- Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Background: This study aims to investigate the clinical efficacy of video-assisted thoracic surgery (VATS) in treating mediastinal mature teratoma (MMT) and explore the clinical factors that increase the difficulties associated with VATS.
Method: We retrospectively reviewed 101 consecutive patients with MMT who underwent surgical excision between November 2001 and June 2021. Follow-up was done by telephone or at an outpatient clinic. The deadline for follow-up was February 2022.
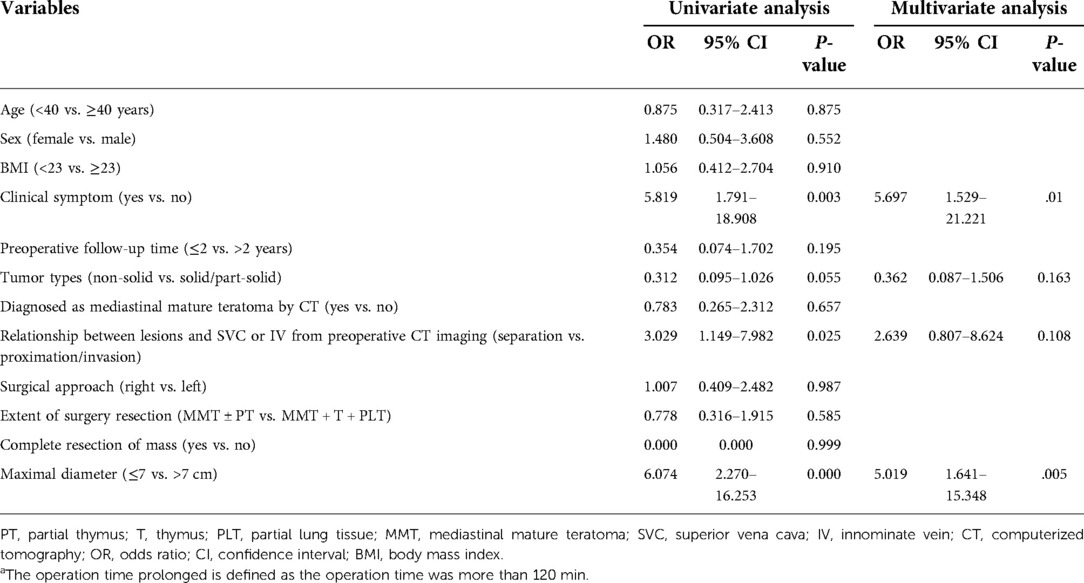
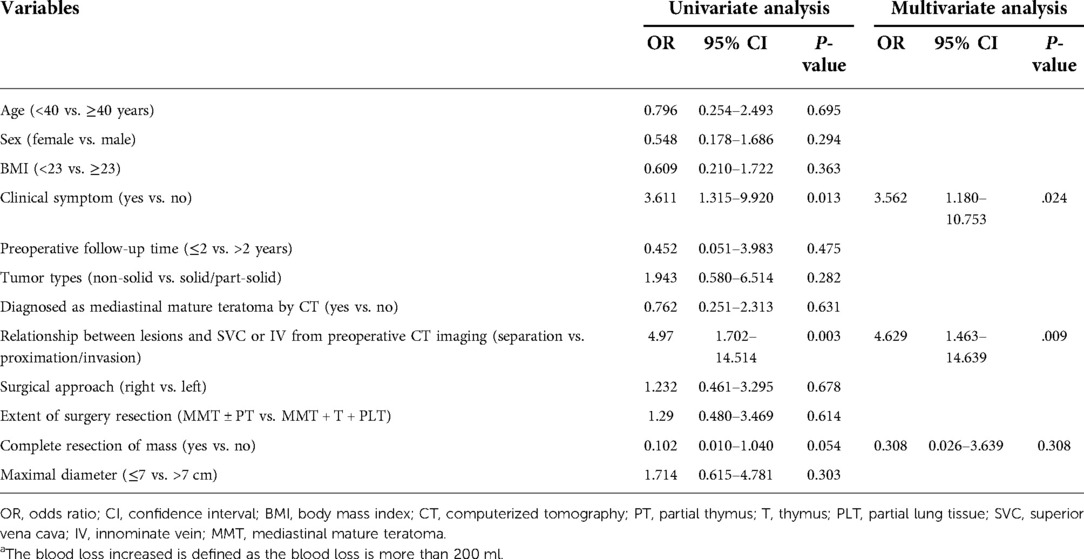
Results: The operative time, the chest tube indwelling time, and the hospital stay duration were significantly shorter in the VATS group compared with the thoracotomy group. The intraoperative and postoperative complication rates in the VATS group were lower than that of the thoracotomy group (P < .05). In thoracoscopic surgery, the clinical symptoms during the course of the disease were significantly associated with bleeding loss increasing [odds ratio (OR) = 3.562; 95% confidence interval (CI) 1.180–10.753, P = .024] and operation time extension (OR = 5.697; 95% CI 1.529–21.221, P = .010). The relationship between lesions and superior vena cava or innominate vein from preoperative CT imaging was significantly associated with bleeding loss increasing (OR = 4.629; 95% CI 1.463–14.639, P = .009). A maximal lesion diameter greater than 7 cm was significantly associated with increased risks of operation time extension (OR = 5.019; 95% CI 1.641–15.348, P = .005).
