- 1Clinical Medical College, Yangzhou University, Yangzhou China
- 2Department of Gastrointestinal Surgery, Northern Jiangsu People’s Hospital, Yangzhou China
- 3General Surgery Institute of Yangzhou, Yangzhou University, Yangzhou China
- 4Yangzhou Key Laboratory of Basic and Clinical Transformation of Digestive and Metabolic Diseases, Yangzhou China
Background: Laparoscopic gastrectomy and robotic gastrectomy are the most widely adopted treatment of choice for gastric cancer. To systematically assess the safety and effectiveness of robotic gastrectomy for gastric cancer, we carried out a systematic review and meta-analysis on short-term and long-term outcomes of robotic gastrectomy.
Methods: In order to find relevant studies on the efficacy and safety of robotic gastrectomy (RG) and laparoscopic gastrectomy (LG) in the treatment of gastric cancer, numerous medical databases including PubMed, Medline, Cochrane Library, Embase, Google Scholar, and China Journal Full-text Database (CNKI) were consulted, and Chinese and English studies on the efficacy and safety of RG and LG in the treatment of gastric cancer published from 2012 to 2022 were screened according to inclusion and exclusion criteria, and a meta-analysis was conducted using RevMan 5.4 software.
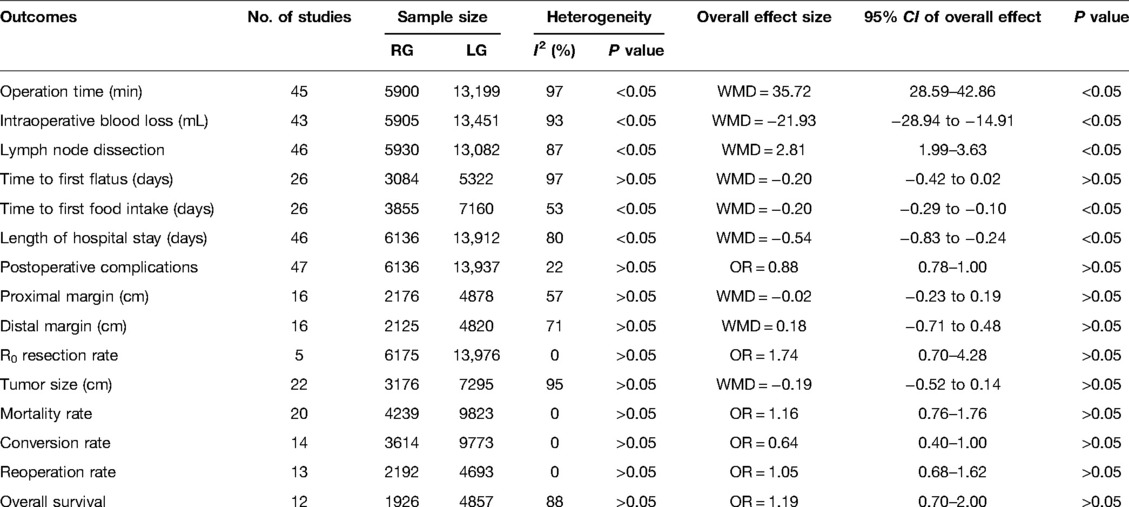
Results: The meta-analysis inlcuded 48 literatures, with 20,151 gastric cancer patients, including 6,175 in the RG group and 13,976 in the LG group, respectively. Results of our meta-analysis showed that RG group had prololonged operative time (WMD = 35.72, 95% CI = 28.59–42.86, P < 0.05) (RG: mean ± SD = 258.69 min ± 32.98; LG: mean ± SD = 221.85 min ± 31.18), reduced blood loss (WMD = −21.93, 95% CI = −28.94 to −14.91, P < 0.05) (RG: mean ± SD = 105.22 ml ± 62.79; LG: mean ± SD = 127.34 ml ± 79.62), higher number of harvested lymph nodes (WMD = 2.81, 95% CI = 1.99–3.63, P < 0.05) (RG: mean ± SD = 35.88 ± 4.14; LG: mean ± SD = 32.73 ± 4.67), time to first postoperative food intake shortened (WMD = −0.20, 95% CI = −0.29 to −0.10, P < 0.05) (RG: mean ± SD = 4.5 d ± 1.94; LG: mean ± SD = 4.7 d ± 1.54), and lower length of postoperative hospital stay (WMD = −0.54, 95% CI = −0.83 to −0.24, P < 0.05) (RG: mean ± SD = 8.91 d ± 6.13; LG: mean ± SD = 9.61 d ± 7.74) in comparison to the LG group. While the other variables, for example, time to first postoperative flatus, postoperative complications, proximal and distal mar gin, R0 resection rate, mortality rate, conversion rate, and 3-year overall survival rate were all found to be statistically similar at P > 0.05.
Conclusions: In the treatment of gastric cancer, robotic gastrectomy is a safe and effective procedure that has both short- and long-term effects. To properly evaluate the advantages of robotic surgery in gastric cancer, more randomised controlled studies with rigorous research methodologies are needed.
Introduction
Gastric cancer is the most common malignant tumor of the digestive tract in far-eastern countries. The global incidence of gastric cancer has declined steadily in recent years, but Asia still has the highest incidence of gastric cancer (1). Due to the lack of early diagnosis methods, most patients are already in the middle and late stages of the disease at the time of their diagnosis. The best method of treatment is currently surgery. The surgical method has evolved from traditional open surgery to laparoscopic surgery (2). Since the mid-1980s, laparoscopic techniques have received increasing recognition for their minimally invasive advantages in treating gastric cancer (3), and laparoscopic gastrectomy (LG) has become the standard treatment for early gastric cancer. Nonetheless, laparoscopic techniques have some limitations and shortcomings, including inflexible operation of surgical instruments, two-dimensional imaging display interface, and a limited range of operation. In recent years, robotic technologies have made tremendous progress in overcoming the technological limitations of traditional laparoscopy. Robot-assisted surgical procedure has visual direction from the bottom to the top and not the other way around as in traditional open surgery, which makes it more advantageous to expose the dirty surface tissue. Although several scholars have conducted meta-analyses of such studies, all of them focused on assessing its immediate efficacy without considering its long-term effectiveness, such as its 3-year survival rate, and some of the results differed from study to study. As the robotic surgery system continues to advance, both its technology and efficacy are continually improving, and the research reports associated with it continue to be updated. In addition, the robotic system was only recently applied to some patients undergoing gastric cancer surgery, and its status in the treatment of gastric cancer has not been conclusively established or included in guidelines. To evaluate the short and long-term efficacy and safety of the robotic surgery system in the clinical treatment of gastric cancer, this study conducted a meta-analysis of published clinical comparative studies (3–31) on RG and LG.
Materials & Methods
Search Strategy
In order to search PubMed, Medline, Cochrane Library, Embase, Google Scholar, and China Journal Full-text Database (CNKI) and other databases according to clinical comparison studies of RG and LG, the search strings “Robotic OR da Vinci OR Robot-Assisted”, “Gastrectomy”, “Gastric “, “Cancer OR Carcinoma OR Tumor OR Neoplasm”, “Laparoscopic OR Laparoscopic-Assisted “ and “Robotic”, Searches were limited to the period 2012–2021, with the “related search” feature being utilized further to exclude omissions.
Literature Inclusion and Exclusion Criteria
Inclusion criteria: (1) published randomized or non-randomized controlled trials comparing RG with LG; (2) patients diagnosed with gastric cancer who have undergone their first surgical procedure; (3) provide clear criteria for the selection of study cases and methods for grouping; (4) provide evidence of clinical efficacy comparison between RG and LG; (5) include data studies of superior quality and detail; (6) describe the raw data, including continuous variables such as mean and standard deviation, and count information such as the number of events and the number of samples. For dichotomous variables, the combined odds ratio (OR) and 95% confidence interval (CI) should be provided, as well as a regression coefficient that can be converted to the combined OR and 95% CI and standard error. Exclusion criteria: (1) comparisons of non-LG and RG cases; (2) study cases containing other benign gastrointestinal diseases; (3) study cases having only undergone palliative major gastrectomy, tumor reduction, or short-circuit surgery; (4) study cases involving emergency surgery; (5) no reliable comparisons could be drawn from the literature; (6) duplicate published studies; (7) no controlled studies conducted simultaneously; and (8) no clear grouping tendency in terms of the extent of lymph node dissection or stage of the disease.
Data Extraction
Extractions are made by two investigators independently, and if a dispute occurs, it is resolved through mutual discussion or by a third party. The following data types can be identified: (1) General information, including the names of the authors, the dates of literature publication, the type of study, the sample size, the tumor site and its size, and the TNM stage; (2) outcome indicators, such as operative time and blood loss, lymph node dissection, transit rate, distal margin length, R0 resection rate, postoperative hospital stay, immediate postoperative gas and food intake, complication rate, 3-year survival rate, and morbidity and mortality rate.
