0.039), but no statistically significant difference was found in ORR. Patients with extra-pelvic metastatic lesions had a better efficacy than those with intra-pelvic metastatic lesions, but the difference was not statistically significant (p > 0.05).
Incidence of Adverse Reactions
The post-treatment adverse reactions mainly involved fatigue, nausea and vomiting, bleeding, leukopenia, anemia, thrombocytopenia, transaminase elevation, hypertension, proteinuria and neurotoxicity, mostly in grade I–II, which were ameliorated after symptomatic therapy. No grade IV adverse reactions and no progressive diseases and deaths related to adverse reactions were observed in both groups. Grade III adverse reactions mainly included pain in 5 cases (7.7%), leukopenia in 17 cases (26.2%), anemia in 22 cases (33.8%), thrombocytopenia in 6 cases (9.2%), hypertension in 5 cases (7.7%) and neurotoxicity in 7 cases (10.8%). The adverse reactions regarding bevacizumab were mainly hypertension, proteinuria and bleeding (Table 3).
Postoperative Follow-up Visits of Patients in the Two Groups
As of May 2021, all patients were followed up for 3–24 months. The median OS and median PFS were 10.6 months and 6.6 months, respectively. Kaplan-Meier method was employed to plot the survival curve (Figure 1).
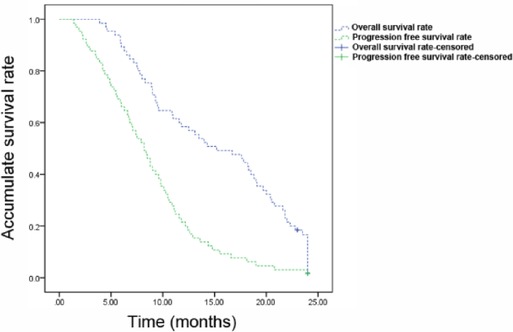
Figure 1. Kaplan-Meier survival curves of patients with recurrent metastatic cervical cancer. The overall survival rate and progression free survival rate of patients were shown.
Discussion
As a common malignant tumor affecting the female reproductive system, cervical cancer ranks second in the mortality rate among tumors in females (6). According to the statistics, about 130,000 cases are newly diagnosed with cervical cancer in China each year, and relapse and metastasis following treatment may occur in 15%–30% of patients. Limitations exist regarding therapeutic regimens for patients with refractory cervical cancer, especially those with continuous progression after multiple courses of radiotherapy and multi-line platinum-based combination chemotherapy. Then, platinum single-agent chemotherapy is usually administered again. However, the therapeutic efficacy is unsatisfactory, and the OS is short. Additionally, the 5-year survival rate is only 10%, and the quality of life is poor. For these reasons, numerous studies have been devoted to the targeted therapy which has become a trend of future development (7, 8).
Bevacizumab is a recombinant humanized monoclonal antibody that can selectively bind to human vascular endothelial growth factor (VEGF) and block its biological activity, and inhibit the binding of VEGF to its receptors Flt-1 and KDR on endothelial cells, thus reducing tumor angiogenesis and suppressing tumor growth (9). The extensive anti-tumor activity of bevacizumab has been reported among various cancers, such as colon cancer, non-small cell lung cancer, epithelial ovarian cancer and breast cancer (10–13). Moreover, bevacizumab is increasingly used in the treatment of cervical cancer. Wright et al. (14) reported that bevacizumab combined with 5-fluorouracil (5-FU) has a DCR reaching 67% and a median PFS of 4.3 months in the treatment of recurrent cervical cancer (14). The mid-term analysis of the U.S. GOG 240 manifested that after chemotherapy combined with bevacizumab, the survival period of patients who suffer from intractable, recurrent or metastatic cervical cancer has been prolonged by about 4 months, the median survival period is 17 months, the effective rate is 48%, and the mortality rate is reduced by 29% (3). Another multi-center study displayed a favorable progression-free survival after administration with bevacizumab, and exhibited survival period exceeding 6 months in 24% of patients, 7.3 months of median OS and a drug response rate of 10.9% (15). Therefore, bevacizumab has successively been approved by the EU and U.S. Food and drug administration for the treatment of advanced cervical cancer. Accordingly, cervical cancer has become the first gynecological tumor whose OS can be effectively prolonged by anti-angiogenesis agents (2). Furthermore, the latest version of NCCN guidelines has issued the application of bevacizumab combined with cisplatin and paclitaxel for recurrent or metastatic cervical cancer as the first-line therapeutic agents (16).