Conclusion: Compared with traditional thoracotomy surgery, VATS can be performed safely in selected patients with MMT. A surgical method for complete resection needs to be determined according to preoperative imaging and intraoperative conditions to reduce the unnecessary damage.
Introduction
Mediastinal teratoma (MT) is one of the most common mediastinal germ cell tumors, with an incidence rate of 5%–10% in primary mediastinal tumors (1). MT often occurs in the anterior mediastinum and contains histologic elements derived from at least two of the three embryonic cell layers: ectoderm, mesoderm, and endoderm. According to histologic differentiation, MT can be divided into mature and immature (2). Mediastinal mature teratoma (MMT) can be cured with complete surgical resection (3). So far, studies on MMT are mostly case reports and few extensive case analyses. Despite the rise of thoracoscopic technology (4), open thoracotomy is still preferred for cases with large tumors. There is still no clear indication in thoracoscopy decisions, including the sizes of the tumor, grades of adhesion, and surgical experience (5). This study aims to clarify the outcomes of video-assisted thoracic surgery (VATS) excision for MMT and to investigate the treatment strategy of MMT with a retrospective analysis over 20 years in our institution. To our knowledge, this is one of the most extensive case series of MMT treated via VATS.
Materials and methods
Based on Peking University People's Hospital institutional database, between November 2001 and June 2021, 101 consecutive patients diagnosed as MMT by histopathology after surgical excision were collected. See Figure 1 for a flowchart of the study screening protocol. The criteria for surgical removal of MMT are the following: (1) patients with symptoms caused by the mediastinal mass; (2) gradual enlargement of the mass during clinical observation; (3) preoperative imaging examination cannot exclude malignant tumor; (4) the patient has severe compression of important mediastinal structures, such as the pulmonary artery or main bronchus.
Preoperative management
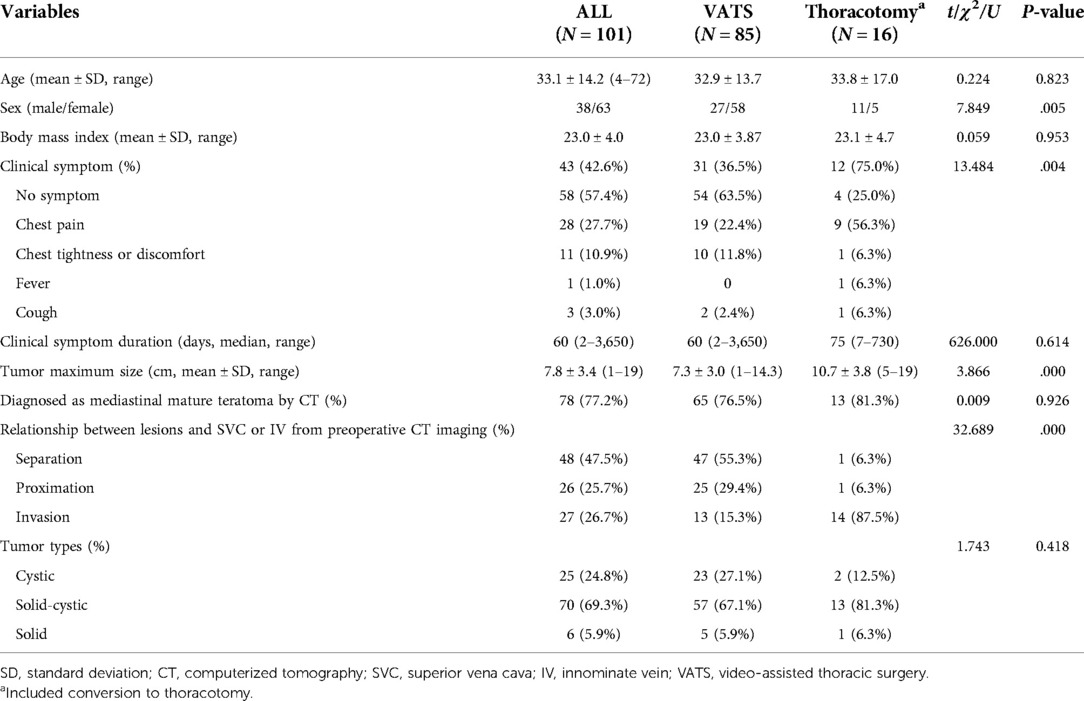
Chest computerized tomography (CT) scans were used for preoperative diagnosis in all patients. If necessary, magnetic resonance imaging (MRI) or PET-CT could be performed to get additional radiological information. According to the imaging characteristics, we divided the relationship between the MMT and superior vena cava or innominate vein (SVC/IV) found by preoperative CT into three types, including separation, proximation, and invasion (Figure 2): (A) separation is defined as a well-defined lesion, at a certain distance from the surrounding blood vessels; (B) proximation is defined as a clear boundary between the lesion and surrounding organs, and the mass partially deforms the blood vessel; (C) invasion is defined as the non-fatty lesion invaded the surrounding vessels. The clinical symptoms, preoperative imaging data, surgical procedure, and prognosis of these patients were analyzed.
