
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg., 30 June 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.898304
This article is part of the Research TopicCo-use of medicines in surgeryView all 114 articles
 Wei Huang1*
Wei Huang1* Jiayu Luo2
Jiayu Luo2 Jianbo Wen1
Jianbo Wen1 Mingjun Jiang1
Mingjun Jiang1
Background: The relationship between systemic immune inflammation index (SII) and the prognosis of cancer has always been a subject of intense interest. However, the prognostic value of SII in non-small cell lung cancer (NSCLC) patients remains a controversial topic.
Objective: To evaluate the effect of SII index on prognosis of NSCLC.
Methods: We conducted a comprehensive search of PubMed, EMBASE, and the Cochrane Library databases to determine correlation between SII index, clinicopathological features, overall survival (OS), and progression-free survival (PFS). Odds ratio (ORs) and 95% confidence interval (CIs) were used to assess the connection between SII and clinicopathological parameters, and HRs and 95% CIs were used to assess the connection between SII and survival.
Results: Seventeen studies with 8,877 cases were included in the analysis. Compared with NSCLC patients with low SII level, patients with NSCLC with high SII level had a poor OS (HR = 1.75, 95% CI, 1.50–2.00; P < 0.001) and had a poor PFS (HR = 1.61, 95% CI, 1.25–1.96; P < 0.001). In addition, patients with higher pathological stage (II–III) had higher SII levels (OR = 2.32, 95% CI, 2.06–2.62; P < 0.001).
Conclusions: The SII index is a promising prognostic biomarker for NSCLC and may help clinicians choose appropriate NSCLC treatments.
Lung cancer has a high incidence and mortality, and non-small cell lung cancer (NSCLC) accounts for about 80% of the total incidence of lung cancer (1). The incidence of NSCLC has risen steadily over the past few decades, but the mortality rate of NSCLC appears to be decreasing, possibly due to the tremendous advances in NSCLC treatment (2, 3). The treatment of NSCLC includes surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy (4). Nonetheless, the efficacy of these therapies in NSCLC patients remains dissatisfactory due to the lack of resultful indicators that can be utilized to predict the disease course and the widespread chemoresistance of NSCLC (5). Hence, it is necessary for researchers to identify exact biomarkers and potential therapeutic targets for NSCLC to improve survival.
In recent years, some indicators reflecting the inflammatory state of the body have been confirmed to be related to the prognosis of various malignant tumors (6). Systemic immune inflammation index (SII) is one of the new inflammatory indexes based on peripheral blood platelet count, neutrophils, and lymphocytes. SII = platelet count × neutrophils/lymphocytes. Studies have confirmed that SII can impersonally reflect the balance between inflammatory response and immune response in tumor patients (7). SII has achieved good results in predicting the prognosis of colorectal cancer, cervical cancer, pancreatic cancer and other malignant tumors (8–10). Furthermore, multiple meta-analyses have shown that SII predicts poor prognosis in a variety of malignancies (11, 12). Nevertheless, studies on SII levels in NSCLC are limited, and the prognostic value of SII levels in NSCLC is still a controversial issue. To solve this problem, we conducted a meta-analysis to synthetically assess the value of SII as a prognostic marker and determine the correlation between SII levels and pathological characteristics of NSCLC patients.
This meta-analysis was based on preferred reporting items for systematic reviews and meta-analysis (PRISMA) (13). This study was based on previously published research data, ethical approval is not necessary.
We performed a comprehensive literature search of published studies using databases such as PubMed, EMBASE, and Cochrane. Studies published before January 2022 were collected. The following keywords were used in the search box: (“Systemic immune inflammation index” OR “SII” OR “neutrophil × platelets/lymphocyte” OR “platelet count × NLR”) AND (“non small cell lung cancer” OR “NSCLC”). In order to find relevant literature, we also checked the references of relevant articles.
