- 1Department of Head and Neck Surgery, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China
- 2Department of Surgery, Dongguan Third Bureau Hospital, Dongguan City, Guangdong, China
- 3Department of Otolaryngology, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, China
Background: Lymph node ratio (LNR) has been reported to reliably predict cancer-specific survival (CSS) in parotid gland cancer (PGC). Our study was designed to validate the significance of LNR in patients with PGC.
Methods: Patients diagnosed with stage I–IV PGC were enrolled from Surveillance Epidemiology and End Results database (SEER, N = 3529), which is the training group, and Sun Yat-sen University Cancer Center database (SYSUCC, N = 99), the validation group. We used X-tile software to choose the optimal cutoff value of LNR; then, univariable and multivariable analyses were performed, assessing the association between LNR and CSS.
Results: The optimal cutoff value of LNR was 0.32 by X-tile based on 3529 patients from SEER. Cox proportional hazard regression analysis revealed better CSS for patients with LNR ≤ 0.32 (adjusted hazard ratio [HR] 1.612, 95% confidence interval [95% CI] 1.286–2.019; p < 0.001) compared with patients with LNR > 0.32 in SEER. In the SYSUCC cohort, patients with LNR ≤ 0.32 also had better CSS over patients with LNR > 0.32 (p < 0.001). In N2 and N3 stage groups, patients with LNR ≤ 0.32 had superior CSS outcomes over those with the LNR > 0.32 group, but this benefit was absent in the N1 stage group.
Conclusions: In conclusion, the lymph node ratio turned out to be an independent prognostic factor for cancer-specific survival of PGC in this study. This valuable information could help clinicians to evaluate the prognosis of PGC and suggest that adequate lymph node dissection is necessary.
Introduction
Among head and neck cancers worldwide, salivary gland cancer has taken up 1%–5% (1). Also, parotid gland cancer (PGC) accounts for 70% of the whole salivary gland cancer, with 24 pathological subtypes (2–4). At present, surgery is the standard option for PGC, and adjuvant radiotherapy is suggested when indicated, which has been previously documented to improve the survival rate and local control effect (5–7). According to some published articles, the 5-year disease-free survival rate was 69%–93.6% (8, 9) and the 5-year overall survival (OS) rate was 76%–94.6% for PGC (9, 10). Nowadays, doctors evaluate treatment outcomes and assess patients’ survival by the standard of the TNM system (11). However, in the TNM system, the lymph node status alone might not reliably predict prognosis (12–14), and some previous studies have shown that the lymph node ratio (LNR, the number of positive lymph nodes divided by the number of neck lymph nodes dissected) is another prognostic factor for head and neck cancers (12, 14–16), even though the number of these studies are still few. According to these studies, patients with different LNR levels had various survival outcomes in the same pathological stage diseases. Practically, clinicians give suggestions on follow-up and the mode of adjuvant therapy based on the evaluation of patients’ survival outcomes.
Therefore, the necessity to precisely predict the prognosis of PGC patients is evident. Thus, we analyzed patients’ data obtained from Surveillance Epidemiology and End Results (SEER) and Sun Yat-sen University Cancer Center (SYSUCC) databases and intended to provide findings derived from different databases to verify the prognostic significance of LNR in PGC. In addition, this present study was designed to choose an appropriate value of LNR with improved prediction efficiency for long-term survival in PGC patients.
Patients and Methods
Patients
The study was approved by the Clinical Research Ethics Committee of Sun Yat-sen University Cancer Center (approval number: B2018-175-01), and the informed consent of patients was waived. A total number of 3529 patients who underwent parotidectomy between 2004 and 2015 were enrolled in this study retrospectively. Patients who were eligible for this cohort study were pathologically confirmed stage I–IV according to the 8th edition of the American Joint Committee on Cancer Staging Manual. In addition, these patients were included in this study who met the following conditions: (1) pathologically diagnosed with parotid gland cancer, (2) one primary only, (3) surgical resection was not performed; and (4) complete follow-up. The exclusion criteria are shown in Figure 1.
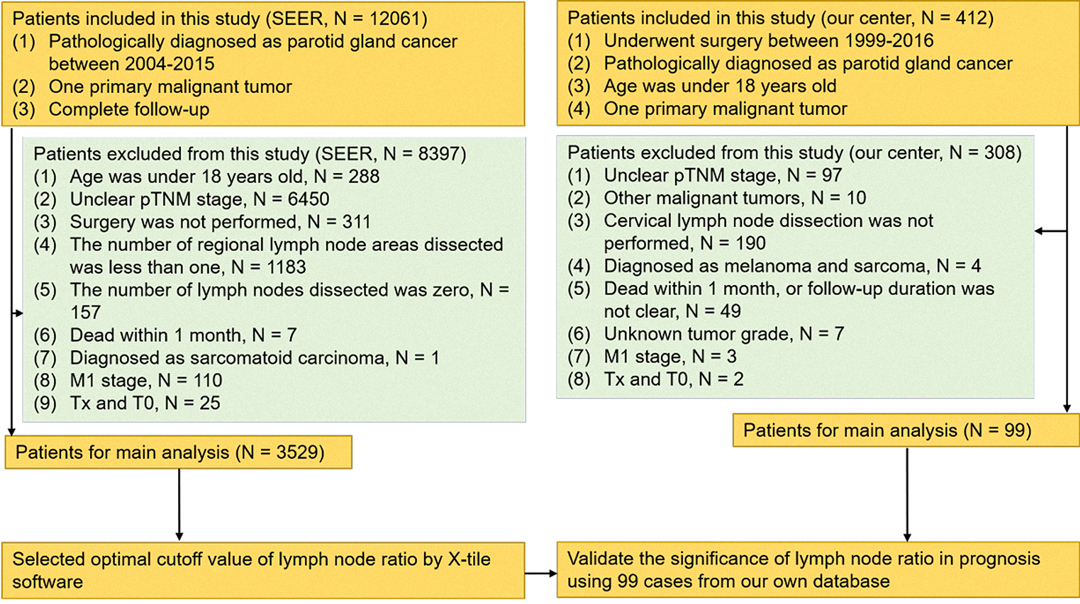
Figure 1. Diagram of the patient screening process in the Surveillance Epidemiology and End Results and our center databases.