Evaluation of the Quality of the Literature
We used the MINORS scoring criteria (32) to assess the quality of the clinical trials (score 0: no description, score 1: inadequate description, and score 2: adequate description). A modified set of MINORS scoring criteria containing 12 items, which yields scores ranging from 0 to 24 was used to evaluate the quality of the literature included in this study (Supplementary file Appendix 1).
Statistical Analysis
We performed the meta-analysis using RevMan 5.4 software, using odds ratio (OR) values for measurement data and weighted mean differences (WMD) for efficacy analysis for count data. The 95% confidence interval (CI) for the effect sizes was calculated. It was checked for heterogeneity between the studies using the χ2 test and I2 values, and in case of heterogeneity (I2 > 50%, P < 0.05), a random-effects model was applied; if there was no heterogeneity (I2 < 50%, P≥ 0.05), a fixed-effects model was applied. The differences were considered statistically significant at P < 0.05.
Results
Search Results
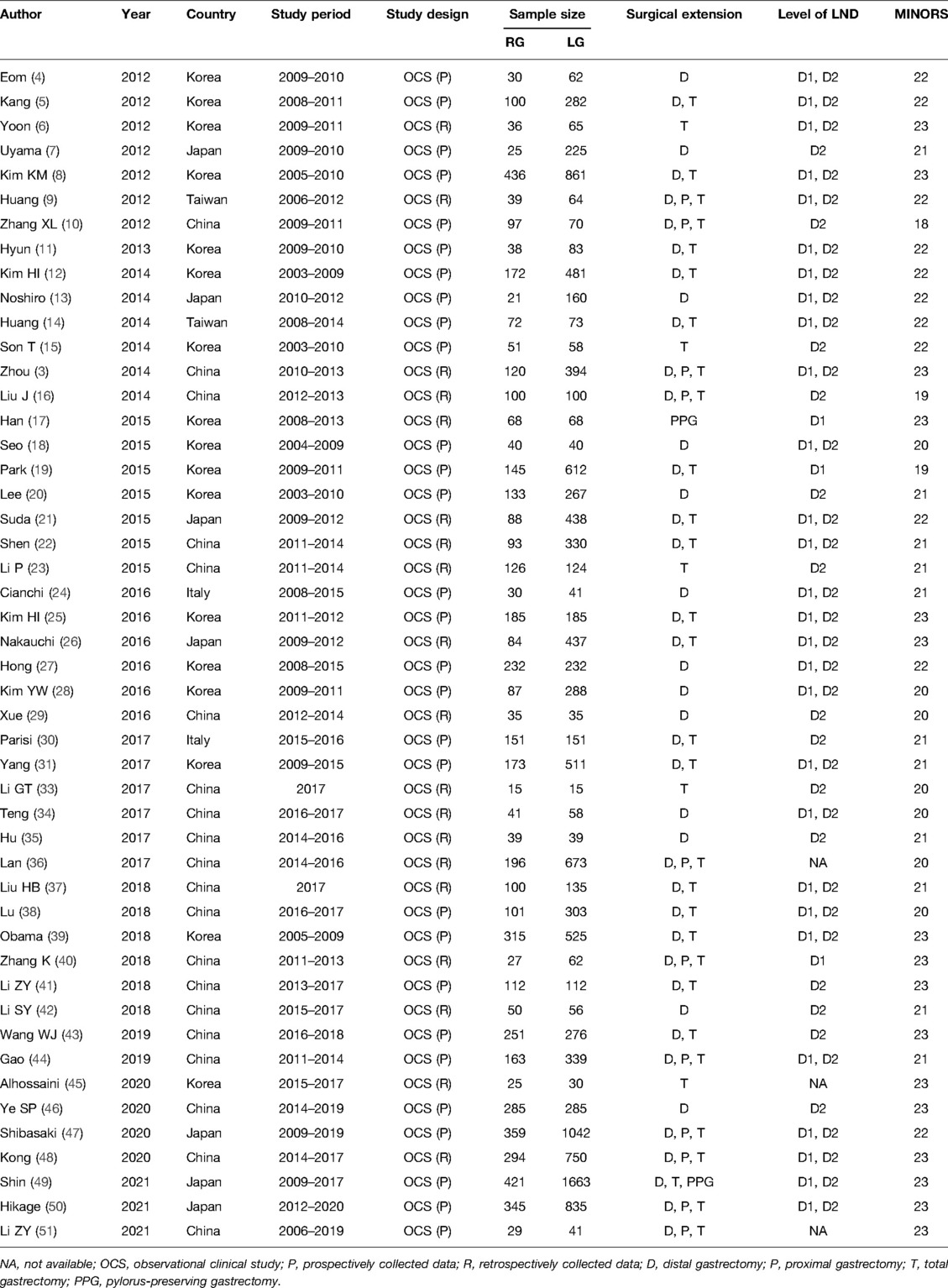
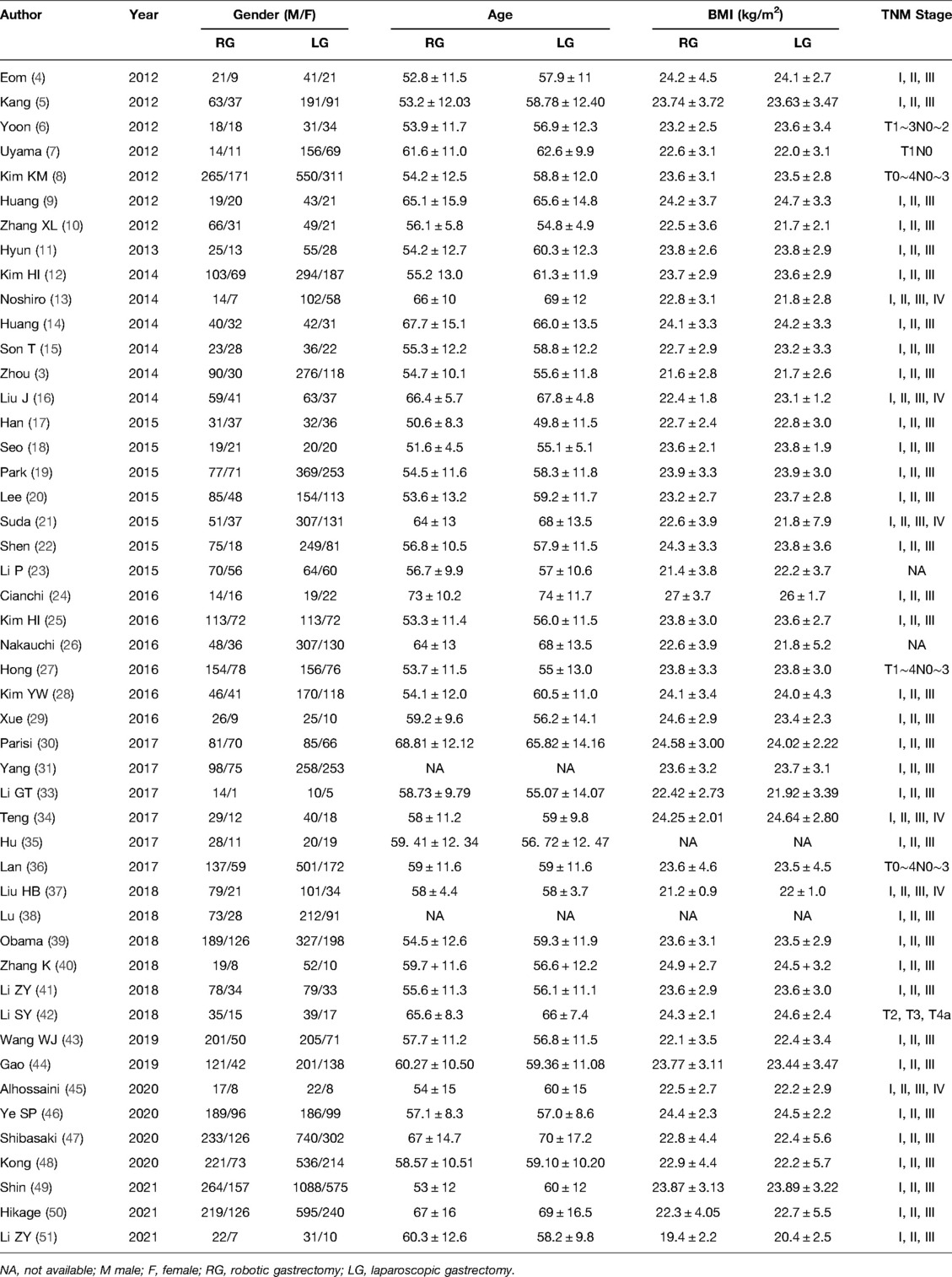
A preliminary search retrieved a total of 5,440 articles published from 2012 to 2021. After reviewing all titles and abstracts, 76 complete articles were found, 28 of which were rejected because they did not meet the inclusion criterion. Supplementary Figure 1 illustrates the search process. Ultimately, 20,151 patients data from 48 retrospective studies were included in the present study, with 6,175 in the RG group and 13,976 in the LG group (3–30). Table 1 presents the basic characteristics of the included literature and MINORS scale for quality assessment, while Table 2 provides the patients' characteristics of the included literature. Supplementary Figure 2 depicts MINORS scores bar graph for the observational studies included in our systematic review.
