
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 10 June 2022
Sec. Reconstructive and Plastic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.895674
Background: Autologous fat transfer is common in breast augmentationor reconstruction. However, AFG recipient site in the breast for fat grafting has not been carefully investigated.
Methods: Forty female patients requiring breast augmentation with fat grafting were randomly assigned into two groups. The retromammary group received 2/3 fat into the retromammary space and the other 1/3 into the subcutaneous and retropectoral planes. The retropectoral group received 2/3 fat into the retropectoral plane and the other 1/3 into the subcutaneous and retromammary planes. The fat grafting result at 6 months was assessed by 3D laser surface scanning and then ultrasound. Any complications were recorded during follow-up. Samples from a patient who underwent fat grafting for 6 months was obtained and histological examination was conducted.
Results: No significant difference in the retention rate after 6 months was observed between the two groups (retromammary group: 35.9% ± 6.6; retropectoral group: 39.3% ± 5.1, p = 0.1076). The retromammary grouphad a higher incidence of oil cyst formation than the retropectoral group. Histological examination showed that there were more oil cysts and mac2 positive macrophage infiltration in the fat cells in retromammary group, while retropectoral group had more small-size adipocytes.
Conclusion: Although fat grafting into the retropectoral plane did not provide a superior fat graft retention rate, it did lower the incidence of complications. The retropectoral space show great potential to become a favorable recipient site.
Autologous fat is considered to be an ideal soft tissue filling material for both cosmetic and reconstructive purposes, and has been used extensively in plastic surgery (1–3). Fat grafting is increasingly used for cosmetic breast augmentation (4). Currently, fat graft retention is more predictable and ideal using standardized techniques (5–7), but complications that occur after breast augmentation with fat grafting have made it controversial for wider use and further study is required (8).
Breast fat grafting can lead to complications, including infection, seroma, palpable cysts, and calcification, which concerns both surgeons and patients (9–12). Various factors can affect the fat grafting outcome, such as fat processing method, injection volume, and patient health status (13–15). The injection site is likely to be among these factors; however, it has not yet been studied.
Recipient sites in the breast that can be selected as the injection planes for fat grafting: beneath the skin, mammary gland, or the pectoralis major. To date, no consensus has been reached as to the optimum injection plane. The retrothoracic plane is usually used to place breast implants in cosmetic and reconstructive surgery. This site is scarce in adipose tissue, which is vascularized by contact with the pectoralis major andminor muscles and intercostal muscles (16). To date, there are no reports of fat graft outcome when injected beneath the pectoralis major.
In this study, we conducted an exploratory study to evaluate efficacy and safty of autologous fat transplantation in breast augmentation with different fat transplantation planes. We used 3D laser scanning to assess fat graft outcome. Additionally, we obtained samples of retropectoraland retromammary surviving fat from the breasts of patients who underwent autologous fat transplantation for breast augmentation.
A preliminary exploratory study on different fat transplantation planes in breast augmentation was made. Forty patients were included in this study and underwent autologous fat grafting for breast augmentation. Since it is an exploratory study, the sample size is not calculated based on statistical principles. These patients were randomly assigned to either the retromammary or the retropectoralgroup.
This study was conducted between September 2019 and October 2020. Inclusion criteria were: healthy adult women, body mass index (BMI) <25 kg/m2, BI-RADS score ≤2. Exclusion criteria were: breast disease (e.g. fibrocystic breast disease and mastitis), diseases of the immune system, and other diseases making surgery unsuitable. This study was approved by the institutional review board of Nanfang Hospital, Southern Medical University, and was conducted in accordance with the guidelines of the Declaration of Helsinki. Each participant received detailed oral and written information about this study, including the risks, benefits, and alternative therapies, and each signed an informed consent form.
Fat tissue was obtained from the patient’s thigh and/or abdomen. Blunt head liposuction needles (needle tube diameter 3.0 mm, side hole diameter 2.0 mm) and 10 mL screw mouth syringe were used for low negative pressure suction. Lipoaspirates were put into a centrifuge and spun at 1,200 g for 3 min. After centrifugation, the oil layer (upper level) was decanted and the aqueous layer (lower level) was drained out of the syringe. The middle layer, predominantly composed of fat grafts, was used for the present study (17).