Furthermore, the data derived from the SYSUCC database were used to validate the results from SEER. According to similar screening criteria, there were 99 patients selected from the SYSUCC database as an external validation cohort. These patients took operation between 1999 and 2016. The flow chart of the study is shown in Figure 1.
Follow-up
In the SEER database, the median follow-up time was 45 months (range 0–155 months). At SYSUCC, those patients were followed up by telephone regularly by the professional follow-up department. The median follow-up time with patients from surgery to the last contact was 64 months (range 1–195 months). The last follow-up date was February 20, 2020, and no patients were lost to follow-up.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 25.0 software (IBM SPSS, Inc., Chicago, IL, USA) and X-tile version 3.6.1 (http://www.tissuearray.org/rimmlab). X-tile software was conducted to choose the optimal cutoff point of LNR (17). Previous studies have shown that X-tile software was similar to the time-varying receiver operating characteristic curve analysis and could provide the best cutoff value for continuous data (18, 19). Chi-squared tests and Fisher exact tests were applied to evaluate the association between clinical variables and LNR groups of different levels. Hazard ratios (HRs) with 95% confidence interval (CI) were calculated by univariable and multivariable Cox proportional hazard regression analyses. Univariable and multivariable analyses were performed to evaluate the effect of sex, age, LNR, marital status, grade, radiotherapy, chemotherapy, race, drinking history, pathological N (pN) stage, pathological T (pT) stage, smoking history, and histological subtypes on CSS. Variables with p < 0.05 in univariable analysis or affecting prognosis (such as sex, approaches of treatment, and tumor differentiation) were selected to enter the multivariable analysis to further confirm the independent prognostic factors. Additionally, Kaplan–Meier analysis and log-rank tests were applied to compare survival curves between groups. The point of CSS as a primary clinical endpoint was considered most clinically relevant, and it was statistically considered significant when the results of all statistical tests met a two-sided p < 0.05. Patients from the SYSUCC database were stratified using the cutoff point of LNR defined in the SEER data set into two subgroups.
Results
Patient Characteristics
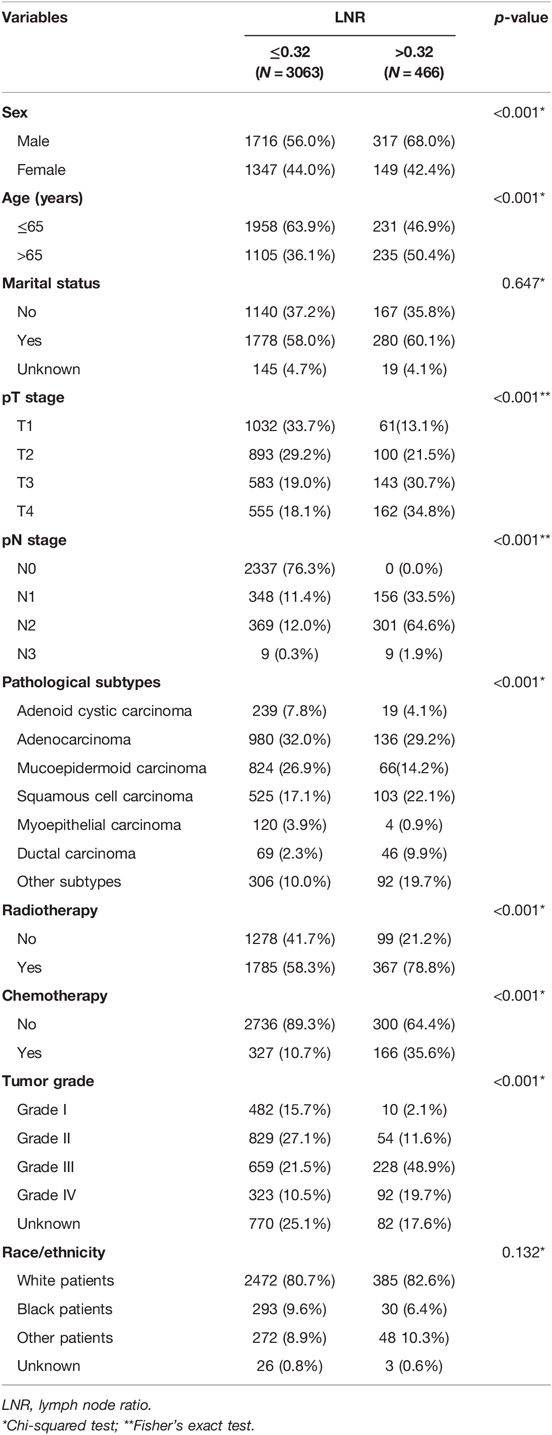
The clinical characteristics of the patients from the SEER database are listed in Table 1. Among the 3529 cases, 2033 (57.6%) of them were men and 1496 (42.4%) of them were women (male vs. female = 1.36:1). The age ranged from 18 to 104 years (median, 60 years). In the SEER cohort, the 1-, 3-, and 5-year CSS rates were 92.0%, 87.0%, and 84.0%, respectively, with the median survival time of 44 months. Lymph node metastasis was reported in 1188 cases (33.7% of the SEER cohort). The optimal cutoff value of LNR was determined by X-tile as 0.32 based on the SEER database.