Meta-Analysis Results
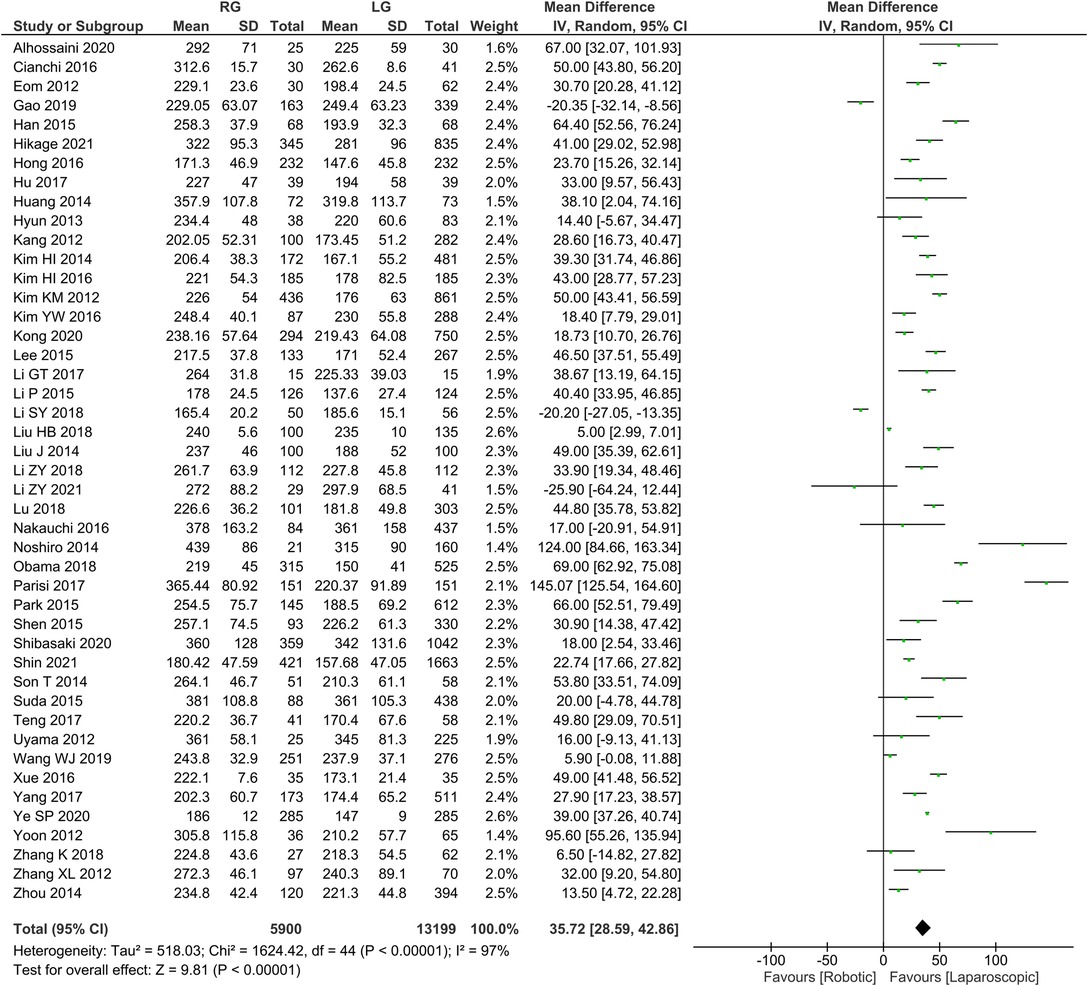
Operation time was reported in 45 publications, with homogeneity test I2 = 97%, P < 0.05. Using a random effect model analysis showed that the RG group had a significantly longer operation time than the LG group (WMD = 35.72, 95% CI = 28.59–42.86, P < 0.05) (Figure 1). The mean ± SD values are 258.69 min ± 32.98 and 221.85 min ± 31.18, for the RG and LG groups, respectively.
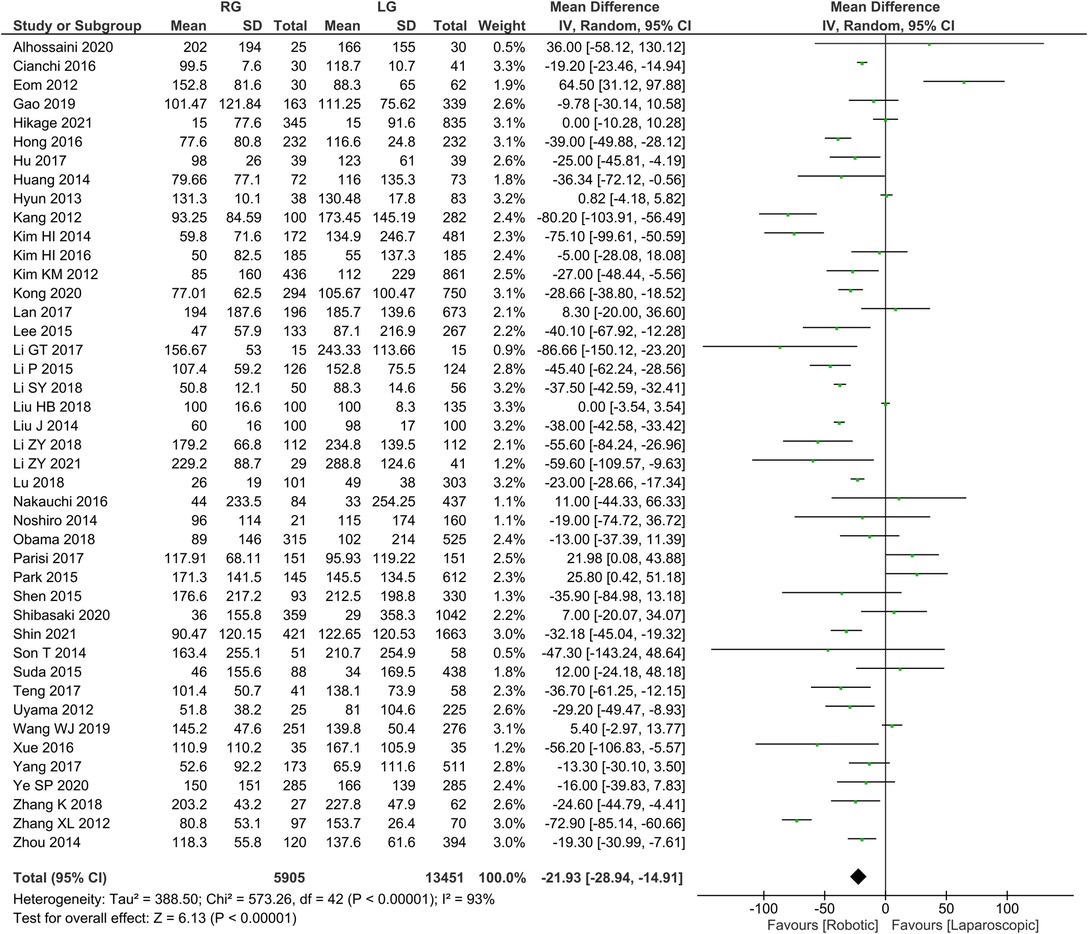
Intraoperative bleeding was reported in 43 publications with homogeneity test I2 = 93%, P < 0.05, and analysis using a random effects model showed that intraoperative bleeding was significantly less in the RG group than in the LG group (WMD = −21.93, 95% CI = −28.94 to −14.91, P < 0.05) (Figure 2). The mean ± SD values are 105.22 ml ± 62.79 and 127.34 ml ± 79.62, for the RG and LG groups, respectively.