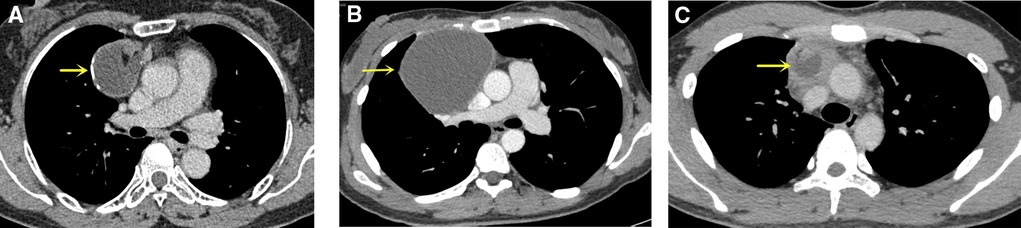
Figure 2. Mediastinal mature teratomas on the CT image. (A) Separation is defined as a well-defined lesion, at a certain distance from the surrounding blood vessels (SVC and IV); (B) Proximation is defined as a clear boundary between the lesion and surrounding organs, and the blood vessel is partially deformed by the mass; (C) Invasion is defined as the non-fatty lesion invaded the surrounding vessels. The yellow arrow points to MMT. CT, computerized tomography; SVC, superior vena cava; IV, innominate vein; MMT, mediastinal mature teratoma.
Operation technique
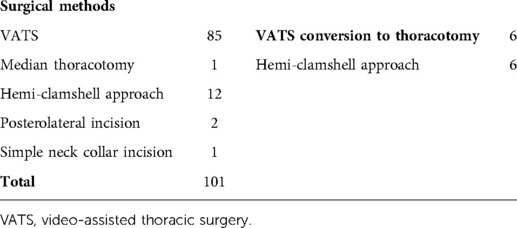
All patients accepted surgical resection. Surgical resections included thoracoscopic surgery with or without conversion to thoracotomy, semi-clamshell, median thoracotomy, simple neck collar incision, and posterolateral thoracotomy, which were performed according to the relationship between the surrounding tissue and tumor location, tumor size, invasion, and pleural adhesions.
After preoperative preparation, patients were under general anesthesia with one-lung ventilation by double-lumen endotracheal intubation. VATS technique was initially performed for selected patients. The surgical procedures were conducted in the 30°–45° lateral recumbent position with a three-port or two-port approach. The camera port (1 cm) was first created in the fifth intercostal space on the mid-axillary line. Under the direct thoracoscopic vision, the operator port (4 cm) and the assisted-operator port (1 cm) were selected according to the site of the teratoma. The operator port usually was made on the midclavicular line at the fourth to fifth intercostal spaces and the assisted-operator port was made on the anterior axillary line at the third intercostal spaces. The assisted-operator port might be omitted for simple cases.
When the tumor was densely attached to the surrounding tissues and could not be resected under the endoscope, or damaged the IV when mobilizing the MMT, conversion to thoracotomy was performed. The operation port was extended to 15 cm and connected to the camera hole, and the fourth sternocostal joint was cut to form a semi-clamshell incision at the lower margin of the breast. If the mass is located in the middle of the anterior mediastinum, adequate exposure of the hilar and thoracic structures on the surgical side is not required, direct median thoracotomy was chosen for patients whose preoperative operation evaluation is too difficult to perform under the endoscope. A simple cervical incision is used if the MMT is located higher, below the thyroid. Difficulty operation was defined as the operation time exceeding 120 min or the intraoperative blood loss more significant than 200 ml.
Our surgical principles are the following: (1) the teratoma should be resected entirely as far as possible, and the extravasation of cystic contents should be avoided; (2) when complete excision could not be performed for severe adhesions, the residual part of the tumor should be destroyed by chemical agents or physical damage; (3) when thymoma or other malignant tumors of the thymus could not be excluded according to preoperative examination or intraoperative exploration, thymectomy should be performed (6). At the end of the surgery, one 24 Fr drainage tube was placed in the thoracic cavity.
Histologic examinations confirmed the diagnosis of mature teratoma based on the existence of multilayer epithelial cells and their appendages such as hair follicles, sebaceous glands, sweat glands on the cystic wall, and three layers of various tissue components microscopically.
Postoperative follow-up
All patients accepted scheduled follow-up done by telephone or at an outpatient clinic. The postoperative complications were documented, and the deadline for follow-up was February 2022.