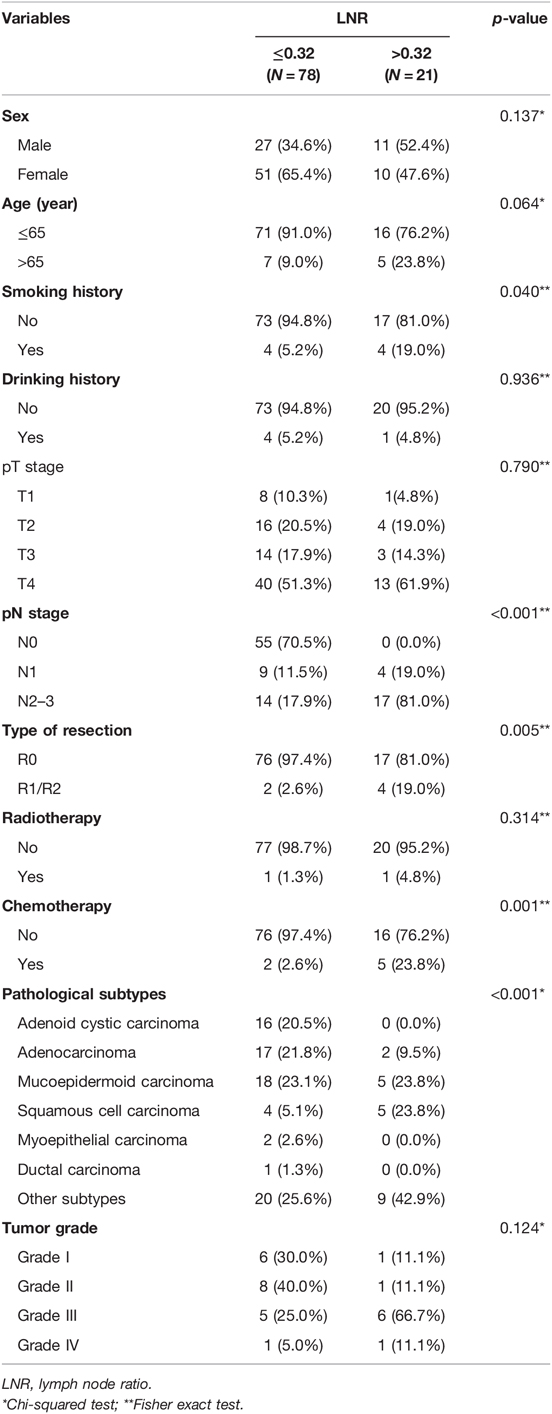
In the SYSUCC cohort, the 1-, 3-, and 5-year CSS rates were 81.0% vs. 72.0% vs. 68.0%, respectively, and the median survival time was 63 months. The clinical characteristics of the SYSUCC cohort are listed in Table 2. Of the whole 99 patients, 43 cases (43.4% of the SYSUCC cohort) with pathologically positive lymph nodes.