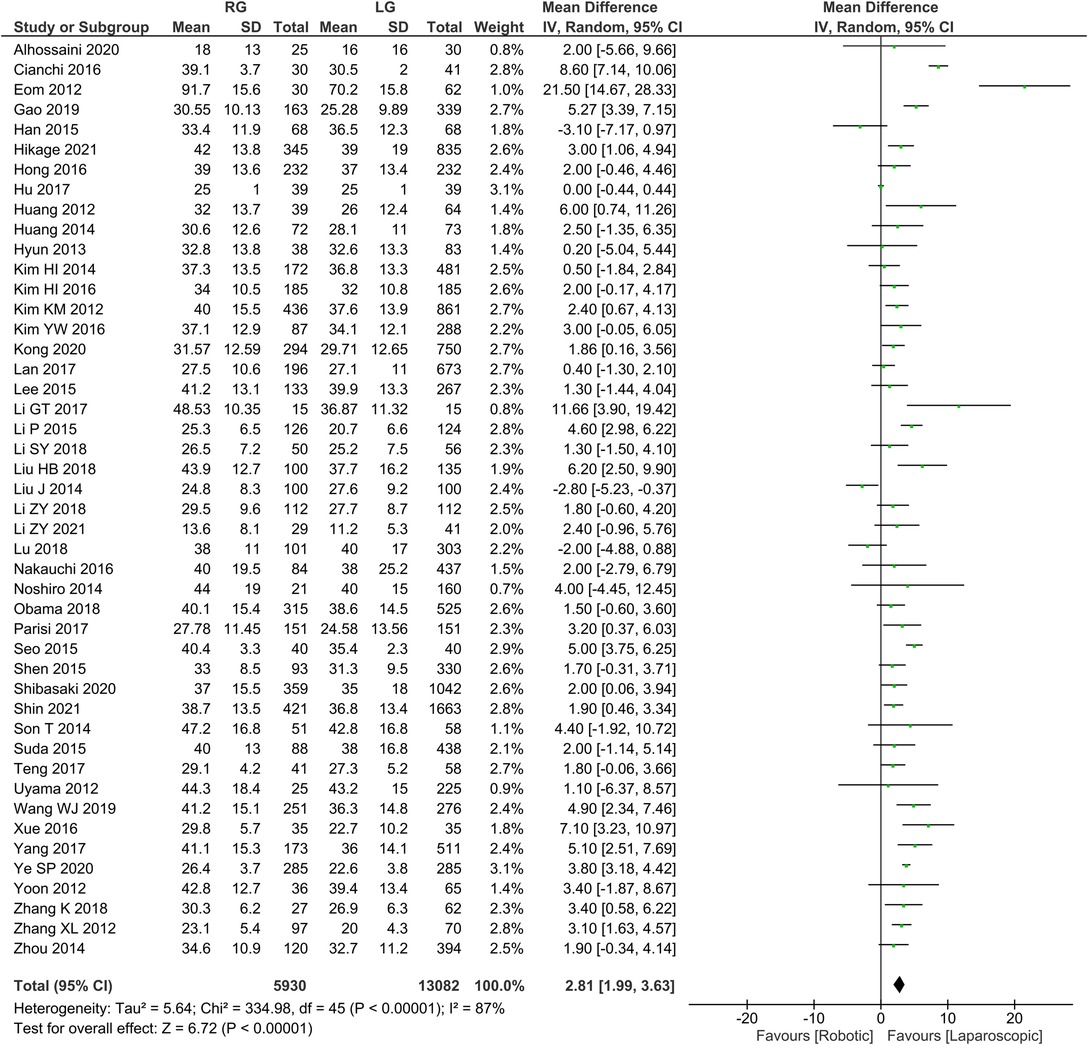
Number of lymph node dissection 46 publications reported the number of lymph node dissections with homogeneity test I2 = 87%, P < 0.05, and analysis using a random effects model showed that the number of lymph node dissections was higher in the RG group than in the LG group (WMD = 2.81, 95% CI = 1.99–3.63, P < 0.05) using random effects model analysis (Figure 3). The mean ± SD values are 35.88 ± 4.14 and 32.73 ± 4.67, for the RG and LG groups, respectively.
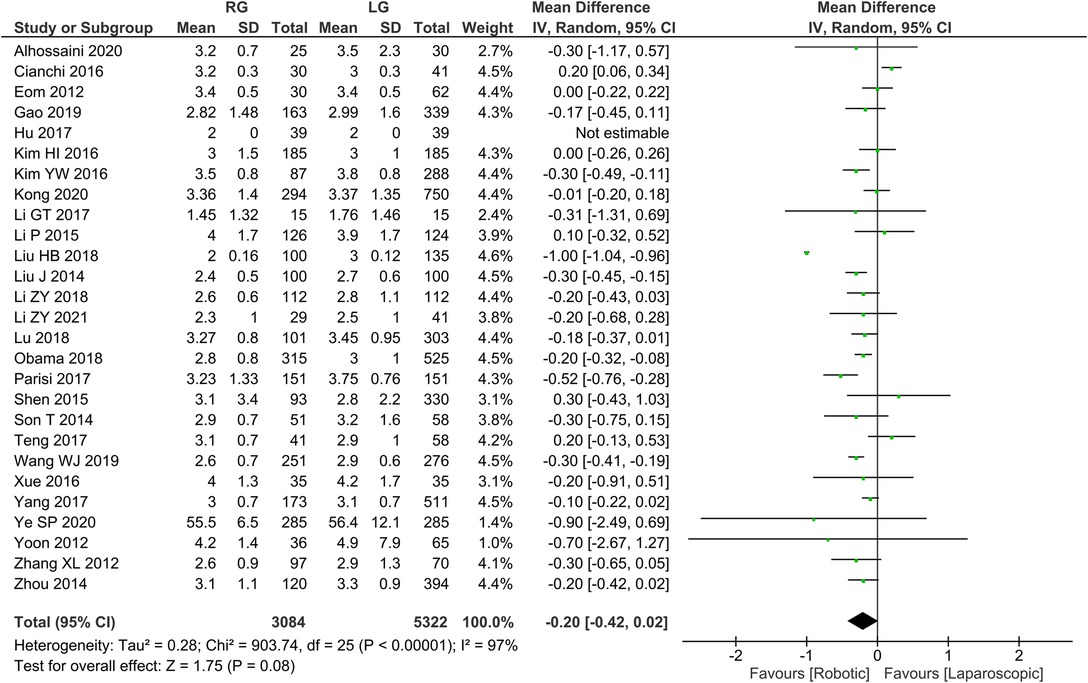
Time to first postoperative flatus 26 publications reported time to first postoperative flatus with homogeneity test I2 = 97%, P < 0.05, and analysis using a random effects model showed not statistically significant in time to first postoperative flatus between the two groups (WMD = −0.20, 95% CI = −0.42 to 0.02, P > 0.05) (Figure 4). The mean ± SD values are 5.02 d ± 1.24 and 5.25 d ± 2.54, for the RG and LG groups, respectively.
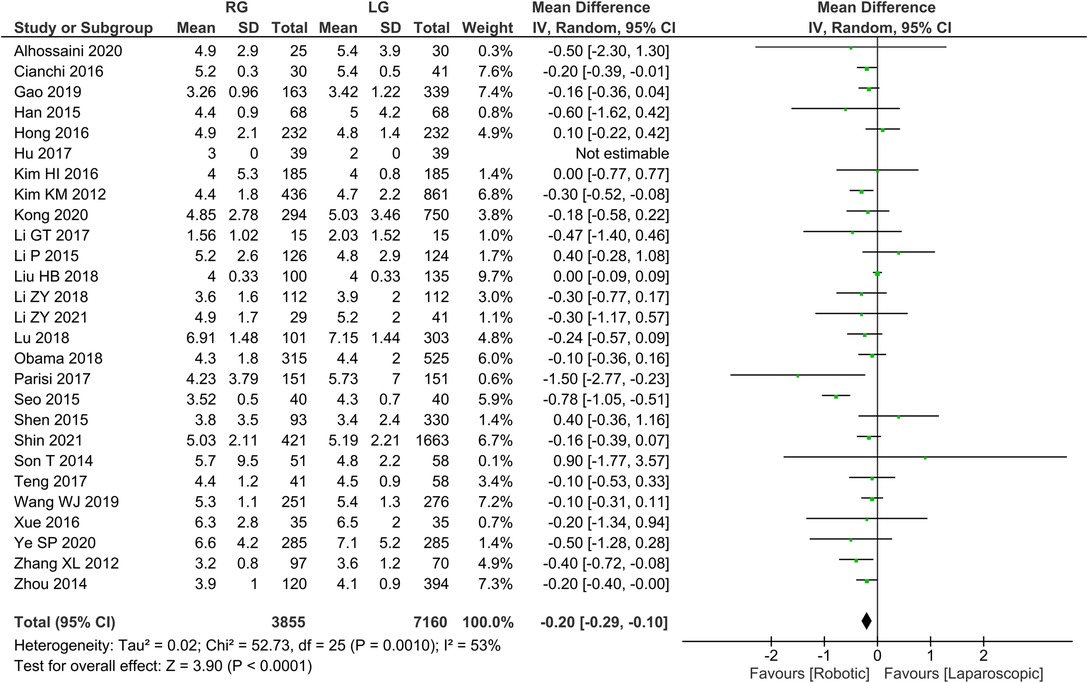
Time to first postoperative food intake 26 publications reported time to first postoperative food intake with homogeneity test I2 = 53%, P < 0.05, and analysis using a random effects model showed that time to first postoperative food intake was significantly shorter in the RG group than in the LG group (WMD = −0.20, 95% CI = −0.29 to −0.10, P < 0.05) (Figure 5). The mean ± SD values are 4.55 d ± 1.94 and 4.76 d ± 1.54, for the RG and LG groups, respectively.