Studies that met the following conditions were considered eligible: (1) The relationship between SII index and survival of NSCLC patients was provided. (2) The critical value of preprocessing SII was provided. (3) The study offered enough data to extract the hazard ratio (HR) and 95% confidence interval (CI) for OS. Articles were excluded if they were reviews or meta-analyses, did not involve non-small cell lung cancer, or only involved animal experiments. If duplicate articles exist, only complete or up-to-date articles were included in this analysis.
All relevant data will be extracted by two data collectors, and if the two collectors were unsure of the data, one researcher wound decided how to extract the data. First author, country, number of cases, patient age, SII index, clinicopathological parameters, HR, and 95% CI were extracted from each study. Quality assessment of each study was performed independently by two data collectors using the Newcastle-Ottawa Scale (NOS), and the quality score was averaged between the two data collectors. The highest NOS score was 9, and studies with a score greater than 6 were considered high quality (14).
HRs and their 95% CIs were used to assess the correlation between SII and survival, and Odds ratio (ORs) and their 95% confidence interval (CIs) were used to determine the correlation between SII and clinicopathological parameters. Heterogeneity between studies was assessed using the chi-square test and I2. Statistics P < 0.1 or I2 > 50% indicated significant heterogeneity between studies and a random effects model (REM) was selected for analysis; otherwise, a fixed effects model (FEM) was selected for analysis. Egger’s test was selected for assess potential publication bias. Review Manager 5.3 (Revman the Cochrane Collaboration) and STATA 16.0 (Stata Corporation) software were selected for Meta-analysis. P < 0.05 means the difference is obviously significant. P values and 95% CI were two-sided tests.
In this study, we collected 100 total potentially relevant articles according to the search methods previously determined. After reviewing the titles and abstracts of these articles, we excluded 67 duplicate or irrelevant studies. After a detailed review of 33 articles, we determined that 17 trials accord with inclusion criteria and were therefore included in the meta-analysis (as shown in Figure 1).
The information of the 17 studies (15–33) are shown in Table 1. Of the 17 studies, the sample size was a minimum of 42 and a maximum of 3,984. 8,877 total patients participated in the study. A total of 17 studies eligible for analysis were retrospective. Four of the studies were from the United States and Japan, and the rest were from China. Nine studies were conducted with patients with advanced stage, and the remaining studies were conducted with patients with early and advanced stage. HRs and 95% CIs were extracted directly from all original articles. The quality of the studies assessed by NOS was all ≥6. Therefore, the study was of high quality.
We analyzed the relationship between SII levels and overall survival (OS) in NSCLC. 8,752 total cases were included in 16 studies. The meta-analysis (as shown in Figure 2) demonstrated that NSCLC patients with high-level SII had a poor OS contrast to NSCLC patients with low-level SII (HR = 1.75, 95% CI, 1.50–2.00; P < 0.001). There was heterogeneity in the results (I2 = 51.32%, P < 0.001), hence, a REM was used for analysis.
We analyzed the relationship between SII levels and progression-free survival (PFS) in NSCLC. Eight studies included a total of 5,496 patients. The meta-analysis (as shown in Figure 3) demonstrated that NSCLC patients with high-level SII had a poor PFS contrast to NSCLC patients with low-level SII (HR = 1.61, 95% CI, 1.25–1.96; P < 0.001). There was heterogeneity in the results (I2 = 70.15%, P = 0.01), hence, a REM was used for analysis.
In this study, we analyzed the relationship between high levels of SII and pathological stage and pathological type. The meta-analysis demonstrated that patients with higher pathological stage (II–III) had higher SII levels (OR = 2.32, 95% CI, 2.06–2.62; P < 0.0001). However, no obvious correlation between SII levels and pathological types (OR = 0.86, 95% CI, 0.55–1.36, P = 0.53), as shown in Figure 4.
