- 1The Department of Pediatric Surgery, Binzhou Medical University Hospital, Binzhou, China
- 2The Department of Surgery, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, China
- 3The Department of Pharmacy, Binzhou Medical University Hospital, Binzhou, China
- 4The Clinical Laboratory, Binzhou Medical University Hospital, Binzhou, China
- 5The Department of Urology, Binzhou Medical University Hospital, Binzhou, China
Anastomotic techniques are of vital importance in restoring gastrointestinal continuity after resection. An alternative asymmetric figure-of-eight single-layer suture anastomotic technique was introduced and its effects were evaluated in an in vitro porcine model. Twelve 15-cm grossly healthy small intestine segments from a porcine cadaver were harvested and randomly divided into asymmetric figure-of-eight single-layer suture (figure-of-eight suture) and single-layer interrupted suture technique (interrupted suture) groups (n = 6 in each group). The anastomosed bowel was infused with methylene blue solution to test anastomotic leakage. Anastomosis construction time, leakage, and suture material cost were recorded and analyzed statistically using Fisher's exact test and Student's t-test. One anastomotic leakage occurred (16.67%) in the figure-of-eight suture group, and two (33.33%) in the interrupted suture group (p > 0.9999). The anastomosis construction time was relatively short in the figure-of-eight suture group, but the difference did not reach a statistically significant level between the two groups. The mean number of suture knots and the cost of suture material in the figure-of-eight suture group were significantly decreased in comparison to the interrupted suture group (15.67 ± 3.30 vs. 22.17 ± 2.03, 167.11 ± 35.20 vs. 236.45 ± 21.70 CNY, p < 0.01, respectively). Our results suggested that the alternative asymmetric figure-of-eight suture technique was safe and economic for intestinal anastomosis. An in vivo experiment is required to elucidate the effects of this suture technique on the physiological anastomotic healing process.
Introduction
Intestinal anastomosis is an essential operative procedure in restoring the continuity of the gastrointestinal tract after resection of bowel lesions, such as necrotic intestinal segments, malignancy, inflammatory bowel disease, and trauma (1–4). In comparison with adult surgery, small intestine anastomosis is difficult and has a high risk of complications due to the smaller diameter of the intestine, inflammation, edema, or immaturity in the pediatric group, especially in premature infants (5–7).
To effectively reduce the occurrence of complications related to anastomosis and to improve patients' surgical outcomes, various modified anastomosis techniques have been developed (8, 9). Double-layer anastomosis may lead to increased tissue damage and impaired blood supply with possibly delayed healing. Single-layer anastomosis is proved to be effective and safe (10, 11). The drawback of hand-sewn single-layer anastomosis is its insecurity in case of high-risk intestinal anastomosis, such as an edematous intestine (11). When the stitches are tied, excessive tension placed on the suture might result in tissue cutting, even anastomotic leakage (12, 13). In the 1950s, Gambee (14) designed a single-layer anastomosis technique. After that, the Gambee stitch and its modification procedures have been widely accepted in gastrointestinal anastomosis (15–17). As an advantage, this technique can improve tissue healing because of minimal disturbance in the blood supply of the anastomotic site (14, 17). However, the Gambee pattern requires more needle manipulation, a modified Gambee stitch that includes the second stitch (through the mucosa on the opposite side to the submucosa), and the third stitch (through the submucosa to the mucosa of the first side) was described by Shureih et al. (16). This technique requires less needle manipulation, being easier to perform with the same good results. Gambee et al. (17) reported subsequent experiences, including the posterior half of the anastomosis sutured within the lumen and the anterior half sutured by the classic Gambee stitch. Its advantage is easy to perform. Liang (18) introduced another single-layer suture technique with simplicity and reliability.
Previous studies revealed that the single-layer continuous Lembert pattern anastomosis is faster to perform and as strong as a two-layer anastomosis (9, 10, 19). Slieker et al. (20) analyzed the literature and concluded that a continuous suture is preferable for completing an anastomosis owing to the technical and time-consuming nature, although clinical and experimental studies have not revealed that the continuous suture technique is superior to the interrupted one. Modified Gambee stitch should remain an option in bowel anastomosis (2). Herein, we describe an alternative asymmetric figure-of-eight single-layer suture method, aiming to create a model that is safe, feasible, and easy to perform. Its feasibility and effectiveness were evaluated in an in vitro porcine anastomosis model.