Statistical analysis
Statistical analyses were performed using the SPSS version 23.0 package (IBM Corporation, Armonk, NY, USA). Descriptive analyses were presented as median ± standard deviation for normally distributed variables and median or ranges for non-normally distributed variables. The Student's t-test was used for the comparisons of normally distributed variables, and the Mann–Whitney U test was used for comparing non-normally distributed variables. Categorical variables were compared by the Chi-square test.
Univariate and multivariable logistic regression analyses were used to identify independent predictors for operative difficulty. Predictors (P < 0.1) in the univariate analysis would be included in a multivariable analysis. P-values <.05 were considered statistically significant.
The study was approved by the ethics committee of the Department of Thoracic Surgery at Peking University People's Hospital. Written consent was obtained from all patients before surgery.
Results
Clinical characteristics
A total of 63 female and 38 male patients had an average age of 33.1 ± 14.2 years (range 4–72 years). Among these patients with mature teratomas, 58 cases (57.4%) were asymptomatic at the first time of diagnosis by radiological examinations incidentally. The most common symptoms included chest pain (27.7%), chest tightness or discomfort (10.9%), and other symptoms, including cough (3.0%) and fever (1.0%). There were 100 teratomas located in the anterior mediastinum and one in the posterior mediastinum. All the patients' preoperative laboratory tests for alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (β-hCG) levels were at normal levels. According to the methods of the operation, we divided the patients into two groups: the VATS group (the whole course of the procedure was completed by thoracoscopy) and the Thoracotomy group (the process was completed by thoracotomy, including VATS conversion to thoracotomy). Only 77.2% (78/101) of patients were correctly diagnosed with teratoma by CT before surgery, and the rate was 81.3% (13/16) in the thoracotomy group and 76.5% (65/85) in the VATS group. Other radiographic diagnoses included mediastinal cyst in six patients, thymoma in six patients, mediastinal abscess in two patients, pericardium cyst in one patient, and mediastinal mass with undefined diagnosis in eight patients. No specific pathological diagnoses were made before surgery. The demographic and preoperative data of these patients are shown in Table 1. And there were significant differences between the VATS group and the thoracotomy group in gender, clinical symptoms, tumor diameter, and details of the mass and vessels shown on preoperative CT (P < .05).
Surgical results
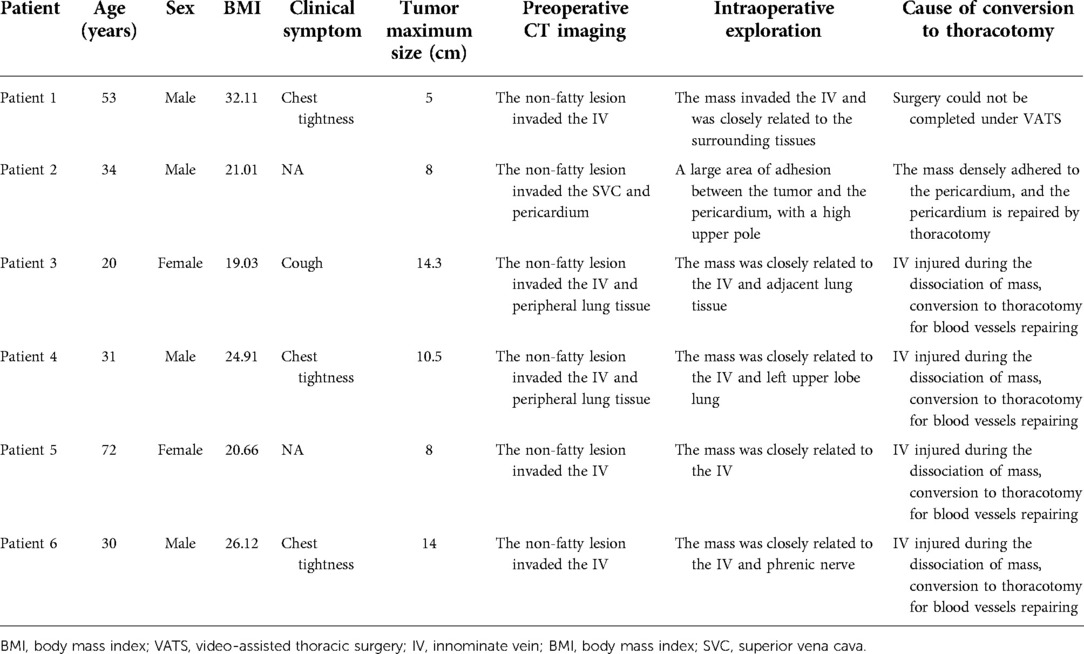
The surgical methods of the 101 patients are summarized in Table 2. Furthermore, six patients were VATS converted to thoracotomy. Among them, two patients were actively converted to thoracotomy because of difficulty in surgery; the remaining four were passively due to intraoperative injury of the IV. The essential data, preoperative imaging data, and intraoperative details of these six patients are summarized in Table 3.