Univariable and Multivariable Analyses of SEER
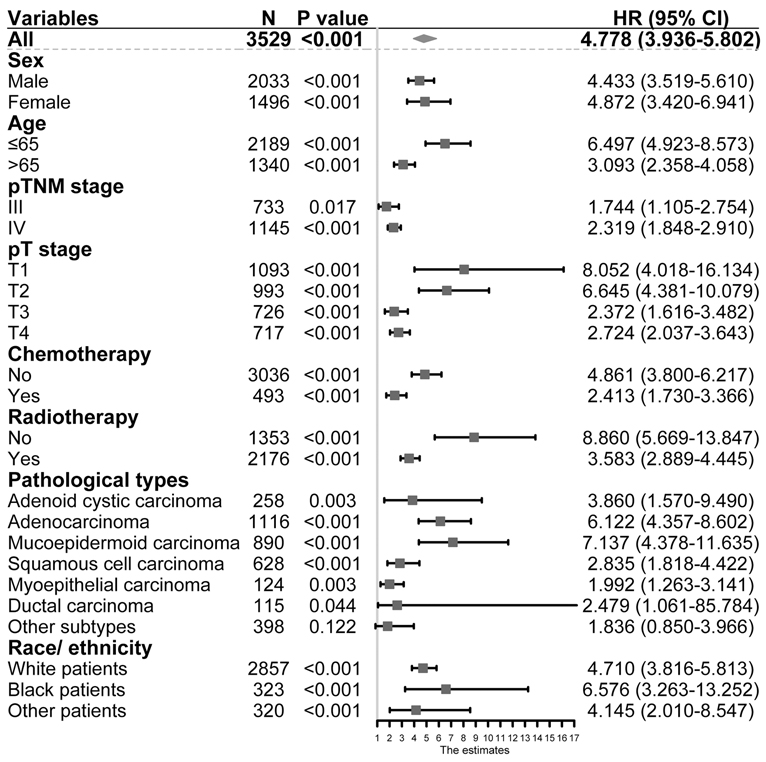
As shown in Table 3, univariable and multivariable analyses identified the following variables as independent prognostic factors for PGC patients: LNR (adjusted HR 4.778, 95% CI 3.936–5.802; p < 0.001), sex, age, radiotherapy, chemotherapy, pT stage, pN stage, tumor grade, and pathological subtypes. Our results revealed that the 12-month, 36-month, and 60-month CSS rates in the subgroup of LNR > 0.32 were 75%, 61%, and 56% compared to 95%, 81%, and 88% in the subgroup of LNR ≤ 0.32. We found that there was a statistically significant difference in CSS rates between the LNR ≤ 0.32 and LNR > 0.32 group (Figure 2A, unadjusted HR 4.778, 95% CI, 3.936–5.802, log-rank test: p < 0.001).
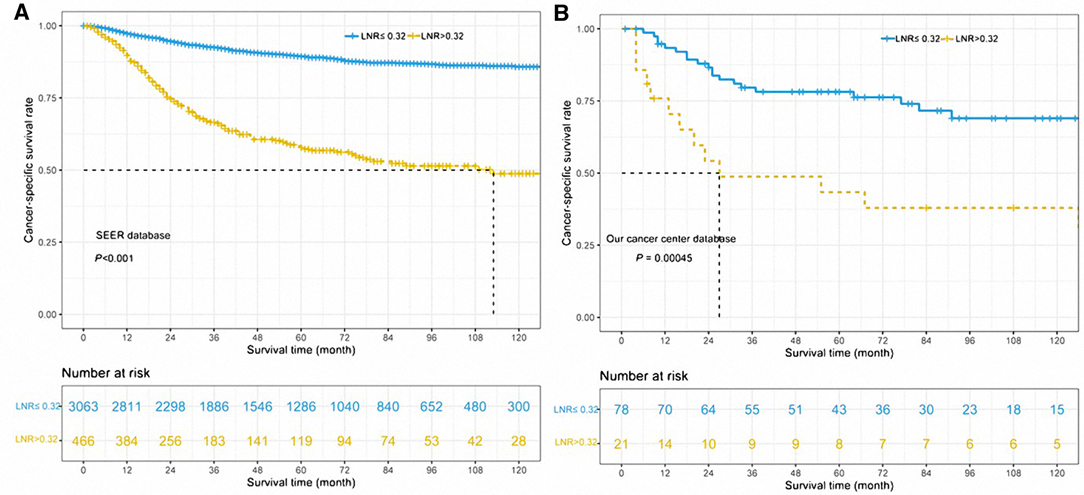
Figure 2. Cancer-specific survival curves for parotid gland cancer patients according to the lymph node ratio in the cohort of Surveillance Epidemiology and End Results database (A) and our center database (B).