Materials and methods
Study design
The research was an in vitro experiment on small intestine segments harvested from a healthy pig sacrificed at an abattoir. The effects of the figure-of-eight suture technique were compared with those of the interrupted suture technique. The study was approved by the Institutional Ethics Committee of Binzhou Medical University Hospital (No. 20210120-01), Shandong Province, China. No live animal was involved in the present study.
Procedures
Small intestine harvest and preservation
The ileum of 180 cm in length was immediately harvested after the healthy pig was sacrificed by electrocution at an abattoir as described in the literature (21, 22). No gross abnormal appearance was observed during the porcine intestine preparation. The specimen was placed in an aseptic plastic bag within a box filled with iced water and was transported to the laboratory and placed in cold normal saline solution with 0.5% povidone-iodine solution (23). Luminal contents were gently milked and then flushed with cold normal saline solution. Ileum transection, anastomosis, and tests were completed within 9 h after the harvest of the specimen, as described by other authors (24, 25).
Sampling and groups
Based on the literature review on experimental intestinal anastomosis techniques, several studies have employed a sample size of six bowel segments per group (23, 26–29). Accordingly, a sample size of six in each group was determined in the present study.
The intestinal segment was then separated into 12 smaller segments (15 cm each), which were randomly divided into figure-of-eight and interrupted suture groups using the random number table. In the middle part of each segment, transection was conducted and anastomoses were then performed using a 4-0 polyglycolic acid suture with an atraumatic taper point needle. Both figure-of-eight and interrupted suture techniques were performed by the same senior surgeon, representing a homogeneous group of anastomoses.
Single-layer asymmetric figure-of-eight suture
This alternative technique involved inserting and pulling out the needle twice in different planes. The steps of the single-layer asymmetric figure-of-eight suture were described as follows. Two 5/0 silk stay sutures were placed at the mesenteric and antimesenteric margins to bring the two intestinal ends together. Thereafter, the anastomosis was performed at the anterior wall with interrupted 4/0 absorbable sutures and the first point was placed on the mesenteric border. The first insertion and withdrawal of the needle were implemented by taking a bite of 2 mm from the wound edge. The needle was obliquely inserted into the serosa, muscularis, and submucosa and then pulled out 1 mm from the mucosa wound edge. Then, taking a bite 1 mm from the wound edge of the contralateral mucosal layer was made and directed obliquely through the submucosa, muscularis, and serosa 2 mm away from the wound edge (3 mm from the wound edge at the mesenteric site). The second insertion from the serosa and pulling out the needle from the submucosa, then from the submucosa through the serosa was taken at a point 1 mm from the edge (2 mm at the mesenteric site) and 1 mm forward from the first stitch plane, making the two stitches at different horizontal levels. The suture was tied on the serosal surface using three square knots with proper strength to avoid bowel strangulation. When the whole anterior wall was anastomosed, the posterior wall was sutured in the same way as described in the literature (30). The anastomotic technique was shown in Figure 1. The mesentery at the anastomosis site was dissected only 3 mm on each side of the bowel wound edge to make a good exposure and to ensure adequate approximation after completion of the anastomosis.