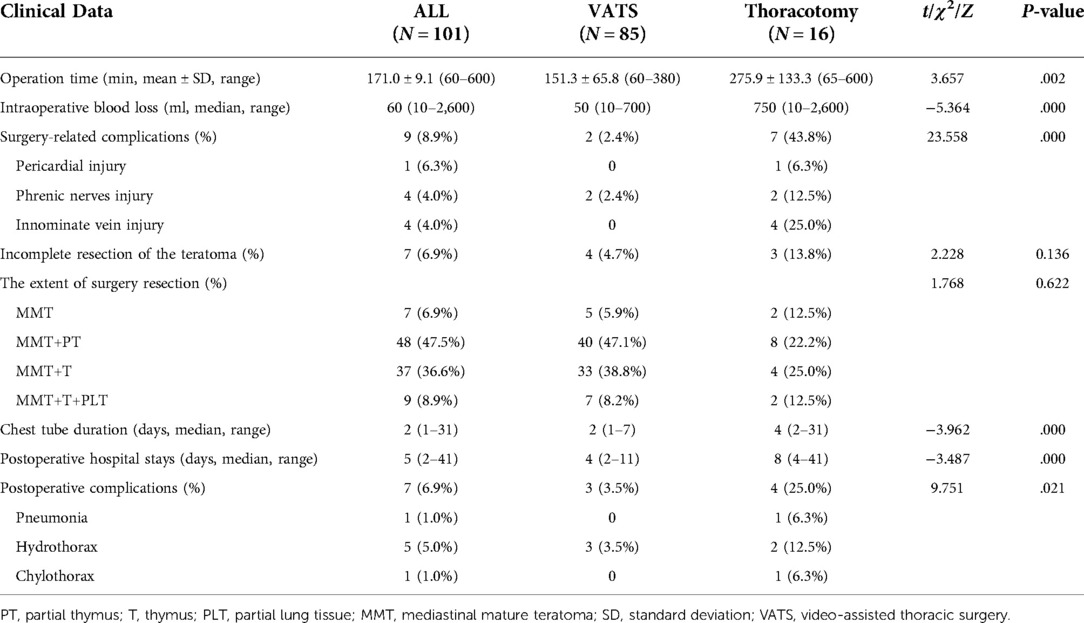
The average operation time was 171.0 ± 9.1 min (range, 60–600 min), and the median intraoperative blood loss was 60 ml (range, 10–2,600 ml). The median postoperative drainage tube removal time was 2 days (range, 1–31 days). The postoperative hospital stay was 5 days (range, 2–41 days). In seven patients with MMT, the resection was incomplete because the teratoma severely adhered to the SCV/IV, pericardium, and lung tissue, and the tumor could not be completely freed. The pericardial injury occurred in one patient, phrenic nerve injury in four patients, and IV injury in four patients during surgery. There were no perioperative period deaths (within 30 days of surgery). After symptomatic treatment, the patients improved. VATS was associated with less intraoperative blood loss and intraoperative complications; postoperative complications, drainage tube removal time, and length of hospital stay were shorter than thoracotomy (P < .05) (Table 4). After the operation, 101 cases were followed up, with three cases being lost to follow-up. The follow-up time was 12–243 months. None of the patients had relapses during follow-up.
Univariate and multivariate logistic regression analyses were performed to identify factors that may increase the operative time (more than 120 min) and intraoperative bleeding (more than 200 ml) in VATS surgery. Multivariate analysis showed that the largest diameter greater than 7 cm [odds ratio (OR) = 5.019; 95% confidence interval (CI) 1.641–15.348, P = .005] and clinical symptoms during the course of the disease (OR = 5.697; 95% CI 1.529–21.221, P = .010) were significantly associated with longer operative time (Table 5). In addition, multivariate analysis showed that clinical symptoms (OR = 3.562; 95% CI 1.180–10.753, P = .024) and preoperative chest CT showed a strong relationship between teratoma and SVC/IV (OR = 4.629; 95% CI 1.463–14.639, P = .009) were significantly associated with an increased risk of blood loss (Table 6).
Discussion
MMT, due to its own characteristics, may have no obvious clinical symptoms for its small size in the early stage, so it is more often found by chance during physical examination. With the increase of age, the enlarged tumor is often found by examination due to compression of surrounding organs, secondary infection, or corresponding clinical symptoms when penetrating the surrounding tissue. At present, the diagnosis mainly relies on chest CT to see calcification in the tumor, and even teeth or bones (7). However, most MMT is cystic or cystic-solid masses, easily misdiagnosed as thymoma before surgery. Only 77.2% of the patients with a mediastinal mass can be correctly diagnosed as MMT before surgery in our center. Surgical resection is the only effective treatment for MMT (8).