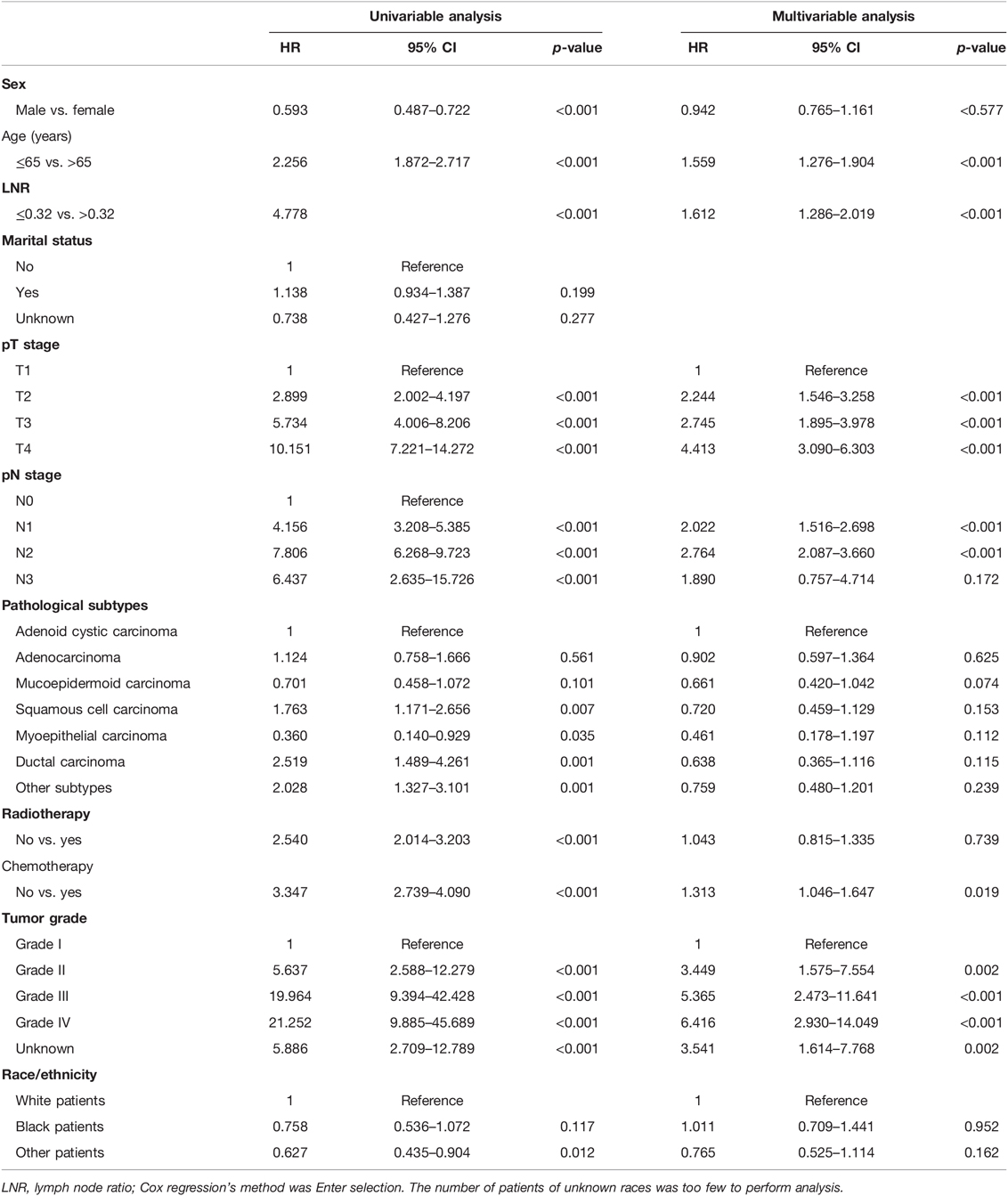
Table 3. Univariable and multivariable Cox proportional hazard regression analyses for overall survival in parotid gland cancer patients with stage I–IV from Surveillance Epidemiology and End Results database.
Validation for the Survival Impact of LNR in the SYSUCC Database
In order to validate the impact of LNR on CSS in parotid gland cancer patients, we enrolled another 99 patients from SYSUCC as an external cohort. We stratified the patients within this validation group into the subgroup of LNR > 0.32 and the subgroup of LNR ≤ 0.32, and the latter was found with worse CSS outcomes (unadjusted HR 2.657, 95% CI, 1.319–5.351, log-rank test: p = 0.00045, Figure 2B). Our results revealed that the 12-month, 36-month, and 60-month CSS rates in the subgroup of LNR > 0.32 were 54%, 48%, and 44% in the subgroup of LNR > 0.32 compared to 88%, 78%, and 74% in the subgroup of LNR ≤ 0.32.
Subgroup Analysis for CSS
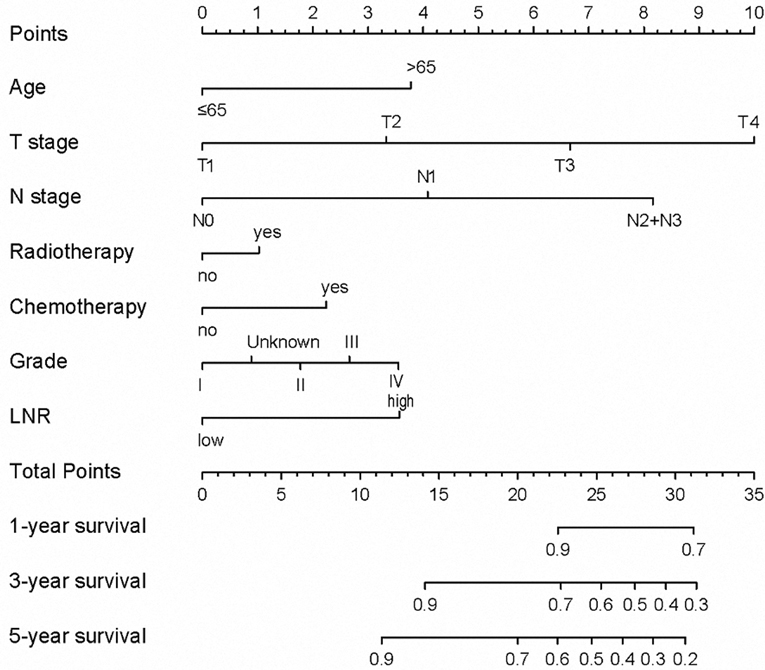
The N stage was an important factor in affecting survival (Table 3). After adjusting for other confounders, LNR was confirmed to be an independent prognostic factor in the present study (Table 3). Based on the results of multivariable Cox proportional hazard regression analyses, a nomogram for prognostic prediction was established for the SEER cohort (Figure 3). The visual nomogram showed the weights of variables affecting the prognosis of PGC.