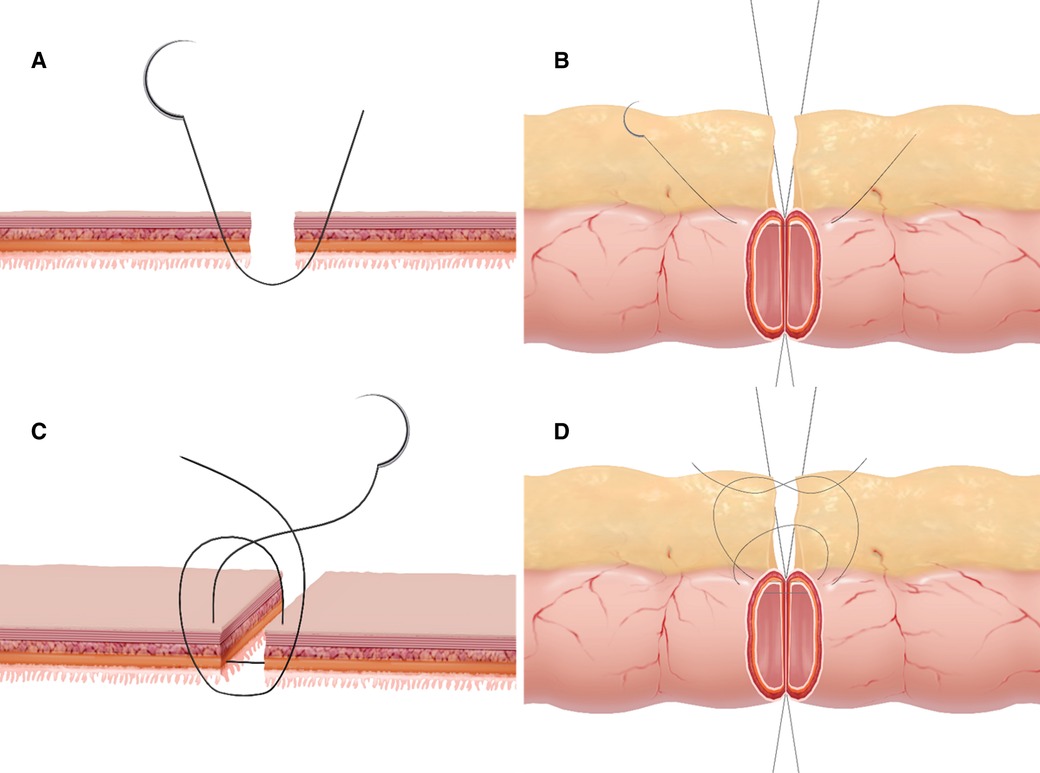
Figure 1. Graphic representation of the asymmetric figure-of-eight suture technic. (A,B) The first insertion and withdrawal of the needle was performed according to the sequence of "serosa, muscular, submucosa, and mucosa to contralateral mucosa, submucosa, muscular, and serosa" to sew more seromuscular and submucosal layers and less mucosal layer. (C,D) The second insertion and withdrawal of the needle was performed according to the sequence of "serosa, muscular, and submucosa to contralateral submucosa, muscular, and serosa" by taking a bite 1 mm from the cutting edge and forward from the first insertion level.
Single-layer interrupted suture anastomosis
The single-layer interrupted suture technique was conducted 2 mm from the wound edge and 2 mm apart between the stitches (3 mm from the wound edge at the mesenteric site).
Anastomosis construction time
The time (in minutes) for the creation of an anastomosis was recorded from the first suture bite to the completion of the anastomosis.
Leakage testing
After anastomosis completion, the specimen was stored in normal saline solution at room temperature and tested within 1 h. The leakage testing was conducted essentially as described previously (23, 31–36). Briefly, a 4-cm flexible pipe was tightly connected to a 50-ml syringe, which was inserted into the proximal end of the anastomosis. A hard plastic conduit, which was inserted into the distal end of the bowel segment, was connected to the pressure manometer. Both ends of the bowel segment with one pipe and one conduit were tightly ligated using a self-made blocking band. A digital pressure manometer (Xuzhou Engel Electronics Engineering Company, China) was used for monitoring the intraluminal pressure. The maximal intraluminal pressure was set at 30 mmHg as described in the literature (32). Methylene blue was mixed with normal saline at 1:250 dilution. When the intraluminal pressure reached 30 mmHg, all specimens were observed by one investigator for leakage and leakage sites (anastomosis line or suture hole), as shown in Figure 2. The intraluminal pressure was continuously recorded using a digital camera.
The number of suture knots and cost of suture materials
The number of suture knots represented the number of sutures used on each anastomosis by simulating the clinical scenario in which sutures cannot be reused in the intraoperative setting. The number of suture knots of the anastomosis multiplied by 10.67 (the price of each suture in Chinese Yuan, CNY) was the cost of suture material.
Statistical methods
All continuous data were analyzed using Student's t-test and the results were expressed as the mean ± standard derivation. Categorical data were analyzed using Fisher's exact test. The anastomosis construction time, the number of suture knots, and suture material cost were compared using a paired Student's t-test. Fisher's exact test was used to compare the occurrence of anastomotic leakage at an intraluminal pressure of 30 mmHg. All statistical analyses were performed using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA) and IBM SPSS Statistics software version 25 (IBM Corp., Armonk, NY, USA). The level of statistical significance was set at p < 0.05.