With the development of thoracoscopy technology for more than 20 years and the advancement of medical devices, VATS has obvious advantages such as minor trauma, less intraoperative blood loss, shorter postoperative hospital stays, and faster recovery than thoracotomy. Therefore, the indications of thoracoscopic surgery have gradually expanded, and it basically covers all fields of modern thoracic surgery (9). For mediastinal tumors, which mainly occur in young people, this group of people has higher aesthetic requirements, so thoracoscopy is more widely used in mediastinal tumor resection. Until now, the largest series of endoscopic treatment of MT is only 22 cases (10); the first case of thoracoscopic MMT resection in our center was carried out in 2001, and 87 cases of thoracoscopic MMT resection were completed in the past 20 years. At present, thoracoscopy mainly adopts the right-side approach. For tumors that are biased to the left, the left thoracic incision or the subxiphoid system can be used. Compared with its advantages, its disadvantage is the poor exposure of the lateral mediastinum and superior mediastinum, especially when the tumor is large or has heavy adhesion to the surrounding tissue, which often leads to incomplete tumor resection, and it is easy to accidentally injure bilateral phrenic nerves and internal mammary artery and IV (11). Among the thoracotomy criteria for anterior mediastinal tumor resection, such as severe compression of important mediastinal structures, median sternotomy has a clear surgical field, which can calmly face various unexpected situations that may occur during surgery and even add extracorporeal circulation and other advantages. In our center, one case of MMT was treated with median sternotomy. However, sternotomy also has certain limitations. The structure of the thoracic cavity and hilum of the operation side is poorly exposed, the damage is extensive, the operation is complicated, the equipment requirements are high, and the probability of sternal complications is also high. For complicated MMT, a Hemi-clamshell incision is mainly used in our center. Its advantage is that the mediastinum and the important viscera of the hilar lung are under the direct view of the surgeon during the operation. When necessary, the sternum can be transected to a clamshell incision to take various emergency measures such as extracorporeal circulation. Low surgical instrument requirements and no need for swinging saws can also avoid sternum infection and sternum nonunion complications. At the same time, if the tumor did invade the SVC and IV, and the tumor could not be completely removed during the operation, we would choose to send the intraoperative frozen pathology. If the pathology indicated a benign condition, incomplete resection would be taken after full communication with the patient's family to ensure the safety of the operation, and a regular postoperation review would be conducted. If the pathology indicates malignancy and vascular replacement is needed, the sternum can be transected to make a clamshell incision. At the same time, if thoracoscopic exploration is required first, the patient only needs a 30–45° lateral decubitus position. When a thoracotomy is required, the patient can directly switch to a recumbent and semi-clamshell incision. Therefore, median sternotomy is rarely used for MMT in our center.
The surgical indication for thoracoscopy is usually defined as a mediastinal mass less than 6 cm (12). Although some centers have expanded the indication for thoracoscopic tumor resection, it is still smaller than 10 cm (1). At the same time, other factors besides tumor size, such as intraoperative exploration of the tumor, the surgeon's experience with thoracoscopy, available equipment and support devices, can also affect the decision of thoracoscopic surgery indications. Our findings suggest that the presence or absence of clinical symptoms and preoperative analysis of computed tomography (tumor size and tumor boundary with essential blood vessels) are two critical indicators of surgical difficulty for MMT. The difficulty in resection of giant MT is that the tumor occupies the thorax and the surgical field is poorly exposed, so it is easy to damage the surrounding important tissues. In our center, the CT size of the largest tumor removed by thoracoscopic surgery was 14.0 cm × 6.0 cm × 6.0 cm, with an average diameter of 7.3 cm. Attention should be paid during the operation: (1) thoracoscopic exploration can be performed first, and the tumor volume can be reduced by endoscopic operation; for cystic lesions, the cyst wall can be punctured and the cyst fluid can be sucked out to provide a wider thoracoscopic field of view, making it easier to remove completely (11); for larger solid tumors, part of the contents can be resected in the capsule to shrink the tumor and obtain more adequate processed after exposure; (2) if the operation under VATS is difficult and the tumor and surrounding adhesions are dense, the surgical incision should be enlarged as much as possible.
MMTs are distinguished from other benign mediastinal tumors by their aggressive growth characteristics. For symptomatic patients diagnosed with MMT, the boundary between the tumor and surrounding tissues is often unclear, and severe adhesions are prone to occur, so the surgical difficulty is also increased. At the same time, studies have shown no difference between the VATS group and the thoracotomy group in patients with teratoma in contact with the left brachiocephalic vein on preoperative CT, indicating the adhesion of the tumor and surrounding organs may be more critical than the tumor location (5). Chang et al. found that thoracoscopic surgery is feasible if no dense adhesions are found on preoperative radiographic evaluation, and symptoms may be a relative contraindication to thoracoscopic surgery because of its association with surgical complications (13). The deficiency of the study is that degree of dense adhesion is not clearly defined. Our study classified the degree of adhesion of the teratoma to the vital blood vessels, namely the SVC and the brachiocephalic vein, into three categories, namely, distant, compressive, and invasion. Multivariate analysis found that when the relationship between the tumor and vital blood vessels was compression and invasion, the difficulty of operation (increased intraoperative blood loss) increased significantly. In our center, six patients had converted to thoracotomy, of which two patients had converted to thoracotomy actively, and four patients had converted to thoracotomy passively due to intraoperative injury of the IV. The main reasons for the conversion of thoracotomy during thoracoscopic thoracotomy reported in other centers are (1) tight adhesion to surrounding tissues (pericardium, lung, phrenic nerve, and vital blood vessels), and the operation cannot be completed by VATS (14); (2) intraoperative damage to essential structures (pericardium, vital blood vessels, etc.) need to be opened for repair (15). Therefore, if combined with the patient's clinical symptoms before surgery and the imaging data suggest that the tumor is large and densely adhered to the surrounding area, it can be converted to minor incision surgery after endoscopic exploration to ensure the safety of the surgery.