In the retromammary group, 2/3 of fat was injected in the retromammary plane while the residual half in subcutaneous plane and retropectoral plane. In the retropectoral group, 2/3 of fat was injected in the retropectoral plane while the residual half in subcutaneous plane and retromammary plane. Fat was injected in the retropectoral plane when cannula was inserted close to the rib surface. To assess the retromammary plane, cannula was inserted upward into the area where glands locate. It was easy to touch the cannula when inserted in subcutaneous plane.
To get access to the retropectoral plane, the intersection of the inframammary fold and the anterior axillary line was chosen as the insertion point, and the lipoinjection cannula was inserted above the ribs and intercostal muscles. To inject lipoaspirates into the retromammary plane, the lipoinjection cannula was inserted at the midline of inframammary fold and the breast was lifted up to enlarge the retromammary space.
At baseline (pre-operation) and 6 months post-operation, photographs were taken by the same photographer in a studio with consistent camera settings, lenses, seating position and lighting. 3D laser surface scanning (MVS-600; CASZM, Shenzhen, China) was also performed with a portable device to objectively calculate the volume of the breasts. The scanning process lasted less than 60 sec and involved multiple viewpoints. Data from these scans were merged for volumetric analysis using software “ZKZM 3D Analyze” (CASZM, Shenzhen, China) on a computer, and each breast per patient was blindly analyzed pre-treatment, and 6 months post-treatment.
Possible complications, including post-operative hematoma, infection, calcification and oil cysts, were assessed by ultrasound scanning (ACUSON Sequoia, Siemens). Oil cysts >10 mm in diameter, as determined by ultrasound, were compared between the two groups of patients. Some patients who required magnetic resonance imaging (MRI) examination were also examined before and 6 months after the breast operation. A 1.5 Tesla scanner (Avanto; Siemens, Germany) was used with 3-mm thick slices.
One patient, who underwent breast augmentation with implant 6 months after autologous fat grafting, provided approval to obtain the transferred fat tissue during the implant surgery. Adipose tissue was obtained beneath the skin and pectoralis major. The tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE) staining according to standard protocols. Immunofluorescence staining was performed with rabbit anti-human Perilipin (ThermoFisher, USA) and mouse anti-human Mac-2 (Abcam, USA) primary antibodies.
For analysis, data were imported into IBM SPSS Version 23.0 software (IBM Corp., Armonk, N.Y.). Statistical testing was performed with values of p < 0.05 considered as statistically significant by means of two-sided testing. Continuous variables were compared using a t-test. Non-normal data were tested for rank equality between strata by Mann-Whitney U tests.
Table 1 shows the demographic and clinical characteristics of the patients. All 20 patients in the retromammary group were non-smoking women with a mean age of 28.0 ± 4.7 years. The mean BMI of these ten patients was 21.6 ± 2.4 kg/m2. The mean injected volume of fat tissue was 307.5 ± 30.4 mL per breast. All 20 patients in the retropectoral group were non-smoking women with a mean age of 29.7 ± 4.9 years. The mean BMI of these 10 patients was 20.4 ± 2.3 kg/m2. The mean injected volume of fat tissue was 311.5 ± 20.2 mL per breast. The two groups were well-matched, with no significant differences in age, BMI, or injected volume (P > 0.05 each).
Illustrative images of both groups demonstrated the improved breast fullness and well-defined breast contour after fat grafting for 6 months (Figures 1, 2). The mean retention rate of the fat graft in the retromammary group after 6 months was 35.9% ± 6.6, and 39.3% ± 5.1 in the retropectoral group (Figures 1, 3). No significant difference was observed between these two groups (P = 0.1076).