Results
The anastomosis construction time was 18.08 ± 5.43 min in the figure-of-eight suture group and 19.95 ± 5.21 min) as shown in Table 1. The mean time of anastomosis construction was relatively short in the figure-of-eight suture group, although a significant difference could not be reached between the two groups.
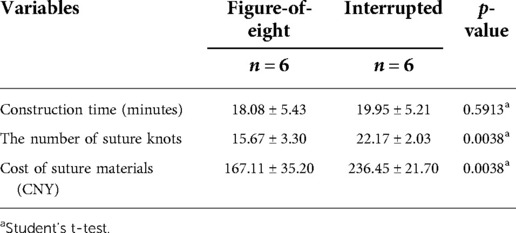
Table 1. Variables of the figure-of-eight and the interrupted suture groups in a porcine model with averages and standard deviations.
When the intraluminal pressure reached 30 mmHg, one (16.67%) leakage at the anastomotic line occurred in the figure-of-eight suture group (24 mmHg), while two (33.33%) leakages (at the anastomotic line and suture hole at 24 and 28 mmHg, respectively) in the interrupted suture group. No significant difference was noted between the two groups (p > 0.9999).
The suture knots in the figure-of-eight suture group were 15.67 ± 3.30, which were significantly reduced in comparison with the interrupted suture group (22.17 ± 2.03; p = 0.0038). The mean cost of suture material was significantly decreased in the figure-of-eight suture group than that in the interrupted suture group (167.11 ± 35.20 vs. 236.45 ± 21.70 CNY, p = 0.0038), as shown in Table 1.
The extra- and intraluminal appearances of the two different anastomosis techniques were all tightly approximations, as shown in Figure 3.
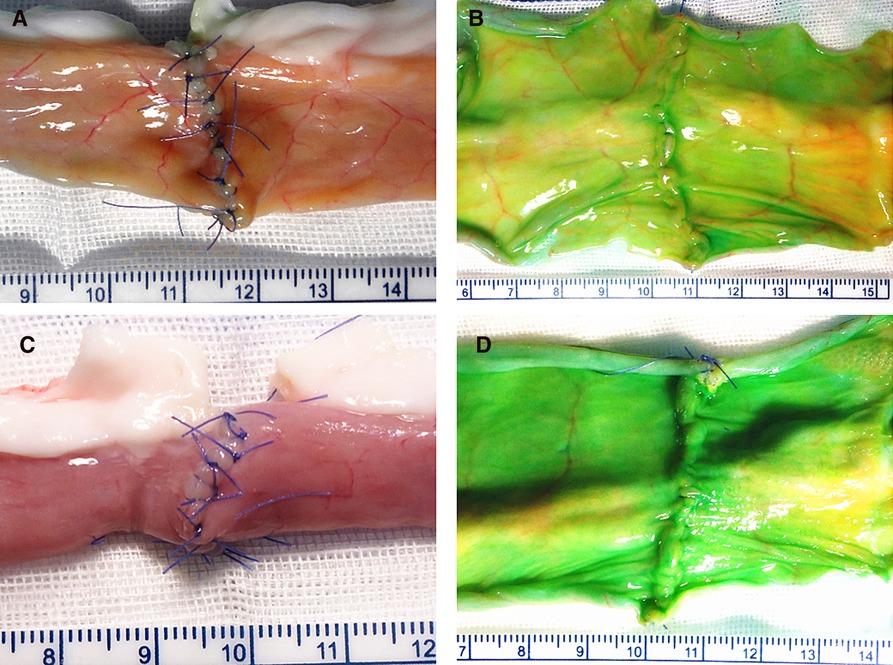
Figure 3. Extra- and intraluminal appearances of the two techniques: figure-of-eight suture technique (A,B); interrupted suture technique (C,D).
Discussion
Intestinal anastomosis for restoring gut continuity is an essential procedure (1–4). Owing to the smaller diameter and immature intestine in small infants and neonates, intestinal anastomosis remains a challenge for surgeons. The single-layer intestinal anastomosis has been proven safe and effective. However, its insecurity in case of high-risk intestinal anastomoses, such as an edematous intestine or severe bowel inflammation, might lead to a change of intraoperative strategy (5, 7, 11, 19). Based on intestinal anastomosis facileness and biomechanics, we designed an alternative asymmetric figure-of-eight suture and evaluated its effects through in vitro experiments on a porcine ileum specimen. Fresh bowel specimens were cleaned, manipulated, and monitored within 9 h after animal sacrifice to minimize the impact of the storage duration on the results (25, 37).