Most teratomas are located in the anterior mediastinum near the bottom of the pericardium and connect with the residual tissue of the thymus (16). Teratomas may contain the pancreas, salivary glands, and other tissues to secrete digestive enzymes that act on surrounding tissues and are easy to form adhesions or perforations with adjacent tissues and organs. Its external penetration range includes the pleural cavity, pericardial cavity, lung, neck, and great vessels (17). Therefore, for an MMT with a smaller diameter, intact capsule, and no serious tissue adhesion, only the tumor and part or all of the thymus need to be removed entirely. When the tumor is huge, preoperative CT combined with intraoperative exploration reveals an invasion of adjacent tissues. (1) Lung: A double-lumen tube is inserted into the trachea during the operation to prevent contralateral aspiration or sowing during the operation (18). Whether or not to perform lobectomy at the same time should be determined according to the specific circumstances of the tumor invading the lung. Pulmonary resection was performed in nine cases in our center. Two cases were decided to undergo right upper lobectomy due to severe adhesion to the right upper lobe and loss of function of the right upper lobe. The remaining seven cases had local adhesion invasion, and wedge resection was the main method. (2) Pericardium: Partial resection of the pericardium can be performed when the tumor invades the pericardium. In our center, there was one case of MMT and teratoma with severe adhesion. After the tumor and partial pericardium resection were performed under VATS, the pericardium was repaired by converting to thoracotomy. (3) IV/SVC: When the tumor invades the IV and SVC, more elaborate actions are required during the operation to avoid rupture and bleeding of blood vessels. If the tumor and blood vessels are closely adhered to or infiltrated, angioplasty or artificial blood vessel replacement may be considered.
There are few reports on the coexistence of malignant components in mature teratomas (19). In seven patients in our center, the tumor could not be completely removed due to the severe adhesion of the tumor to the SVC, IV, lung tissue, and pericardium. No recurrence or metastasis was found in postoperative follow-up. It has been reported in previous literature that when the residual tumor wall of MMT is infiltrated with large blood vessels and is difficult to separate, if the intraoperative pathology confirms that the tumor is benign, in order to reduce the incidence of intraoperative secondary injury. After scraping the tumor wall endothelium, iodine or carbolic acid should be applied. Regular follow-up after surgery is recommended (8).
Our study has several limitations. First, although the study spans a long time, it is still a single-center retrospective study, and selection bias is inevitable, so there may be a lack of homogeneity among different groups of patients. To confirm the findings, it is worth conducting multicenter studies in more MMT patients. Second, with the increase in the volume of single-center mediastinal mass operations, the surgical techniques may become more proficient, and the indications for surgical thoracoscopic surgery for teratoma will expand accordingly.
This is the single-center study with the largest sample size of VATS in mature teratomas to date. In our 20-year experience with mediastinal mature teratomas, we determined that VATS resection is a feasible choice, with a shorter postoperative recovery time if there are no dense adhesions present in the preoperative CT scan. For large tumors and preoperative clinical symptoms, when the relationship between the tumor and surrounding blood vessels is found to be oppressed or invaded by preoperative imaging combined with intraoperative exploration, in order to ensure the safety of the operation, conversion to thoracotomy after VATS exploration can be considered. For patients with incomplete resection, although the intraoperative pathology proved to be benign, regular follow-up is required after surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University People's Hospital. Written informed consent to participate in this study was provided by the legal guardian/next of kin of the participants. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