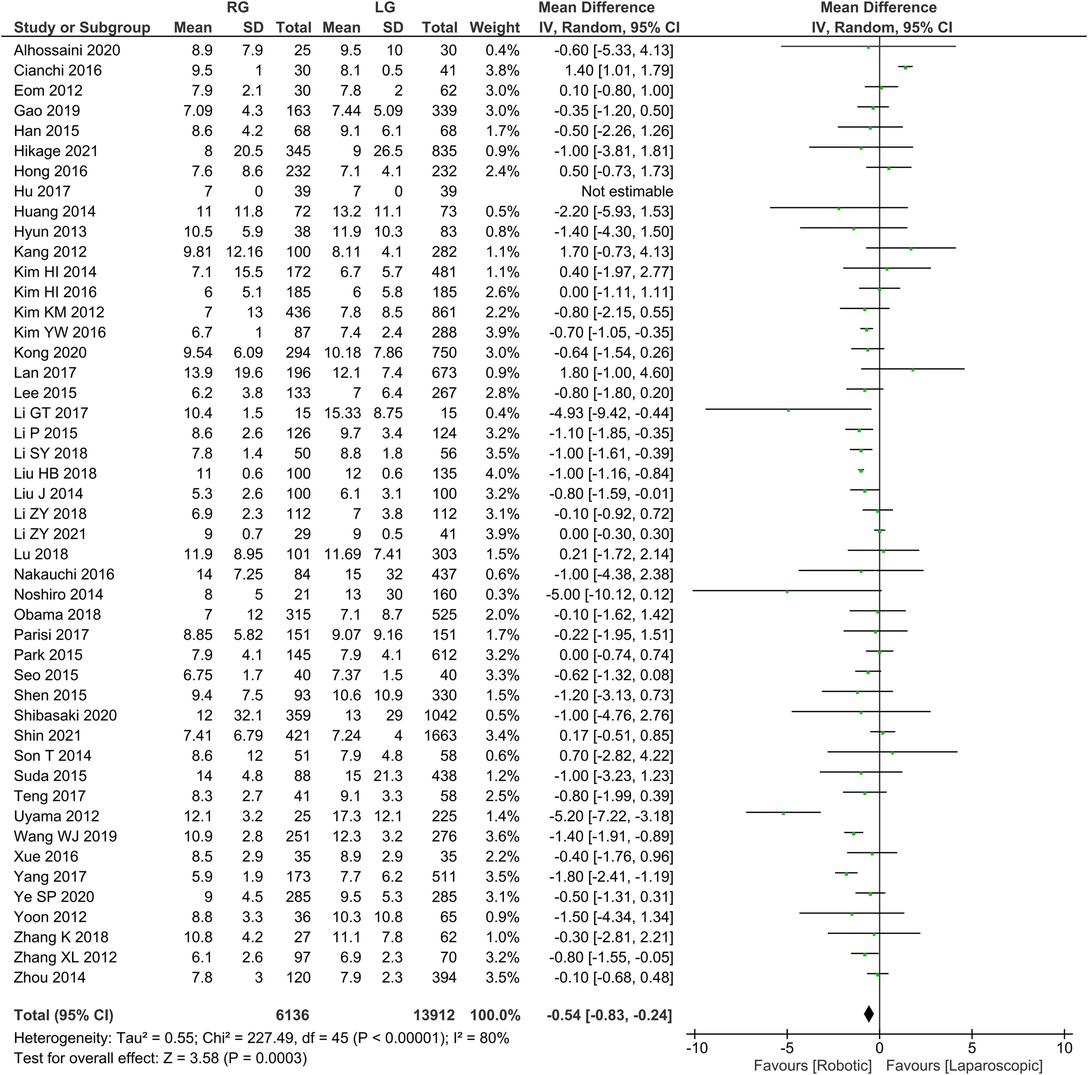
Postoperative length of hospital stays 46 publications reported postoperative length of stay, homogeneity test I2 = 80%, P < 0.05, and a random effects model analysis showed significantly lower length of hospital stay in the RG group than in the LG group (WMD = −0.54, 95% CI = −0.83 to −0.24, P < 0.05) (Figure 6). The mean ± SD values are 8.91 d ± 6.13 and 9.61 d ± 7.74, for the RG and LG groups, respectively.
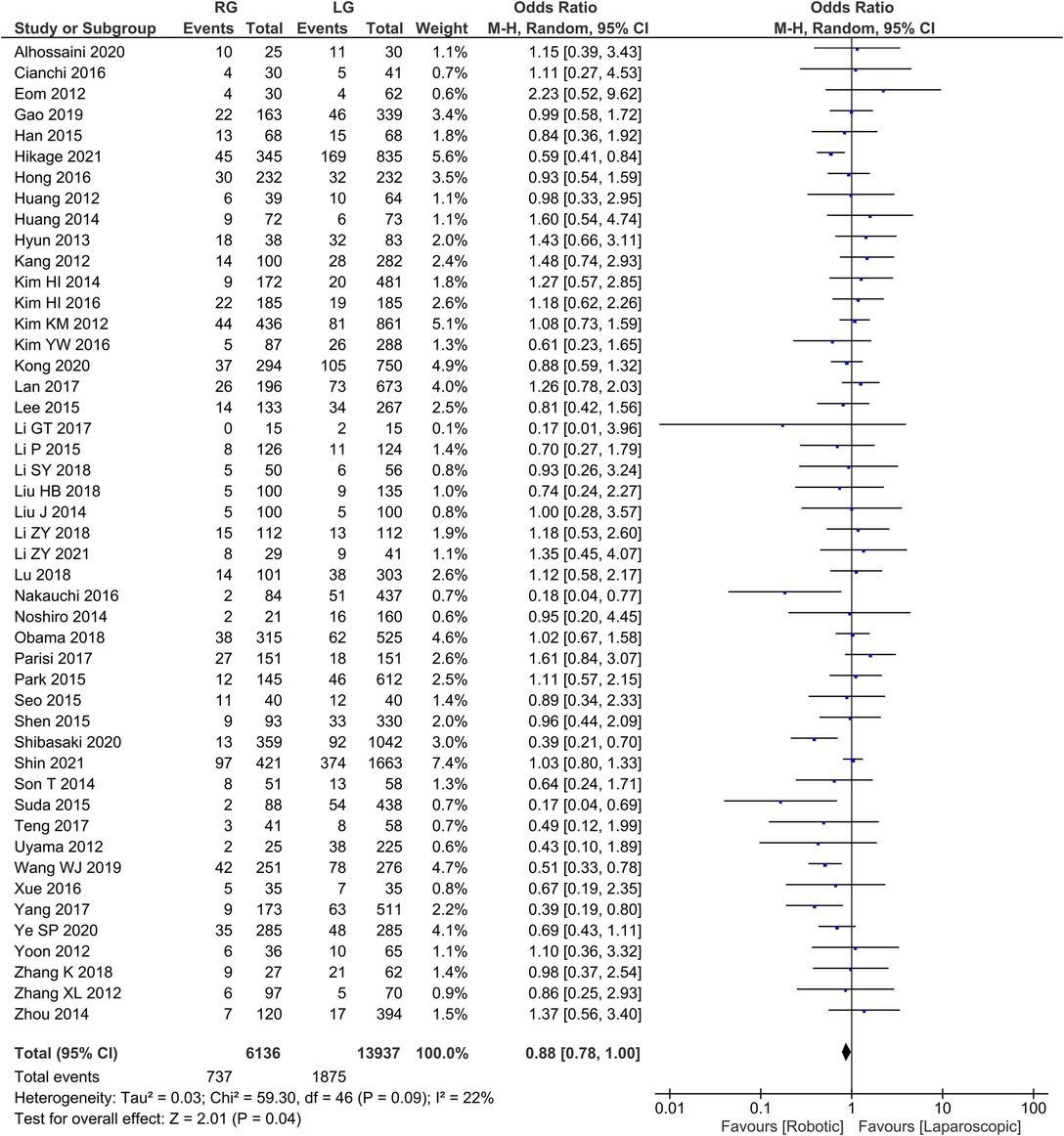
Postoperative Complication rates 47 publications reported complication rates with homogeneity test I2 = 22%, P > 0.05, and a random effects model analysis showed no statistically significant difference in complication rates between the two groups (OR = 0.88, 95% CI = 0.78–1.00, P < 0.05) (Figure 7). The average complication rate was 15.68 in RG group and 39.89 in LG group.
Proximal margin distance 16 publications reported proximal margin distance with homogeneity test I2 = 57%, P < 0.05, and analysis using a random effects model analysis showed no statistically significant difference in proximal margin distance between the two groups (WMD = −0.02, 95% CI = −0.23 to 0.19, P > 0.05) (Supplementary Figure 3). The mean ± SD values are 4.05 cm ± 1.15 and 4.05 cm ± 0.94, for the RG and LG groups, respectively.
Distal margin distance 16 publications reported distal margin distance with homogeneity test I2 = 71%, P < 0.05, and a random effects model analysis showed not statistically significant in distal margin distance between the two groups (WMD = 0.18, 95% CI = −0.71 to 0.48, P > 0.05) (Supplementary Figure 4). The mean ± SD values are 5.98 cm ± 1.56 and 5.66 cm ± 1.89, for the RG and LG groups, respectively.