In the present study, the results showed that the asymmetric figure-of-eight suture required fewer suture knots in the anastomosis, which should save time by lesser needle manipulation and knot tying, although the time difference did not reach a statistically significant level owing to the small sample size. Rapid completion of an anastomosis with minimal trauma to the bowel is an important determinant of success in intestinal anastomosis, especially in those subjects that are suffering from a life-threatening gastrointestinal problem related to serious systemic condition, such as hemodynamic instability (38).
Anastomotic leakage is a severe postoperative complication that may lead to diffuse peritonitis, sepsis, and even life-threatening conditions (37, 39). Studies, including techniques, materials, and perioperative care have aimed to improve the outcome of intestinal anastomosis (20, 31, 40, 41). Intraoperative detection of the anastomosis quality and repairing defects without delay can significantly reduce the risk of postoperative leakage (31, 42).
Air testing, methylene blue perfusion testing, and intraoperative colonoscopy were selected to detect anastomotic leaks in both the in vitro experimental model and clinical research (2, 31, 42). In the present study, intraluminal leakage pressure with methylene blue perfusion was conducted to detect any potential anastomotic leakage as described by the previous study (42). Roumen et al. (32) reported that no adverse effects on the anastomosis occurred with the intraluminal pressure set at 40 cmH2O (approximately 29.41 mmHg). Avoiding luminal overexpansion will decrease the risk of potential induction of suture tract leakage (43). Accordingly, the intraluminal leakage pressure value at 30 mmHg was set in the present study. One leakage in the figure-of-eight suture group and two in the interrupted suture group occurred at the suture hole or anastomotic line. Other research suggests that the leakage may be linked to the needle size or a technique related to tissue tearing (44). The first stitch in the asymmetric figure-of-eight suture technique included whole layers, and the second stitch covered three layers except the mucosal layer. Furthermore, the two stitches were not at a horizontal level, which may lead to a shorter intersuture distance and tension-free while tying the knot, resulting in a secure anastomosis. This alternative suture technique might minimize the tension and avoid cutting through the fragile tissue (45). Anti-tension of the anastomosis caused by suturing the submucosa twice might improve the security of the anastomosis. Early and tight mucosal apposition may protect the bowel anastomosis from luminal content stimulation and potential infection (46, 47). Although this single-layer suture technic for intestinal anastomosis was feasible, the knot must be carefully tied with adequate strength to avoid any possible tissue strangulation at the anastomotic site (30), and an additional Lembert's suture should be placed if needed (48).
Limitations
This study has several limitations. First, it is an in vitro experiment with small sample size. Second, an in vitro study could not simulate the pathophysiological process of intestinal anastomosis, including intra-abdominal adhesion formation. Although the new synthetic absorbable sutures seem to generate less adhesion response, steps such as avoiding excessive suture materials could play a role in preventing adhesion formation (49–51). The effects of suture material exposure on adhesion formation need to be clarified in animal models. Third, all cadaveric ileum specimens were harvested from one pig, which might not cover the intestine specimens from the general population of pigs.
In conclusion, the preliminary in vitro study showed that the single-layer asymmetric figure-of-eight suture anastomosis technique was feasible and low-cost, but essentially it is a safe pattern for intestinal anastomosis, which presented less leakage than the single-layer interrupted suture technique.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study was reviewed and approved by the Institutional Ethics Committee of Binzhou Medical University Hospital (No. 20210120-01), China.
Author contributions
HY, TF, LG, and CL were involved in the study design. YW, A-rZ, F-aH, QF, GH, and GD equally contributed to the experiments and data analysis. CL contributed to writing the manuscript. CL and TF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Key Research Plan Fund from Binzhou Medical University (BY2012KJZD02) in Yantai, Shandong, China.