Pemetrexed is a synthetic multi-target antifolate and contains pyrrolidine grouping in structure, which can effectively inhibit dihydrofolate reductase, glycinamide ribonucleotide formyl transferase and thymidylate synthase and block thymidine and purine synthesis, thus affecting nucleic acid synthesis in tumor cells to exert anti-tumor effects (17). Previous studies have confirmed the effectiveness of single agent pemetrexed in the treatment of multiple tumors (18). The research on pemetrexed as a second-line treatment of cervical cancer manifested that 15% (4/27) and 59% (16/27) of patients achieve PR and SD, and PFS and OS are 3.1 months and 7.4 months, respectively (19). Pemetrexed was recommended for the second-line and above chemotherapy of cervical cancer by the NCCN guidelines in 2016.
In this study, ORR and DCR were 21.5% (14/65) and 76.9% (50/65), respectively, in patients with recurrent and metastatic cervical cancer receiving bevacizumab combined with pemetrexed as the second-line treatment. The follow-up results denoted that median OS and median PFS were 10.6 months and 6.6 months, respectively. DCR was superior in patients with squamous cell carcinoma to that in those with adenocarcinoma (p = 0.039), but no statistically significant difference was found in ORR. Patients with extra-pelvic metastatic lesions had a better efficacy than those with intra-pelvic metastatic lesions, but the difference was not statistically significant (p > 0.05). The findings demonstrated the certain efficacy of chemotherapy combined with targeted drugs. As reported, bevacizumab combined with chemotherapy exhibits better PR than pemetrexed or bevacizumab alone, greater SD than bevacizumab single agent but similar to pemetrexed, and more favorable PFS and OS than either of the two (15). Therefore, combination of bevacizumab and pemetrexed as a second-line treatment is superior to its single-agent therapy in terms of short-term clinical efficacy for patients with recurrent and metastatic cervical cancer. With respect to safety, this study revealed that the adverse reactions during treatment were mainly in grade I–II, and grade III–IV severe adverse reactions mainly included leukopenia, anemia, thrombocytopenia, hypertension and neurotoxicity, which could be basically tolerated. In addition, no death related to treatment was observed. These findings confirmed the safety of combined therapy.
The present study was a retrospective study with limited sample sizes and relatively short follow-up period, and the follow-up content was not comprehensive enough. Thus, in the future, multi-center, large-sample, prospective clinical trials should be carried out to validate the conclusions of this study.