R0 resection rates 48 publications reported R0 resection rates with homogeneity test I2 = 0%, P > 0.05, and analysis using a fixed effect model showed no statistically significant difference in R0 resection rates between the two groups (OR = 1.74, 95% CI = 0.70–4.28, P > 0.05) (Supplementary Figure 5). The average R0 resection rate was 128.52 in RG group and 290.81 in LG group.
Tumor size 22 publications reported tumor size with homogeneity test I2 = 95%, P < 0.05, and analysis using a random effects model analysis showed no statistically significant difference in tumor size between the two groups (WMD = −0.19, 95% CI = −0.52 to 0.14, P > 0.05) (Supplementary Figure 6). The mean ± SD values are 3.27 cm ± 0.82 and 3.31 cm ± 0.76, for the RG and LG groups, respectively.
Mortality rate 20 publications reported mortality rate with homogeneity test I2 = 0%, P > 0.05, and analysis using a fixed effect model showed no statistically significant difference in mortality rate between the two groups (OR = 1.16, 95% CI = 0.76–1.76, P > 0.05) (Supplementary Figure 7). The average mortality rate was 1.32 in RG group and 3 in LG group.
Conversion rate 14 publications reported conversion rate with homogeneity test I2 = 0%, P > 0.05, and a fixed effect model analysis showed no statistically significant difference in conversion rate between the two groups (OR = 0.64, 95% CI = 0.40–1.00, P > 0.05) (Supplementary Figure 8). The average conversion rate was 0.88 in RG group and 3.03 in LG group.
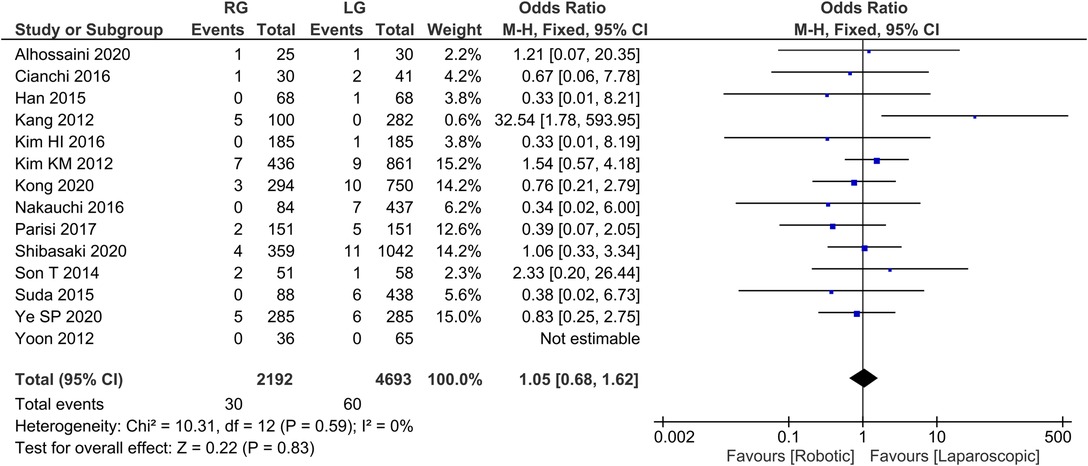
Reoperation rate 13 publications reported reoperation rate with homogeneity test I2 = 0%, P > 0.05, and a fixed effect model analysis showed no statistically significant difference in reoperation rate between the two groups (OR = 1.05, 95% CI = 0.68–1.62, P > 0.05) (Figure 8). The average reoperation rate was 2.14 in RG group and 4.28 in LG group.
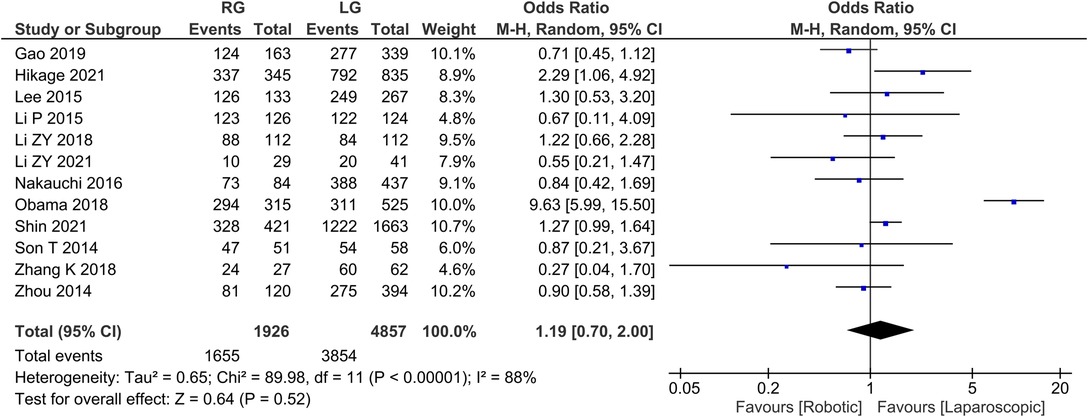
Overall survival 12 publications reported 3-year survival rates with homogeneity test I2 = 88%, P < 0.05, and a random effects model analysis showed no statistically significant difference in 3-year survival between the two groups (OR = 1.19, 95% CI = 0.70–2.00, P > 0.05) (Figure 9). The average overall survival was 137.91 in RG group and 321.16 in LG group.
Heterogeneity and Sensitivity Analysis
Heterogeneity is considered to be significant when I2 > 50% and P < 0.05. Our results suggest that there was heterogeneity in the time to first flatus, proximal margin, distal margin, tumor size, and overall survival (I2 > 50%, P > 0.05) (Table 3). Furthermore, substantial heterogeneity was also in operation time, intraoperative bleeding loss, lymph node dissection, and time to first food intake (I2 > 50%, P < 0.05) (Table 3). According to the MINORS score, high-quality literature with a score of more than 18 points was selected for sensitivity analysis.
Publication of Bias
Evaluation of publication bias was accomplished using a funnel plot of intraoperative blood loss, lymph node dissection, and postoperative complications. There was no evidence of publication bias in the bilaterally symmetrical funnel plots of overall complications (Supplementary Figures 9, 10, 11).
Discussion
In most cases of gastric cancer, gastrostomies are the mainstay of treatment. Almost thirty years ago, minimally invasive gastrostomies were introduced to reduce patient burden. As a result of the increasing availability of surgical robots, a robotic-assisted gastrectomy was performed for the first time in Japan in 2002 (52). Currently, robotic surgery is widely used in general surgery as well as other applications (22). In comparison to laparoscopic gastrectomy (LG), the feasibility and safety of the robotic-assisted (RG) technique were explored in this study.
Robotic surgery has become increasingly popular in a variety of surgeries due to its increased surgical precision and safety. Since the earliest application of robotics in surgery, it has evolved in five distinct categories: endoscopic, stereotactic, bioinspired, millimeter-scale microbots, and autonomous systems. Robotic surgery has shown to have dramatically superior clinical results when compared to laprascopic and open surgical techniques. In our study, of the 48 publications examined, 38 researches employed the Da Vinci surgical systems while the other 10 did not specify the surgical systems used (supplementary Figure 12.) According to the results of the comparative analysis of RG and LG gastric cancer treatment found in this study, there are disparities in efficacy between these treatments.
Based on the meta-analysis, it was found that RG requires a longer surgical procedure time than LG. One of the main reasons is that the robotic surgical system necessitates machine assembly at the beginning of the operation, and Jiménez-Rodrguez et al. (53) reported that the average preparation time for RG was 62.9% ± 24.6%min, but with experience the preparation time gradually decreases. Huang et al. (14) reported that the preparation time could be reduced to thirty minutes after 25 surgical operations. A study by Kang et al. (5) reported that the experienced RG group had a considerably shorter mean operation time than the inexperienced RG group. Furthermore, robotic surgery is a relatively new minimally invasive procedure that necessitates a learning process to master which is significantly shorter than LG. As reported by Mege et al. (54) and Huang et al. (14), the learning curve for LG surgery ranges from 30 to 50 cases, whereas surgeons performing 10–20 RG cases would accomplish a stable level of operative time. Huang et al. (14) compared LG to RG in the middle and later stages of the learning curve, finding LG to have a longer operative time than RG regardless of the stage. Consequently, once the learning curve is passed, the time spent intraoperatively in RG would be shorter than that in LG.