Acknowledgments
We acknowledge Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen C. The art of bowel anastomosis. Scand J Surg. (2012) 101:238–40. doi: 10.1177/145749691210100403
2. Kieves NR, Krebs AI, Zellner EM. A comparison of ex vivo leak pressures for four enterotomy closures in a canine model. J Am Anim Hosp Assoc. (2018) 54:71–6. doi: 10.5326/JAAHA-MS-6459
3. Cha J, Shademan A, Le HN, Decker R, Kim PC, Kang JU, et al. Multispectral tissue characterization for intestinal anastomosis optimization. J Biomed Opt. (2015) 20:106001. doi: 10.1117/1.JBO.20.10.106001
4. Kono T, Ashida T, Ebisawa Y, Chisato N, Okamoto K, Katsuno H, et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn's disease. Dis Colon Rectum. (2011) 54:586–92. doi: 10.1007/DCR.0b013e318208b90f
5. Xu WH, Xie BS, Li YX, Tong HX, Ma SJ, Fang ZN. Experimental research of modified single-layer intestinal anastomosis and its clinical application in neonates with intestinal atresia. Chin J Pediatr Surg. (1997) 18:94–6. doi: 10.3760/cma.j.issn.0253-3006.1997.02.011.
6. Wiggins T, Majid MS, Markar SR, Loy J, Agrawal S, Koak Y. Benefits of barbed suture utilisation in gastrointestinal anastomosis: a systematic review and meta-analysis. Ann R Coll Surg Engl. (2020) 102:153–9. doi: 10.1308/rcsann.2019.0106
7. Ordorica-Flores RM, Bracho-Blanchet E, Nieto-Zermeno J, Reyes-Retana R, Tovilla-Mercado JM, Leon-Villanueva V, et al. Intestinal anastomosis in children: a comparative study between two different techniques. J Pediatr Surg. (1998) 33:1757–9. doi: 10.1016/S0022-3468(98)90279-2
8. Natale C, Ferrozzi L, Pellegrino C, Bruno L. Digestive anastomosis in general surgery. G Chir. (1998) 19:175–83.9628068
9. Auletta L, Lamagna F, Uccello V, Lamagna B, Pasolini MP. In vitro comparison of three suture techniques for anastomosis of the equine small intestine. Equine Vet J Suppl. (2011) 40:46–50. doi: 10.1111/j.2042-3306.2011.00494.x
10. Kar S, Mohapatra V, Singh S, Rath PK, Behera TR. Single layered versus double layered intestinal anastomosis: a randomized controlled trial. J Clin Diagn Res. (2017) 11(6):PC01–4. doi: 10.7860/JCDR/2017/24817.9983.28764239
11. Abdella M, Fathi M, El-Sayed A, Shehata A. Is single-layer better than double-layer interrupted intestinal anastomosis? A comparative study in pediatric patients. Egypt J Surg. (2018) 37:9. doi: 10.4103/ejs.ejs_78_17
12. Huh YJ, Kim HY, Lee SC, Park KW, Jung SE. Comparison of outcomes according to the operation for type A esophageal atresia. Ann Surg Treat Res. (2014) 86:83–90. doi: 10.4174/astr.2014.86.2.83
13. Shao Q, Lin G. Surgical skills in the prevention of anastomotic leakage after rectal neoplasm surgery. Zhonghua Wei Chang Wai Ke Za Zhi. (2018) 21:399–403. Chinese.29682710
14. Gambee LP. A single-layer open intestinal anastomosis applicable to the small as well as the large intestine. West J Surg Obstet Gynecol. (1951) 59:1–5.14798834
15. Wheeless CR Jr. The Gambee intestinal anastomosis in gynecologic surgery. Obstet Gynecol. (1975) 46:448–52.1101121
16. Shureih SF, Wilson TH Jr., Howard WH. Modified Gambee stitch. Safe, easy and fast modification. Am J Surg. (1981) 141:304. doi: 10.1016/0002-9610(81)90182-3
17. Gambee LP, Garnjobst W, Hardwick CE. Ten years’ experience with a single layer anastomosis in colon surgery. Am J Surg. (1956) 92:222–7. doi: 10.1016/S0002-9610(56)80063-9
18. Liang H. An alternative to single-layer hand-sewn anastomotic technique. Chin J Gastrointest Surg. (2017) 20:932–4. doi: 10.3760/cma.j.issn.1671-0274.2017.08.023. Chinese.