To investigate the association between LNR and N stage, we performed the Kaplan–Meier method to compare the survival of patients with different LNR levels in the cohorts with stages N1, N2, and N3. The results showed that in N2 and N3 stage groups, patients with LNR ≤ 0.32 had superior CSS outcomes over those with the LNR > 0.32 group, but this benefit was absent in the N1 stage group (Figure 4). Besides, our results showed that LNR could be a risk indicator among the population of different histological subtypes except for the cohort with other subtypes (all p’s < 0.05, Figure 5). Limited by the number of patients, the subgroup survival curve was not drawn in our center. Our results showed that unadjusted HR exceeded 1 or, in other words, LNR > 0.32 could be a risk indicator among the population of different histological subtypes except for cohorts with other subtypes (Figure 5).
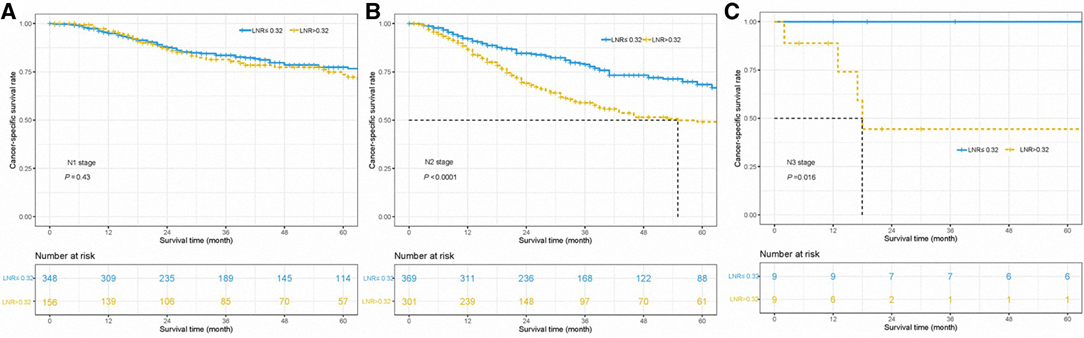
Figure 4. Cancer-specific survival curves for parotid gland cancer patients with stage N1 (A), N2 (B), and N3 (C) according to the lymph node ratio in the cohort of Surveillance Epidemiology and End Results database and Sun Yat-sen University Cancer Center.
Discussion
In the present study, we analyzed the data of patients with PGC from two research databases: SYSUCC and SEER. The data of SEER was set as the training group and analyzed by univariable and multivariable Cox regression, and then, the results were validated in the SYSUCC database. LNR and tumor grading were considered independent prognostic factors, and in this study, we found that LNR ≤ 0.032 was associated with better CSS for patients with PGC. Furthermore, LNR was found to be a prognostic indicator among the population of different histological subtypes except for cohorts with other subtypes and LNR was also identified to have an excellent discriminated efficiency in stage III–IV groups and each T stage by the subgroup analysis. Actually, the relative data for LNR could be easily obtained from the medical record, which makes it rather practical to validate our results by multi-institution study and be applied in clinical work.
Previous studies had revealed that LNR could be a prognostic indicator for PGC patients. Meyer et al. retrospectively analyzed the medical data of 128 patients with PGC by multivariable Cox regression and suggested that LNR < 0.1 was an independent protective prognostic factor for OS (HR, 0.332, p = 0.043) (13). However, the TNM stage was not a significant prognostic factor in their study after adjusting for other factors. Another study by Elhusseiny et al. revealed that LNR > 0.3333 was a risk prognostic factor for major salivary gland cancer, including 4608 PGC patients (20). In addition, the diseases’ stage was also proved an independent prognosticator for this malignancy in Elhusseiny’s research. As for our study, T stage, N stage, and LNR were both proved to be independent prognostic factors by analyzing the SEER data. Our findings were similar to Elhusseiny’s, and different from Meyer’s, and his disparity might be derived from the difference in sample size. In Meyer’s study, adjuvant radiotherapy followed surgery did not provide a survival benefit for PGC patients. The findings were similar to our results. The results of the present study revealed that radiotherapy could not serve as a protective prognostic factor for PGC patients after adjusting for other confounders. Besides, Elhusseiny’s study did not conclude that adjuvant radiotherapy is a protective factor for PGC patients. Our research excluded patients who did not receive surgery and patients whose number of lymph-node-dissection areas was less than one. Elhusseiny’s research included patients without surgery yet and did not restrict the lymph-node-dissection area on patients. Though our patient screening process was different from theirs, we got similar findings. Therefore, prospective studies are still needed to demonstrate the significance of radiotherapy in patients undergoing surgical resection of PGCs.