This meta-analysis revealed that the intraoperative blood loss in RG was less than that in LG, and the number of lymph nodes dissected in RG was higher than that in LG. There are abundant blood vessels and lymphatic vessels in the perigastric tissue. The process of LG perigastric tissue separation and lymph node dissection is prone to haemorrhage, which may affect the operator's ability to identify the tissue structure and to view the operation field. Due to the advantages of the robotic, these issues have been resolved (3–30), such as: (1) jitter filtering, the robotic arm eliminates the natural tremor in the human hand and improves the stability of the operation; (2) High-definition three-dimensional images, 3D three-dimensional images magnify the surgical field by 10–15 times, revealing the small blood vessels and tissue structure around the stomach, making the blood vessels around the stomach more secure, and improving the accuracy of the procedure; (3) Robotic arms have seven degrees of freedom to simulate the mechanical wrist, which allows for greater flexibility of operation and the ability to work in confined spaces; (4) The operator controls the robotic arm individually, eliminating the problem of incompatibility between the mirror arm and the operator; (5) The operator adopts a sitting position that provides both physical comfort and improves the efficiency of his or her operation; (6) Remote control by the operator so to avoid direct contact with the patient; (7) Reconstruction of the digestive tract to achieve a full endoscopic anastomosis which is suitable for obesity, barrel chests, high esophageal cut planes, a small costal arch angle, and anterior and posterior abdominal walls. There are several advantages to total endoscopic in vivo anastomosis for patients with the same diameter and width. These attributes, without a doubt, improve surgical precision and stability, minimise mistake rates, and promote minimally invasive surgery.
A patient's prognosis and degree of surgical cure are affected by the number of lymph nodes dissected at the time of surgery for early gastric cancer. As a treatment for intermediate and advanced gastric cancer, D2 lymph node dissection remains the standard procedure. Nonetheless, it is difficult to dissect D2 lymph nodes in LG. The included studies (3, 8, 22, 24, 28) showed that more lymph nodes had been cleared in the RG group than in the LG group, while the remaining studies showed no significant differences between the two groups in terms of lymph node clearance (6, 7, 11, 14, 17, 55). Across the included studies (3–30), the number of surgically cleared lymph nodes in RG ranged between 13.6 and 91.7, while all were able to reach the range of clearance of D2 lymph nodes. It has been revealed that RG can have therapeutic benefits that are comparable to LG and may even exceed them (for example in dissection, abdominal reduction, suturing etc).
We found that the RG group had a shorter first postoperative food intake period than the LG group. We found substantial discrepancies between Kim et al. (8) and Zhang et al. (10) among the independent literature examined. Possible reasons are (8, 10): (1) The robotic arm moves stably and flexibly during RG operation, helping to avoid overstretching and separation of tissues and accidental injury to blood vessels, thus causing less trauma to patients; (2) Adopting the concept of enhanced recovery surgery after perioperative management, Zhang et al. (10) reported earlier postoperative time to get out of bed, first gassing and eating time in the RG group compared to the LG group. As a result of the meta-analysis, however, the potential factor could not be the cause of the different postoperative hospital stay between the groups of RG and LG. A slight statistically significant difference was found between the two groups in terms of hospital stay, but the RG did appear to be preferred.
A meta-analysis of the data revealed that there was no difference in the rest of the data between the RG and LG group. Despite this, there is heterogeneity in first postoperative flatus time, postoperative complications, proximal incision margin distance, distal incision margin distance, tumor size, and 3-year survival rate. There may be a variety of reasons for this: (1) The operators included in the study may be in different stages of their RG development, and the indicators are heterogeneous. (2) The tumor size, location, and stage of included studies are different; (3) Preoperative management and surgical methods are also different, contributing to varying results. The findings of a high-quality non-randomized controlled trial, however, were also convincing when evaluating the short-term effects of surgery, as shown by Abraham et al. (56). After reviewing the high-quality literature, it was discovered that there was no significant difference between the two groups in terms of the number of lymph nodes dissected (WMD = 1.87, 95% CI = −1.24, 3.97, P > 0.05), and the rest of the results remained unchanged, indicating that systematic analysis results are relatively reliable.
This study has some limitations (1) the inclusion of the most recent literature and exclusion of studies with duplicate cases; (2) the inclusion of a relatively large number of studies, which increased the number of relevant cases; and (3) the systematic analysis of long-term survival information, such as the 3-year survival rate. Several limitations exist in this meta-analysis, including: (1) the included literature was retrospective, lacking high-quality randomized controlled trials, some of which had a small number of patients, which may have contributed to publication bias, and (2) the recurrence rate was not examined.
Conclusion
Based on our meta-analysis, RG appears to have superior therapeutic effects than traditional LG for treating gastric cancer and both are safe and practical. Its future application opportunities will improve as more experience is gathered. In the future, large-scale, multi-sampled multicenter randomised controlled studies will be required to increase the reliability of RG in clinical therapy.
Author Contributions
SB: administrative support, provision of study materials or subjects, and manuscript writing. QS; MHA: administrative support, collection and assembly of data, and manuscript writing. YW; QS: provision of study materials or subjects, data analysis, and interpretation. MJ; LW; MA: collection and assembly of data, data analysis and interpretation, and manuscript writing. DW: conception and design, and final approval of the manuscript. All authors have read and approved the manuscript.
Funding
The author(s) declare that no funding was received from any government or private institution, organization, and any other entity.
Acknowledgments
The authors are thankful to Yangzhou University for providing the necessary facilities to carry our the meta-analysis. We are also thankful to Negenome Bio Solutions Pvt Ltd for the statistical analyses and for their constant support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.895976/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. Chen K, Wu D, Pan Y, Cai JQ, Yan JF, Chen DW, et al. Totally laparoscopic gastrectomy using intracorporeally stapler or hand-sewn anastomosis for gastric cancer: a single-center experience of 478 consecutive cases and outcomes. World J Surg Oncol. (2016) 14:115. doi: 10.1186/s12957-016-0868-7
3. Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. (2014) 28(6):1779–87. doi: 10.1007/s00464-013-3385-6
4. Eom BW, Yoon HM, Ryu KW, Lee JH, Cho SJ, Lee JY, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol. (2012) 38(1):57–63. doi: 10.1016/j.ejso.2011.09.006
5. Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of surgical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: the learning curve of robotic surgery. J Gastric Cancer. (2012) 12(3):156–63. doi: 10.5230/jgc.2012.12.3.156
6. Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. (2012) 26(5):1377–81. doi: 10.1007/s00464-011-2043-0
7. Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. (2012) 36(2):331–7. doi: 10.1007/s00268-011-1352-8
8. Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. (2012) 99(12):1681–7. doi: 10.1002/bjs.8924
9. Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. (2012) 16(7):1303–10. doi: 10.1007/s11605-012-1874-x
10. Zhang XL, Jiang ZW, Zhao K. [Comparative study on clinical efficacy of robot-assisted and laparoscopic gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. (2012) 15(8):804–6. doi: 10.3760/cma.j.issn.1671-0274.2012.08.016
11. Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. (2013) 20(4):1258–65. doi: 10.1245/s10434-012-2679-6
12. Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. (2014) 40(10):1346–54. doi: 10.1016/j.ejso.2013.09.011
13. Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc. (2014) 28(4):1180–7. doi: 10.1007/s00464-013-3304-x
14. Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Li AF, et al. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One. (2014) 9(10):e111499. doi: 10.1371/journal.pone.0111499
15. Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. (2014) 28(9):2606–15. doi: 10.1007/s00464-014-3511-0
16. Liu J, Ruan H, Zhao K, Wang G, Li M, Jiang Z. [Comparative study on da vince robotic and laparoscopic radical gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. (2014) 17(5):461–4. doi: 10.3760/cma.j.issn.1671-0274.2014.05.013