19. Nieto JE, Dechant JE, Snyder JR. Comparison of one-layer (continuous Lembert) versus two-layer (simple continuous/Cushing) hand-sewn end-to-end anastomosis in equine jejunum. Vet Surg. (2006) 35:669–73. doi: 10.1111/j.1532-950X.2006.00206.x.17026553
20. Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. JAMA Surg. (2013) 148:190–201. doi: 10.1001/2013.jamasurg.33
21. Conte S, Pomar C, Paiano D, Duan Y, Zhang P, Lévesque J, et al. The effects of feeding finishing pigs of two genders with a high fiber and high fat diet on muscle glycolytic potential at slaughter and meat quality. Meat Sci. (2021) 177:108484. doi: 10.1016/j.meatsci.2021.108484.33756246
22. Lin RQ. Live pig slaughtering and protection of stress syndrome. Meat Ind. (2009) 5:11–3. doi: 10.3969/j.issn.1008-5467.2009.05.006. Chinese.
23. Regier PJ, Fealey MJ, Kim SE, Case JB, Garcia-Pereira F. Comparison of intestinal leak pressure between cadaveric canine and commercial synthetic intestinal tissue that did and did not undergo enterotomy. Am J Vet Res. (2020) 81:827–31. doi: 10.2460/ajvr.81.10.827
24. Yan ZQ, Zou XM, Li G, Song ML, Li XL, Li YL, et al. Perfusion and preservation of isolated piglets small intestine graft for transplantation. Chin J Comp Med. (2008) 18:55–6. Chinese.
25. Tesi RJ, Jaffe BM, McBride V, Haque S. Histopathologic changes in human small intestine during storage in Viaspan organ preservation solution. Arch Pathol Lab Med. (1997) 121:714–8.9240907
26. Hansen LA, Monnet EL. Evaluation of serosal patch supplementation of surgical anastomoses in intestinal segments from canine cadavers. Am J Vet Res. (2013) 74:1138–41. doi: 10.2460/ajvr.74.8.1138
27. Mullen KM, Regier PJ, Waln M, Fox-Alvarez WA, Colee J. Gastrointestinal thickness, duration, and leak pressure of six intestinal anastomoses in dogs. Vet Surg. (2020) 49:1315–25. doi: 10.1111/vsu.13490
28. Ikeda T, Kumashiro R, Oki E, Taketani K, Ando K, Aishima S, et al. Evaluation of techniques to prevent colorectal anastomotic leakage. J Surg Res. (2015) 194:450–7. doi: 10.1016/j.jss.2014.11.045
29. Hansen LA, Monnet EL. Evaluation of a novel suture material for closure of intestinal anastomoses in canine cadavers. Am J Vet Res. (2012) 73:1819–23. doi: 10.2460/ajvr.73.11.1819
30. Höllwarth ME. Short bowel syndrome. In: Puri P, Höllwarth ME, editors. Pediatric surgery: Springer surgery atlas series. Berlin: Springer (2006). p. 259–60.
31. Dong H, Wang YL, Zhang X, Zhang WJ, Dong SH, Zhang FP, et al. The effect of air test and methylene blue perfusion test on detecting the quality of anastomosis during laparoscopic rectal cancer excision (Dixon). Zhonghua Yi Xue Za Zhi. (2019) 99:939–42. doi: 10.3760/cma.j.issn.0376-2491.2019.12.012. Chinese.30917445
32. Roumen RM, Rahusen FT, Wijnen MH, van Uchelen FAC. “Dog ear” formation after double-stapled low anterior resection as a risk factor for anastomotic disruption. Dis Colon Rectum. (2000) 43:522–5. doi: 10.1007/BF02237198
33. Kryzauskas M, Degutyte AE, Abeciunas V, Lukenaite B, Jasiunas E, Poskus E, et al. Experimental study of mechanical integrity testing in stapled large bowel: methylene blue leak test is not inferior to air leak test. Visc Med. (2021) 37:189–97. doi: 10.1159/000510660
34. Hiradfar M, Shojaeian R, Zabolinejad N, Gharavifard M, Sabzevari A, Joodi M, et al. “Tie over ring” sutureless compression based gastrointestinal anastomotic method: experimental rat model. J Pediatr Surg. (2014) 49:405–9. doi: 10.1016/j.jpedsurg.2013.07.007