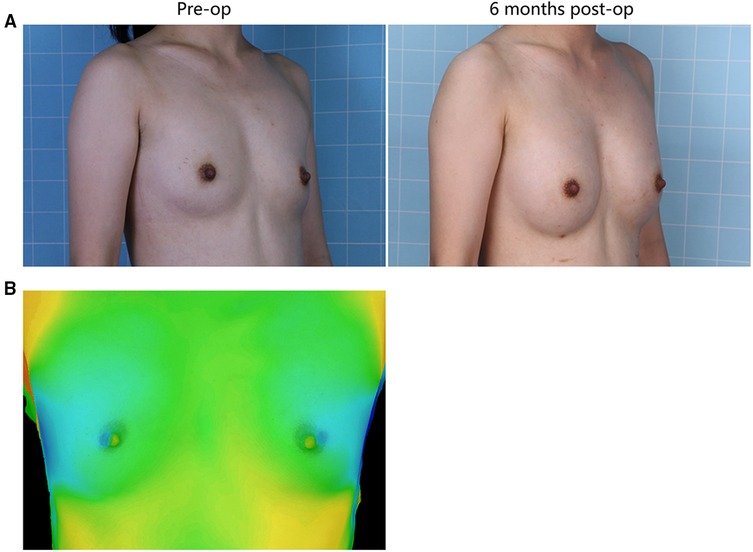
Figure 1. (A) A 28-year-old female patient from the retromammary group presented with involutional atrophy of the breasts. She received 330 mL lipoaspirates per breast, with 220 mL into the retromammary space, 55 mL into the subcutaneous plane and 55 mL into the retropectoral space. Six months after surgery, each breast was augmented. (B) The 3D laser scanner demonstrated the volume change before and after surgery.
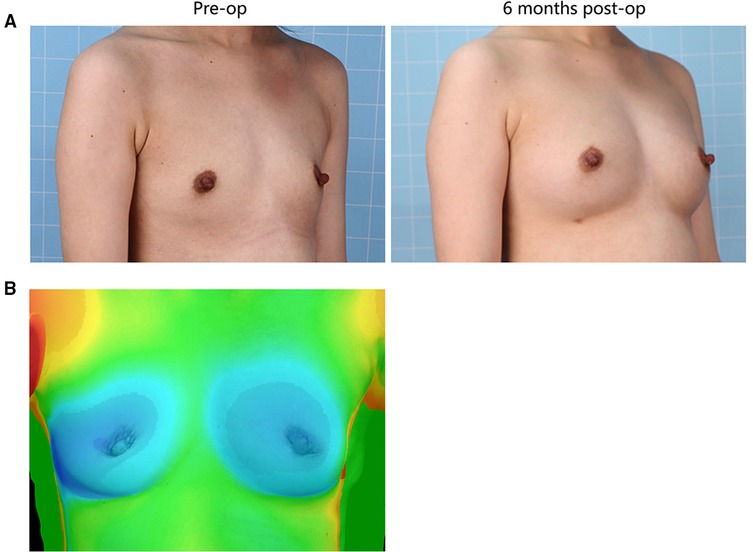
Figure 2. (A) 25-year-old female patient from the retropectoral group presented with involutional atrophy of the breasts. She received 300 mL lipoaspirates per breast, with 200 mL into the retropectoral space, 50 mL into the subcutaneous plane and 50 mL into the retromammary space. Six months after surgery, the breasts gained fullness and a well-defined shape. (B) The 3D laser scanner demonstrated the volume change before and after surgery.

Figure 3. The mean retention rate of the fat graft in the retromammary group after 6 months was 35.87% ± 6.60, and 41.28% ± 7.64 in the retropectoral group. No significant difference was observed between these two groups (p = 0.1076).
During follow-up, no patient in either group experienced adverse events, such as calcificationor hematoma (Table 2). One patient in the retromammary group had infection, while no patient in retropectoralgroup had infection (P > 0.05). Ultrasound examination showed that oil cysts were present in all patients of both groups, although their number and size differed significantly between the two groups. In the retropectoral group, the majority of the cysts were <1 cm in diameter and only one patient had an oil cyst >1 cm in diameter. By contrast, oil cysts in the retromammary group were generally larger in size. Six patients in the retromammary group developed cysts >1 cm in diameter (*P < 0.05).