We also found that histological subtypes could significantly affect the CSS by analyzing the data of the SEER database. However, the results in the univariable analysis and multivariable analysis were not exactly consistent. In the results of univariable analysis, squamous cell carcinoma, ductal carcinoma, and other subtypes were considered risk indicators affecting CSS, while myoepithelial carcinoma was associated with better CSS (Table 3). However, after adjusting for other confounders, the pathological subtypes did not play a role in impacting the survival of PGC patients. In fact, the mucoepidermoid carcinoma and myoepithelial carcinoma were seen as low-risk histological subtypes according to the risk stratification of WHO-recognized salivary gland malignancies, which were not prone to lymph node metastasis (21). Qian et al. suggested that histological subtypes could affect the cancer-specific survival of salivary gland cancer patients independently (22). However, in this study, we could not conclude similar conclusions to theirs. The first reason might be that the selected cases of the two studies were different. We selected patients with PGC; however, they selected patients with all salivary gland carcinoma. The second reason was that they classified pathological subtypes as high- and low-risk groups; however, we separated each subtype in order to explore the role of LNR in different pathological subtypes. Therefore, we suggest that future research should focus on the influence of pathological subtypes on the prognosis of PGC patients.
There are some limitations to our study. First, the sample size of PGC patients was relatively limited in the SYSUCC database, and the data distribution of the TNM stage was not balanced in the two databases. Therefore, the data of SYSUCC were only used to perform the Kaplan–Meier analysis in order to validate the results from the SEER database. To improve this aspect, the sample size would need to be expanded in further studies. Second, the patients’ data used in this study originated from limited academic databases, which might also impact the accuracy of our findings, so a larger scale of patients’ information from multiple centers was necessary to confirm our results. Third, these findings could only provide certain reference information to the clinicians but not the treatment recommendations. Doctors would need to make decisions on the patients’ treatment according to the relevant guidelines and clinical experience. Fourth, data on molecular diagnosis were absent, which might be effective prognostic factors contributing to a more accurate prediction. Therefore, substantial research at the molecular level is still needed to further improve the proposed findings.
Conclusions
In conclusion, the lymph node ratio turned out to be an independent prognostic factor for cancer-specific survival in this study. This valuable information could help clinicians evaluate the prognosis of parotid gland cancer and suggest that adequate lymph node dissection is necessary.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The study was approved by the Clinical Research Ethics Committee of Sun Yat-sen University Cancer Center (approval number: B2018-175-01), and the informed consent of patients was waived by the Clinical Research Ethic Committee of Sun Yat-sen University Cancer Center. Patient information was anonymized and deidentified prior to analysis. The human data and the study were in accordance with the Declaration of Helsinki in the manuscript. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Author Contributions
Conception and design: W-MJ, Y-FC, and QZ. Administrative support: Y-FC and QZ. Provision of study materials or patients: QZ and Y-FC. Collection and assembly of data: J-FX, JC, G-LL, and Y-FG. Data analysis and interpretation, including all tables and all figures: W-MJ, J-FX, JC, and G-LL. Manuscript writing: all authors. Final approval of manuscript: all authors. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors sincerly thank colleagues in the Department of Head and Neck Surgery, Sun Yat-sen University Cancer Center, and staff in the Surveillance Epidemiology and End Results database. W-MJ sincerely thanks Dr. WLL for his kindly help and spiritual support. Last but not least, the authors thank their patients who are willing to provide personal information for medical research and they are the best teachers for doctors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA, et al. Salivary gland carcinoma in Denmark 1990–2005: outcome and prognostic factors. Results of the Danish Head and Neck Cancer Group (DAHANCA). Oral Oncol. (2012) 48:179–85. doi: 10.1016/j.oraloncology.2011.09.005
2. Fang Q, Liu F, Seng D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manage Res. (2019) 11:1081–85. doi: 10.2147/CMAR.S192788
3. Seethala RR, Stenman G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the salivary gland. Head Neck Pathol. (2017) 11:55–7. doi: 10.1007/s12105-017-0795-0
4. Niu X, Fang Q, Liu F. Role of intraparotid node metastasis in mucoepidermoid carcinoma of the parotid gland. BMC Cancer. (2019) 19:417. doi: 10.1186/s12885-019-5637-x
5. Spiro IJ, Wang CC, Montgomery WW. Carcinoma of the parotid gland: analysis of treatment results and patterns of failure after combined surgery and radiation therapy. Cancer. (1992) 71:2699–2705. doi: 10.1002/1097-0142(19930501)71:9<2699::AID-CNCR2820710902>3.0.CO;2-T
6. Noh JM, Ahn YC, Nam H, Park W, Baek CH, Son YI, et al. Treatment results of major salivary gland cancer by surgery with or without postoperative radiation therapy. Clin Exp Otorhinolaryngol. (2010) 3:96–101. doi: 10.3342/ceo.2010.3.2.96
7. Nagliati M, Bolner A, Vanoni V, Tomio L, Lay G, Murtas R, et al. Surgery and radiotherapy in the treatment of malignant parotid tumors: a retrospective multicenter study. Tumori. (2009) 95:442–448. doi: 10.1177/030089160909500406
8. Chang JW, Hong HJ, Ban MJ, Shin YS, Kim WS, Koh YW, et al. Prognostic factors and treatment outcomes of parotid gland cancer: a 10-year single-center experience. Otolaryngol Head Neck Surg. (2015) 153:981–89. doi: 10.1177/0194599815594789
9. Poorten VV, Hart A, Vauterin T, Jeunen G, Schoenaers J, Hamoir M, et al. Prognostic index for patients with parotid carcinoma: international external validation in a Belgian-German database. Cancer. (2009) 115:540–50. doi: 10.1002/cncr.24015
10. Stenner M, Molls C, Luers JC, Beutner D, Klussmann JP, Huettenbrink KB. Occurrence of lymph node metastasis in early-stage parotid gland cancer. Eur Arch Otorhinolaryngol. (2012) 269:643–48. doi: 10.1007/s00405-011-1663-2
11. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2020).