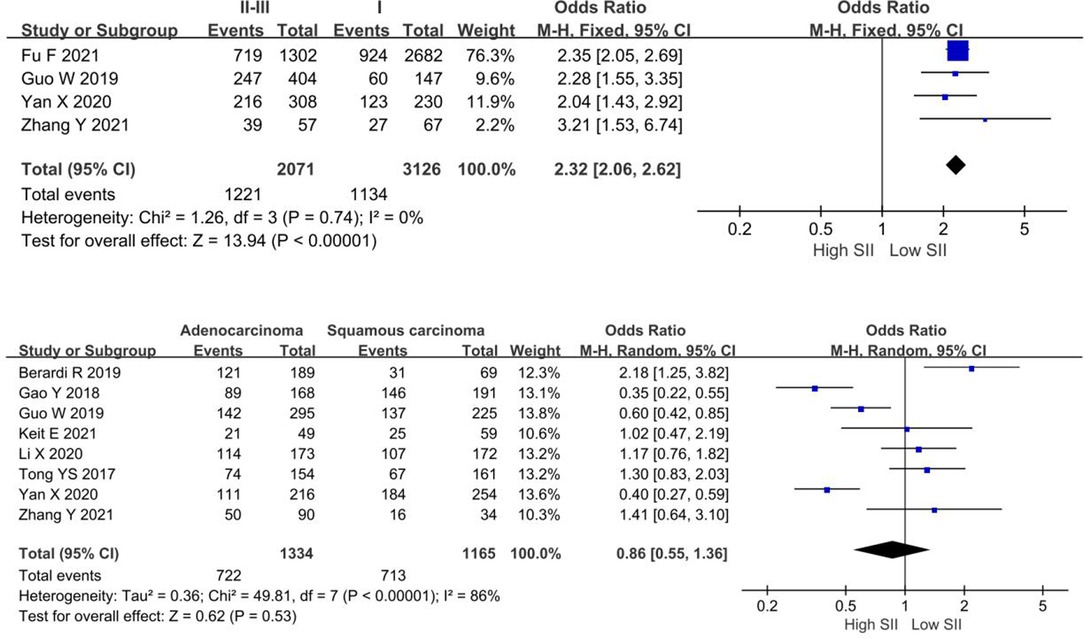
Figure 4. Forest plot depicting the relationship between SII levels and clinicopathological characteristics of NSCLC patients.
Sensitivity analysis, which involves deleting one study at a time to assess the stability of the results. After removing the literature, none of the individual studies obviously affected the whole population, Indicating that the results of the current meta-analysis were credible.
Egger’s test indicated that the included studies exhibited publication bias affecting the hazard ratio for OS, with a P value of 0.0001, as shown in Figure 5.
A large number of studies have reported the relationship between inflammation and tumor and found that inflammation is one of the factors promoting the occurrence and development of tumor (34). For example, neutrophils, lymphocytes, and platelets play important roles in tumor progression. These indicators can promote tumor cell proliferation, invasion, and distant metastasis (35). For the past few years, some systemic inflammatory cell-based biomarkers, such as platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), have been shown to correlate with many The prognosis of this type of cancer is relevant (36). Nevertheless, these indicators are based on two inflammatory indices, and SII is a novel biomarker based on three indices (platelet, neutrophil, lymphocyte count) that comprehensively reflects the host immune and inflammatory status. SII is a relatively objective index and has good prognostic reliability (8–10).
As far as we know, a meta-analysis of the effect of SII index on survival in patients with NSCLC has not been reported. In this context, a comprehensive literature search was conducted and incorporated into 17 total studies with 8,877 cases. From the results of the meta-analysis, we found a obvious correlation between the SII index and the prognosis of NSCLC patients. The OS and PFS of patients with high SII levels were shorter than those with low SII levels, suggesting that SII index may be a promising prognostic factor for NSCLC patients. Berardi et al. (15), Deng et al. (17), Hong et al. (22), Li et al. (26), Tong et al. (29), Yucel et al. (32) and other results demonstrated that the median OS of patients with high SII level was obviously lower than that of patients with low SII level. In addition, this meta-analysis also observed that the SII index of NSCLC patients with higher pathological stages (II–III) was significantly higher than that of stage I patients, suggesting that SII index may be a risk factor for disease development in patients with NSCLC.