35. Ikeuchi D, Onodera H, Aung T, Kan S, Kawamoto K, Imamura M, et al. Correlation of tensile strength with bursting pressure in the evaluation of intestinal anastomosis. Dig Surg. (1999) 16:478–85. doi: 10.1159/000018773
36. Gandini M, Bertuglia A. In vitro evaluation of an inverted end-to-end equine jejunojejunal anastomosis using skin staples. Vet Surg. (2006) 35:678–82. doi: 10.1111/j.1532-950X.2006.00208.x
37. Curran KM, Fransson BA, Gay JM. A comparison of in situ and in vitro techniques for bursting pressure testing of canine jejunum. Am J Vet Res. (2010) 71:370–3. doi: 10.2460/ajvr.71.3.370
38. Freeman DE. Surgery of the small intestine. Vet Clin North Am Equine Pract. (1997) 13:261–301. doi: 10.1016/S0749-0739(17)30240-7.9290184
39. Han S, Cai Z, Ning X, He L, Chen J, Huang Z, et al. Comparison of a new high-frequency electric welding system for intestinal closure with hand-sewn in vivo pig model. J Laparoendosc Adv Surg Tech A. (2015) 25:662–7. doi: 10.1089/lap.2015.0101
40. Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis. (2014) 16:95–109. doi: 10.1111/codi.12411
41. Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. (2017) 19:288–98. doi: 10.1111/codi.13476
42. Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. (1990) 77:1095–7. doi: 10.1002/bjs.1800771006
43. Saile K, Boothe HW, Boothe DM. Saline volume necessary to achieve predetermined intraluminal pressures during leak testing of small intestinal biopsy sites in the dog. Vet Surg. (2010) 39:900–3. doi: 10.1111/j.1532-950X.2010.00730.x
44. Mitsou K, Papazoglou LG, Savvas I, Tzimtzimis E. Investigation of leakage holes created by four needle types used for closure of canine enterotomies. Open Vet J. (2018) 8:411–4. doi: 10.4314/ovj.v8i4.10
45. Awad S, El-Rahman AIA, Abbas A, Althobaiti W, Alfaran S, Alghamdi S, et al. The assessment of perioperative risk factors of anastomotic leakage after intestinal surgeries; a prospective study. BMC Surg. (2021) 21:29. doi: 10.1186/s12893-020-01044-8
46. Lam A, Fleischer B, Alverdy J. The biology of anastomotic healing-the unknown overwhelms the known. J Gastrointest Surg. (2020) 24:2160–6. doi: 10.1007/s11605-020-04680-w
47. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. (2008) 453:314–21. doi: 10.1038/nature07039
48. Sugimachi K, Yaita A, Nakamura T, Inokuchi K. An alternative one layer inverting suture technique for intestinal anastomosis. Jpn J Surg. (1979) 9:322–6. doi: 10.1007/BF02468632
49. Fabri PJ, Rosemurgy A. Reoperation for small intestinal obstruction. Surg Clin North Am. (1991) 71:131–46. doi: 10.1016/S0039-6109(16)45338-7
50. Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. (2011) 17:4545–53. doi: 10.3748/wjg.v17.i41.4545
Keywords: intestinal anastomosis, single-layer suture, asymmetric figure-of-eight suture, anastomotic leakage, in vitro experiment
Citation: Liu C, Wang Y, Zhao A, Hu F, Fan Q, Han G, Ding G, Fu T, Geng L and Yin H (2022) An alternative asymmetric figure-of-eight single-layer suture technique for bowel anastomosis in an in vitro porcine model. Front. Surg. 9:896542. doi: 10.3389/fsurg.2022.896542
Received: 15 March 2022; Accepted: 5 September 2022;
Published: 28 September 2022.
Edited by:
Cihangir Akyol, Ankara University, TurkeyReviewed by:
Carlos Arturo Rodríguez-Alarcón, Universidad Autónoma de Ciudad Juárez, MexicoMustajab Hussain Mirza, Louisiana State University, United States
© 2022 Liu, Wang, Zhao, Hu, Fan, Han, Ding, Fu, Geng and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Geng MzgxODExNDFAcXEuY29t Hongshan Yin aHMxMjMxOGJ5QDE2My5jb20=
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Chen Liu1,2
Chen Liu1,2 Guojian Ding
Guojian Ding Tingliang Fu
Tingliang Fu