12. Patel SG, Amit M, Yen TC, Liao CT, Chaturvedi P, Agarwal JP, et al. Lymph node density in oral cavity cancer: results of the international consortium for outcomes research. Br J Cancer. (2013) 109:2087–2095. doi: 10.1038/bjc.2013.570
13. Meyer MF, Kreppel M, Meinrath J, Grünewald I, Stenner M, Drebber U, et al. Prediction of outcome by lymph node ratio in patients with parotid gland cancer. Clin Otolaryngol. (2017) 42:98–103. doi: 10.1111/coa.12672
14. Gil Z, Carlson DL, Boyle JO, Kraus DH, Shah JP, Shaha AR, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. (2009) 115:5700–5710. doi: 10.1002/cncr.24631
15. Liao CT, Hsueh C, Lee LY, Lin CY, Fan KH, Wang HM, et al. Neck dissection field and lymph node density predict prognosis in patients with oral cavity cancer and pathological node metastases treated with adjuvant therapy. Oral Oncol. (2012) 48:329–336. doi: 10.1016/j.oraloncology.2011.10.017
16. Amit M, Tam S, Boonsripitayanon M, Cabanillas ME, Busaidy NL, Grubbs EG, et al. Association of lymph node density with survival of patients with papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg. (2018) 144:108–114. doi: 10.1001/jamaoto.2017.2416
17. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. (2004) 10:7252–59. doi: 10.1158/1078-0432.CCR-04-0713
18. Wu LL, Liu X, Jiang WM, Huang W, Lin P, Long H, et al. Stratification of patients with stage IB NSCLC based on the 8th edition of the American joint committee on cancer (AJCC) staging manual. Front Oncol. (2020) 10:571. doi: 10.3389/fonc.2020.00571
19. Lv Y, Wang Z, Li K, Wang Q, Bai W, Yuan X, et al. Risk stratification based on CLIF consortium acute decompensation score in patients with child-pugh B cirrhosis and acute variceal bleeding. Hepatology. (2021) 73(4):1478–93. doi: 10.1002/hep.31478
20. Elhusseiny KM, Abd-Elhay FA, Kamel MG, Abd El Hamid Hassan HH, El Tanany HHM, Hieu TH, et al. Examined and positive lymph nodes counts and lymph nodes ratio are associated with survival in major salivary gland cancer. Head Neck. (2019) 41:2625–35. doi: 10.1002/hed.25742
21. Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. (2009) 3:69–77. doi: 10.1007/s12105-009-0102-9
22. Qian K, Sun W, Guo K, Zheng X, Sun T, Chen L, et al. The number and ratio of positive lymph nodes are independent prognostic factors for patients with major salivary gland cancer: results from the surveillance, epidemiology, and end results dataset. Eur J Surg Oncol. (2019) 45:1025–32. doi: 10.1016/j.ejso.2018.11.008
Keywords: Parotid gland cancer, lymph node ratio, survival, stage I–IV, SEER
Citation: Jiang W, Xu J, Chen J, Li G, Gao Y, Zhang Q and Chen Y (2022) Prediction of Long-Term Survival Outcome by Lymph Node Ratio in Patients of Parotid Gland Cancer: A Retrospective study. Front. Surg. 9:903576. doi: 10.3389/fsurg.2022.903576
Received: 24 March 2022; Accepted: 19 April 2022;
Published: 11 May 2022.
Edited by:
Małgorzata Wierzbicka, Poznan University of Medical Sciences, PolandReviewed by:
K Thomas Robbins, School of Medicine, Southern Illinois University Carbondale, USARance Fujiwara, David Geffen School of Medicine, University of California, USA
Copyright © 2022 Jiang, Xu, Chen, Li, Gao, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Feng Chen Y2hlbnlmQHN5c3VjYy5vcmcuY24= Quan Zhang emhhbmdxdWFuQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
Specialty section: This article was submitted to Otorhinolaryngology—Head and Neck Surgery, a section of the journal Frontiers in Surgery
Abbreviations: PGC, parotid gland cancer; LNR, lymph node ratio; SEER, Surveillance Epidemiology and End Results; SYSUCC, Sun Yat-sen University Cancer Center; OS, overall survival; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis.
 Wen-Mei Jiang
Wen-Mei Jiang Jian-Feng Xu2†
Jian-Feng Xu2† Yun-Fei Gao
Yun-Fei Gao Yan-Feng Chen
Yan-Feng Chen