We tried to do a comprehensive analysis, but there are still limitations to this study. First, treatment strategies not analyzed in this research may have influenced the results. Second, the included studies were limited to articles published in English and mostly from China, which may lead to publication bias. Third, the cutoff values used to determine high levels of SII also inconsistent. Finally, the sample size of the studies included in the analysis may have contributed to heterogeneity. However, this meta-analysis has proved an correlation between high levels of SII and clinicopathological factors in NSCLC. The results of this study may improve the prognosis of NSCLC. However, despite the robustness of our findings, caution is required in interpreting the validity of SII in NSCLC prognosis.
In conclusion, this meta-analysis identified pretreatment or preoperative SII index as a prognostic factor for OS and PFS in NSCLC patients. SII index may be an effective survival indicator of NSCLC. Larger multicenter studies are needed in the future to further verify the clinical application value of the SII index.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
WH is the mainly responsible for the writing, the research is completed by JW and data analysis of the article is completed by MJ. JL is responsible for the guidance of the entire research. The corresponding author is WH and he is responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Amiri A, Pourhanifeh MH, Mirzaei HR, Nahand JS, Moghoofei M, Sahebnasagh R, et al. Exosomes and lung cancer: roles in pathophysiology, diagnosis and therapeutic applications. Curr Med Chem. (2021) 28(2):308–28. doi: 10.2174/0929867327666200204141952
2. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. (2019) 94(8):1623–40. doi: 10.1016/j.mayocp.2019.01.013
3. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. (2020) 198(6):897–907. doi: 10.1007/s00408-020-00407-5
4. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. (2018) 52(Pt 1):103–9. doi: 10.1016/j.semcancer.2017.11.019
5. Chen Y, Zhou H, Yang S, Su D. Increased ABCC2 expression predicts cisplatin resistance in non-small cell lung cancer. Cell Biochem Funct. (2021) 39(2):277–86. doi: 10.1002/cbf.3577
6. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. (2019) 18(3):121–6. doi: 10.4103/aam.aam_56_18
7. Xie QK, Chen P, Hu WM, Sun P, He WZ, Jiang C, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. (2018) 16(1):273. doi: 10.1186/s12967-018-1638-9
8. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
9. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. (2019) 9(1):3284. doi: 10.1038/s41598-019-39150-0
10. Murthy P, Zenati MS, Al Abbas AI, Rieser CJ, Bahary N, Lotze MT, et al. Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. (2020) 27(3):898–906. doi: 10.1245/s10434-019-08094-0
11. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. (2018) 9(18):3295–302. doi: 10.7150/jca.25691
12. Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. (2020) 12:1758835920937425. doi: 10.1177/1758835920937425
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. (2021) 18(3):e1003583. doi: 10.1371/journal.pmed.1003583
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Berardi R, Santoni M, Rinaldi S, Bower M, Tiberi M, Morgese F, et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Ann Transl Med. (2019) 7(20):572. doi: 10.21037/atm.2019.09.18
16. Chen X, Hong X, Chen G, Xue J, Huang J, Wang F, et al. The pan-immune-inflammation value predicts the survival of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer treated with first-line ALK inhibitor. Transl Oncol. (2022) 17:101338. doi: 10.1016/j.tranon.2021.101338
17. Deng C, Zhang N, Wang Y, Jiang S, Lu M, Huang Y, et al. High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine (Baltimore). (2019) 98(33):e16875. doi: 10.1097/MD.0000000000016875
18. Fu F, Deng C, Wen Z, Gao Z, Zhao Y, Han H, et al. Systemic immune-inflammation index is a stage-dependent prognostic factor in patients with operable non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10(7):3144–54. doi: 10.21037/tlcr-21-267
19. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. (2018) 484:272–7. doi: 10.1016/j.cca.2018.05.059
20. Guo D, Zhang J, Jing W, Liu J, Zhu H, Fu L, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol. (2018) 14(25):2643–50. doi: 10.2217/fon-2018-0285
21. Guo W, Cai S, Zhang F, Shao F, Zhang G, Zhou Y, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer. (2019) 10(4):761–8. doi: 10.1111/1759-7714.12995
22. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. (2015) 236(4):297–304. doi: 10.1620/tjem.236.297
23. Ju Q, Huang T, Zhang Y, Wu L, Geng J, Mu X, et al. Systemic immune-inflammation index predicts prognosis in patients with different EGFR-mutant lung adenocarcinoma. Medicine (Baltimore). (2021) 100(6):e24640. doi: 10.1097/MD.0000000000024640
24. Keit E, Coutu B, Zhen W, Zhang C, Lin C, Bennion N, et al. Systemic inflammation is associated with inferior disease control and survival in stage III non-small cell lung cancer. Ann Transl Med. (2021) 9(3):227. doi: 10.21037/atm-20-6710
25. Li A, Mu X, He K, Wang P, Wang D, Liu C, et al. Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol. (2020) 16(30):2433–44. doi: 10.2217/fon-2020-0423
26. Li X, Hu P, Liu J, Zhang J, Liu Q. Systemic immune-inflammation index predicted overall survival and radiosensitivity in advanced non-small-cell lung cancer. Future Oncol. (2020) 16(5):103–15. doi: 10.2217/fon-2019-0761
27. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. (2019) 33(8):e22964. doi: 10.1002/jcla.22964
28. Takeda T, Yamada T, Tanimura K, Nakano T, Ishida M, Tachibana Y, et al. Prognostic markers of survival among Japanese patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer receiving first-line alectinib. Diagnostics (Basel). (2021) 11(12):2170. doi: 10.3390/diagnostics11122170
29. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. (2017) 15(1):221. doi: 10.1186/s12967-017-1326-1
30. Watanabe K, Noma D, Masuda H, Masuda M. Preoperative inflammation-based scores predict early recurrence after lung cancer resection. J Thorac Dis. (2021) 13(5):2812–23. doi: 10.21037/jtd-20-3458
31. Yan X, Li G. Preoperative systemic immune-inflammation index predicts prognosis and guides clinical treatment in patients with non-small cell lung cancer. Biosci Rep. (2020) 40(3):BSR20200352. doi: 10.1042/BSR20200352
32. Yucel S, Bilgin B. The prognostic values of systemic immune-inflammation index and derived neutrophil-lymphocyte ratio in EGFR-mutant advanced non-small cell lung cancer. J Oncol Pharm Pract. (2021) 27(1):71–7. doi: 10.1177/1078155220913106
33. Zhang Y, Chen Z, Jin F, Guo D, Chen Q, Liu Z, et al. The value of the systemic immune-inflammation index in predicting survival outcomes in patients with brain metastases of non-small-cell lung cancer treated with stereotactic radiotherapy. Mediators Inflamm. (2021) 2021:2910892. doi: 10.1155/2021/2910892
34. Liao CP, Booker RC, Brosseau JP, Chen Z, Mo J, Tchegnon E, et al. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. (2018) 128(7):2848–61. doi: 10.1172/JCI99424
35. Caziuc A, Schlanger D, Amarinei G, Dindelegan GC. Neutrophils-to-lymphocytes, lymphocytes to-monocytes and platelets-to-lymphocytes ratios - predictive biomarkers for response to neoadjuvant chemotherapy in breast cancer. J BUON. (2020) 25(1):182–7. PMID: 32277630
36. Zheng J, Cai J, Li H, Zeng K, He L, Fu H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. (2017) 44(3):967–81. doi: 10.1159/000485396
Keywords: systemic immune inflammatory index, non-small cell lung cancer, prognosis, meta-analysis, systematic review
Citation: Huang W, Luo J, Wen J and Jiang M (2022) The Relationship Between Systemic Immune Inflammatory Index and Prognosis of Patients With Non-Small Cell Lung Cancer: A Meta-Analysis and Systematic Review. Front. Surg. 9:898304. doi: 10.3389/fsurg.2022.898304
Received: 17 March 2022; Accepted: 3 June 2022;
Published: 30 June 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Wenjie Song, Fourth Military Medical University, ChinaCopyright © 2022 Huang, Luo, Wen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang aHVhbmdrZWltZW5nQDE2My5jb20=
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.