17. Han DS, Suh YS, Ahn HS, Kong SH, Lee HJ, Kim WH, et al. Comparison of surgical outcomes of robot-assisted and laparoscopy-assisted pylorus-preserving gastrectomy for gastric cancer: a propensity score matching analysis. Ann Surg Oncol. (2015) 22(7):2323–8. doi: 10.1245/s10434-014-4204-6
18. Seo HS, Shim JH, Jeon HM, Park CH, Song KY. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res. (2015) 194(2):361–6. doi: 10.1016/j.jss.2014.10.022
19. Park JY, Ryu KW, Reim D, Eom BW, Yoon HM, Rho JY, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg. (2015) 39(7):1789–97. doi: 10.1007/s00268-015-2998-4
20. Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. (2015) 29(11):3251–60. doi: 10.1007/s00464-015-4069-1
21. Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. (2015) 29(3):673–85. doi: 10.1007/s00464-014-3718-0
22. Shen W, Xi H, Wei B, Cui J, Bian S, Zhang K, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. (2016) 30(2):574–80. doi: 10.1007/s00464-015-4241-7
23. Peng L, Bing L, Hongyi L, Baoqing J. Application of the Da vinci robot operation system in gastric cancer. J Clin Pathol. (2015) 35(06):1103–6. doi: 10.3978/j.issn.2095-6959.2015.06.045
24. Cianchi F, Indennitate G, Trallori G, Ortolani M, Paoli B, Macri G, et al. Robotic vs laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer: a retrospective comparative mono-institutional study. BMC Surg. (2016) 16(1):65. doi: 10.1186/s12893-016-0180-z
25. Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg. (2016) 263(1):103–9. doi: 10.1097/SLA.0000000000001249
26. Nakauchi M, Suda K, Susumu S, Kadoya S, Inaba K, Ishida Y, et al. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc. (2016) 30(12):5444–52. doi: 10.1007/s00464-016-4904-z
27. Hong SS, Son SY, Shin HJ, Cui LH, Hur H, Han SU. Can robotic gastrectomy surpass laparoscopic gastrectomy by acquiring long-term experience? A propensity score analysis of a 7-year experience at a single institution. J Gastric Cancer. (2016) 16(4):240–6. doi: 10.5230/jgc.2016.16.4.240
28. Kim YW, Reim D, Park JY, Eom BW, Kook MC, Ryu KW, et al. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc. (2016) 30(4):1547–52. doi: 10.1007/s00464-015-4372-x
29. Xue Y, Zhang B, Zhang J, Li P, Liu H, Jia B. Evaluation of clinical short-term outcomes of da vinci robotic gastrectomy for advanced gastric cancer. J Abdom Surg. (2016) 29(1):8–12. doi: 10.3969/j.issn.1003-5591.2016.01.003
30. Parisi A, Reim D, Borghi F, Nguyen NT, Qi F, Coratti A, et al. Minimally invasive surgery for gastric cancer: a comparison between robotic, laparoscopic and open surgery. World J Gastroenterol. (2017) 23(13):2376–84. doi: 10.3748/wjg.v23.i13.2376
31. Yang SY, Roh KH, Kim YN, Cho M, Lim SH, Son T, et al. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. (2017) 24(7):1770–7. doi: 10.1245/s10434-017-5851-1
32. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
33. Gaitian L, Peng C, Lin X, Hongbin L. Da vinci robot and laparoscopic radical total stomach comparison of short-term efficacy of resection. J Laparosc Surg. (2017) 22(10):721–5. doi: 10.3969/j.issn.1003-5591.2016.01.003
34. da T, Songyan L, Shidong H, Yuxuan L, Xiaohui D. Short-term outcomes of robot-assisted versus laparoscopic distal gastrectomy for gastric cancer. J Chin People's Lib Army Med Coll. (2017) 38(12):1095–7. doi: 10.3969/j.issn.2095-5227.2017.12.001
35. Shidong H, Zilong H, Guijun Z, Youjun W, Xiaowei X, Yuxuan L, et al. Comparison of short-term outcome of da vinci robotic-assistedand laparoscopic-assisted radical gastrectomy for gastric cancer. Chin J Clin Res. (2017) 30(06):721–4. doi: 10.13429/j.cnki.cjcr.2017.06.001
36. Lan X, Xi H, Zhang K, Cui J, Li M, Chen L. [Comparison of complications following open, laparoscopic and robotic gastrectomy]. Zhonghua Wei Chang Wai Ke Za Zhi. (2017) 20(2):184–9. doi: 10.3760/cma.j.issn.1671-0274.2017.02.014
37. Liu HB, Wang WJ, Li HT, Han XP, Su L, Wei DW, et al. Robotic versus conventional laparoscopic gastrectomy for gastric cancer: a retrospective cohort study. Int J Surg. (2018) 55:15–23. doi: 10.1016/j.ijsu.2018.05.015
38. Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, et al. A propensity score-matched comparison of robotic versus laparoscopic gastrectomy for gastric cancer: oncological, cost, and surgical stress analysis. J Gastrointest Surg. (2018) 22(7):1152–62. doi: 10.1007/s11605-018-3785-y
39. Obama K, Kim YM, Kang DR, Son T, Kim HI, Noh SH, et al. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. (2018) 21(2):285–95. doi: 10.1007/s10120-017-0740-7
40. Zhang K, Huang X, Gao Y, Liang W, Xi H, Cui J, et al. Robot-Assisted versus laparoscopy-assisted proximal gastrectomy for early gastric cancer in the upper location: comparison of oncological outcomes, surgical stress, and nutritional Status. Cancer Control. (2018) 25(1):1073274818765999. doi: 10.1177/1073274818765999
41. Li Z, Li J, Li B, Bai B, Liu Y, Lian B, et al. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched analysis. Cancer Manag Res. (2018) 10:705–14. doi: 10.2147/CMAR.S161007
42. Song-yan SL-j L, Xing-bang N, Yu Y, Hong-liang Z, Xiao-hui D. Comparison of da vinci robotic surgery system versus laparoscopic D2 radical distal gastrectomy for gastric cancer. J Chin Pract Diagn Ther. (2018) 32(3):245–7. doi: 10.13507/j.issn.1674-3474.2018.03.011
43. Wang WJ, Li HT, Yu JP, Su L, Guo CA, Chen P, et al. Severity and incidence of complications assessed by the clavien-dindo classification following robotic and laparoscopic gastrectomy for advanced gastric cancer: a retrospective and propensity score-matched study. Surg Endosc. (2019) 33(10):3341–54. doi: 10.1007/s00464-018-06624-7
44. Gao Y, Xi H, Qiao Z, Li J, Zhang K, Xie T, et al. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: updated short- and long-term results. Surg Endosc. (2019) 33(2):528–34. doi: 10.1007/s00464-018-6327-5
45. Alhossaini RM, Altamran AA, Cho M, Roh CK, Seo WJ, Choi S, et al. Lower rate of conversion using robotic-assisted surgery compared to laparoscopy in completion total gastrectomy for remnant gastric cancer. Surg Endosc. (2020) 34(2):847–52. doi: 10.1007/s00464-019-06838-3
46. Ye SP, Shi J, Liu DN, Jiang QG, Lei X, Tang B, et al. Robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer based on propensity score matching: short-term outcomes at a high-capacity center. Sci Rep. (2020) 10(1):6502. doi: 10.1038/s41598-020-63616-1
47. Shibasaki S, Suda K, Nakauchi M, Nakamura K, Kikuchi K, Inaba K, et al. Non-robotic minimally invasive gastrectomy as an independent risk factor for postoperative intra-abdominal infectious complications: a single-center, retrospective and propensity score-matched analysis. World J Gastroenterol. (2020) 26(11):1172–84. doi: 10.3748/wjg.v26.i11.1172
48. Kong Y, Cao S, Liu X, Li Z, Wang L, Lu C, et al. Short-Term clinical outcomes after laparoscopic and robotic gastrectomy for gastric cancer: a propensity score matching analysis. J Gastrointest Surg. (2020) 24(3):531–9. doi: 10.1007/s11605-019-04158-4
49. Shin HJ, Son SY, Wang B, Roh CK, Hur H, Han SU. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2084 consecutive patients. Ann Surg. (2021) 274(1):128–37. doi: 10.1097/SLA.0000000000003845
50. Hikage M, Fujiya K, Kamiya S, Tanizawa Y, Bando E, Notsu A, et al. Robotic gastrectomy compared with laparoscopic gastrectomy for clinical stage I/II gastric cancer patients: a propensity score-matched analysis. World J Surg. (2021) 45(5):1483–94. doi: 10.1007/s00268-020-05939-8
51. Li ZY, Liu JJ, Yu PW, Zhao YL, Shi Y, Luo ZY, et al. Robotic total gastrectomy for carcinoma in the remnant stomach: a comparison with laparoscopic total gastrectomy. Gastroenterol Rep. (2021) 9(6):583–8. doi: 10.1093/gastro/goab021
52. van Boxel GI, Ruurda JP, van Hillegersberg R. Robotic-assisted gastrectomy for gastric cancer: a European perspective. Gastric Cancer. (2019) 22(5):909–19. doi: 10.1007/s10120-019-00979-z
53. Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis. (2013) 28(6):815–21. doi: 10.1007/s00384-012-1620-6
54. Mege D, Hain E, Lakkis Z, Maggiori L, Prost ALDJ, Panis Y. Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? A learning curve with case-matched study in 68 patients. Colorectal Dis. (2018) 20(6):O143–51. doi: 10.1111/codi.14238
55. Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, et al. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. (2010) 24(10):2594–602. doi: 10.1007/s00464-010-1014-1
Keywords: robotic, laparoscopic, gastrectomy, gastric cancer, meta-analysis
Citation: Baral S, Arawker MH, Sun Q, Jiang M, Wang L, Wang Y, Ali M and Wang D (2022) Robotic Versus Laparoscopic Gastrectomy for Gastric Cancer: A Mega Meta-Analysis. Front. Surg. 9:895976. doi: 10.3389/fsurg.2022.895976
Received: 14 March 2022; Accepted: 9 June 2022;
Published: 28 June 2022.
Edited by:
Salman Yousuf Guraya, College of Medicine University of Sharjah, United Arab EmiratesReviewed by:
Airazat M. Kazaryan, Østfold Hospital, NorwayJacopo Andreuccetti, Civil Hospital of Brescia, Italy
Copyright © 2022 Baral, Arawker, Sun, Jiang, Wang, Wang, Ali and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daorong Wang d2Rhb3Jvbmc2NjZAc2luYS5jb20=
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Shantanu Baral
Shantanu Baral Mubeen Hussein Arawker
Mubeen Hussein Arawker Qiannan Sun2,3,4
Qiannan Sun2,3,4 Daorong Wang
Daorong Wang