Conclusion
Bevacizumab combined with pemetrexed exhibits certain efficacy in the treatment of recurrent and metastatic cervical cancer, with tolerable adverse reactions. Therefore, this therapeutic option deserves clinical popularization and application.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of The First Affiliated Hospital of Hebei North University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH and QX designed the study and performed the experiments, YH and JW collected the data, QX and SX analyzed the data, YH and QX prepared the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. J Buon. (2016) 21(2):320–5. https://pubmed.ncbi.nlm.nih.gov/27273940/27273940
2. Liu FW, Cripe J, Tewari KS. Anti-angiogenesis therapy in gynecologic malignancies. Oncology (Williston Park). (2015) 29(5):350–60. https://pubmed.ncbi.nlm.nih.gov/25979545/25979545
3. Tewari KS, Sill MW, Long HR, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. (2014) 370(8):734–43. doi: 10.1056/NEJMoa1309748
4. Park CK, Oh IJ, Kim KS, Choi YD, Jang TW, Kim YS, et al. Randomized phase III study of docetaxel plus cisplatin versus pemetrexed plus cisplatin as first-line treatment of nonsquamous non-small-cell lung cancer: a TRAIL trial. Clin Lung Cancer. (2017) 18(4):e289–96. doi: 10.1016/j.cllc.2017.01.002
5. Lester JF, Casbard AC, Al-Taei S, Harrop R, Katona L, Attanoos RL, et al. A single centre phase II trial to assess the immunological activity of TroVax(R) plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma - the SKOPOS trial. Oncoimmunology. (2018) 7(12):e1457597. doi: 10.1080/2162402X.2018.1457597
6. Wuerthner BA, Avila-Wallace M. Cervical cancer: screening, management, and prevention. Nurse Pract. (2016) 41(9):18–23. doi: 10.1097/01.NPR.0000490390.43604.5f
7. Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, et al. Emerging biological treatments for uterine cervical carcinoma. J Cancer. (2014) 5(2):86–97. doi: 10.7150/jca.7963
8. Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. (2020) 112(2):229–32. doi: 10.1016/j.jnma.2020.03.002
9. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
10. Pfeiffer P, Yilmaz M, Moller S, Zitnjak D, Krogh M, Petersen LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. (2020) 21(3):412–20. doi: 10.1016/S1470-2045(19)30827-7
11. Reck M, Mok T, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
12. Pfisterer J, Shannon CM, Baumann K, Rau J, Harter P, Joly F, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. (2020) 21(5):699–709. doi: 10.1016/S1470-2045(20)30142-X
13. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. (2007) 357(26):2666–76. doi: 10.1056/NEJMoa072113
14. Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol. (2006) 103(2):489–93. doi: 10.1016/j.ygyno.2006.03.023
15. Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. (2009) 27(7):1069–74. doi: 10.1200/JCO.2008.18.9043
16. Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. NCCN guidelines insights: cervical cancer, version 1.2020. J Natl Compr Cancer Network. (2020) 18(6):660–6. doi: 10.6004/jnccn.2020.0027
17. Qian T, Huang XE. Study of pemetrexed-based chemotherapy for patients with locally advanced or metastatic cancers. Asian Pac J Cancer Prev. (2015) 16(11):4791–5. doi: 10.7314/apjcp.2015.16.11.4791
18. Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Konaka K, Okada N, et al. Pemetrexed-induced rash may be prevented by supplementary corticosteroids. Biol Pharm Bull. (2015) 38(11):1752–6. doi: 10.1248/bpb.b15-00435
19. Miller DS, Blessing JA, Bodurka DC, Bonebrake AJ, Schorge JO. Evaluation of pemetrexed (Alimta, LY231514) as second line chemotherapy in persistent or recurrent carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. (2008) 110(1):65–70. doi: 10.1016/j.ygyno.2008.03.009
Keywords: bevacizumab, pemetrexed, cervical cancer, efficacy, treatment
Citation: He Y, Wang J, Xie S and Xue Q (2022) Efficacy of Bevacizumab Combined with Pemetrexed in the Treatment of Recurrent and Metastatic Cervical Cancer. Front. Surg. 9:908101. doi: 10.3389/fsurg.2022.908101
Received: 30 March 2022; Accepted: 27 April 2022;
Published: 16 May 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Meifeng Lv, Jinan Second Maternal and Child Health Hospital, ChinaChun Ye, Fuyang Orthopaedics and Traumatology Affiliated Hospital of Zhejiang Chinese Medical University, China
Copyright © 2022 He, Wang, Xie and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianlong Xue Y2VrdG15QGJlbmp1bGlnaHRpbmcuY29t
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Ying He1
Ying He1 Qianlong Xue
Qianlong Xue