The concept and design of the study were done by ZDZ, XW, and YL. Acquisition, analysis, or interpretation of data and drafting of the manuscript were done by ZDZ and XW. Statistical analysis was carried out by ZDZ, XW, ZLZ, and GJ. Critical revision of the manuscript for important intellectual content was done by YL. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Takeda S, Miyoshi S, Ohta M, Minami M, Masaoka A, Matsuda H. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer. (2003) 97(2):367–76. doi: 10.1002/cncr.11068
2. Harms D, Zahn S, Göbel U, Schneider DT. Pathology and molecular biology of teratomas in childhood and adolescence. Klin Padiatr. (2006) 218(6):296–302. doi: 10.1055/s-2006-942271
3. Lewis BD, Hurt RD, Payne WS, Farrow GM, Knapp RH, Muhm JR. Benign teratomas of the mediastinum. J Thorac Cardiovasc Surg. (1983) 86(5):727–31. doi: 10.1016/S0022-5223(19)39090-7
4. Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumor resection: Taiwan experience. Ann Cardiothorac Surg. (2016) 5(2):107–11. doi: 10.21037/acs.2016.03.10.27134836
5. Shintani Y, Funaki S, Nakagiri T, Inoue M, Sawabata N, Minami M, et al. Experience with thoracoscopic resection for mediastinal mature teratoma: a retrospective analysis of 15 patients. Interact Cardiovasc Thorac Surg. (2013) 16(4):441–4. doi: 10.1093/icvts/ivs543
6. Wang X, Chen K, Li Y, Yang F, Zhao H, Wang J. The video-assisted thoracic surgery for mediastinal bronchogenic cysts: a single-center experience. World J Surg. (2018) 42(11):3638–45. doi: 10.1007/s00268-018-4660-4
7. Park JW, Jeong WG, Lee JE, Lee HJ, Ki SY, Lee BC, et al. Pictorial review of mediastinal masses with an emphasis on magnetic resonance imaging. Korean J Radiol. (2021) 22(1):139–54. doi: 10.3348/kjr.2019.0897
8. Allen MS, Trastek VF, Pairolero PC. Benign germ cell tumors of the mediastinum. In: Shields TW, Joseph L, Reed CE, Fein RH, editors. General thoracic surgery. Lippincott Williams & Wilkins (2009). p. 2266–510.
9. Rothermel L, Gilkeson R, Markowitz AH, Schröder C. Thoracoscopic resection of a giant teratoma compressing the right heart. Interact Cardiovasc Thorac Surg. (2013) 17(3):594–7. doi: 10.1093/icvts/ivt225
10. Tian Z, Liu H, Li S, Chen Y, Ma D, Han Z, et al. Surgical treatment of benign mediastinal teratoma: summary of experience of 108 cases. J Cardiothorac Surg. (2020) 15(1):36. doi: 10.1186/s13019-020-1075-8
11. Hwang SK, Park SI, Kim YH, Kim HR, Choi SH, Kim DK. Clinical results of surgical resection of mediastinal teratoma: efficacy of video-assisted thoracic surgery. Surg Endosc. (2016) 30(9):4065–8. doi: 10.1007/s00464-015-4721-9
12. Tsubochi H, Endo S, Nakano T, Minegishi K, Tetsuka K, Hasegawa T. Extraction of mediastinal teratoma contents for complete thoracoscopic resection. Asian Cardiovasc Thorac Ann. (2015) 23(1):42–5. doi: 10.1177/0218492314536173
13. Chang CC, Chang YL, Lee JM, Chen JS, Hsu HH, Huang PM, et al. 18 Years surgical experience with mediastinal mature teratoma. J Formos Med Assoc. (2010) 109(4):287–92. doi: 10.1016/S0929-6646(10)60054-X
14. Yang CJ, Cheng MS, Chou SH, Tsai KB, Huang MS. Primary germ cell tumors of the mediastinum: 10 years of experience in a tertiary teaching hospital. Kaohsiung J Med Sci. (2005) 21(9):395–400. doi: 10.1016/S1607-551X(09)70140-8
15. Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. (1997) 80(4):681–90. doi: 10.1002/(SICI)1097-0142(19970815)80:4<681::AID-CNCR6>3.0.CO;2-Q
16. Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR Am J Roentgenol. (1997) 169(4):985–90. doi: 10.2214/ajr.169.4.9308448
17. Serraj M, Lakranbi M, Ghalimi J, Ouadnouni Y, Smahi M. Mediastinal mature teratoma with complex rupture into the lung, bronchus and skin: a case report. World J Surg Oncol. (2013) 11:125. doi: 10.1186/1477-7819-11-125
18. Dulmet EM, Macchiarini P, Suc B, Verley JM. Germ cell tumors of the mediastinum. A 30-year experience. Cancer. (1993) 72(6):1894–901. doi: 10.1002/1097-0142(19930915)72:6<1894::AID-CNCR2820720617>3.0.CO;2-6
Keywords: video-assisted thoracic surgery, surgical treatment, minimally invasive surgical procedures, mediastinum, mature teratoma
Citation: Zhang Z, Wang X, Zhou Z, Jiang G and Li Y (2022) Surgical management of primary mediastinal mature teratoma: A single-center 20 years’ experience. Front. Surg. 9:902985. doi: 10.3389/fsurg.2022.902985
Received: 28 March 2022; Accepted: 11 August 2022;
Published: 5 September 2022.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Angela De Palma, University of Bari Medical School, ItalyYener Aydin, Atatürk University, Turkey
© 2022 Zhang, Wang, Zhou, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li c3VyZ2VvbmxpQGhvdG1haWwuY29t
†These authors share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zhedong Zhang
Zhedong Zhang Xun Wang†
Xun Wang† Zuli Zhou
Zuli Zhou