A28-year-old female patient who underwent breast augmentation with implant 6 months after autologous fat grafting provided approval for us to obtain the transferred fat tissue during their implant surgery. Before implant surgery, her MRI results indicated that a thick fat pad had survived under the pectoral major (Figure 4). During the surgery, the transplanted fat tissue was clearly observed after opening the pectoralis major during the implant operation (Figure 5). Retropectoraland retromammary adipose tissue were obtained for histological examination. The retropectoralsample had smaller sized adipocytes and less inflammatory cell infiltration than the retromammary one. In the retromammary transferred fat tissue, numerous oil cysts were found, while few were found in the retropectoralfat graft (Figure 6). The expression levels of perilipin and Mac-2 were detectedby immunofluorescence. In the retropectoralsample, several multilocular adipocytes and few MAC-2 positive (+) macrophages were found. In the retromammary sample, less adipocytes and more MAC-2+ macrophages were found (Figure 7).
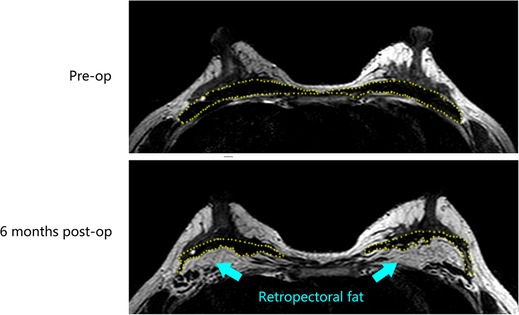
Figure 4. Magnetic resonance images show that a thick fat pad has survived under the pectoral major 6 months after fat grafting. The yellow-dotted line indicates the border of the pectoral major muscle.
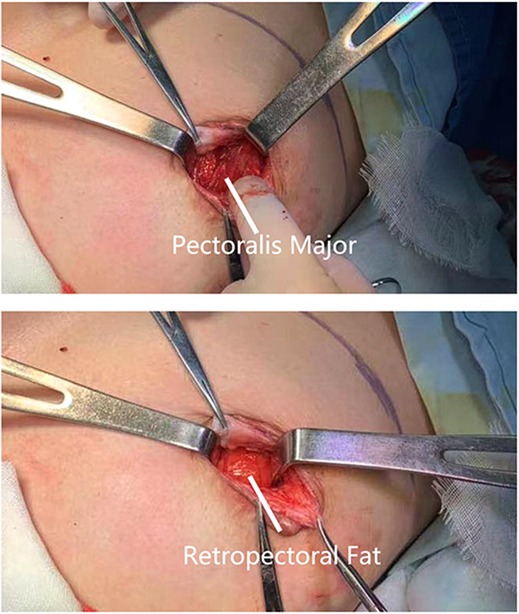
Figure 5. Collection of fat specimen during an implant-based breast augmentation 6 months after fat grafting. During the surgery, the transplanted fat tissue was clearly observed beneath the pectoralis major muscle.
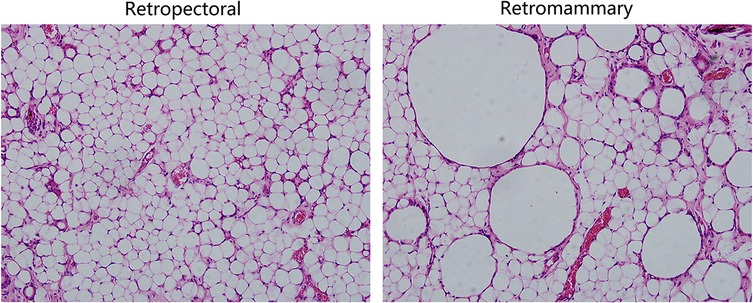
Figure 6. Hematoxylin and eosin (HE) staining results of the retromammary and retropectoral fat. (Left) HE staining results of retropectoral fat tissue. The morphology of the adipocytes is uniform, and few oil cysts can be observed. (Right) HE staining results of the retromammary fat tissue. Oil cysts with different size can be observed in the surviving fat tissue.
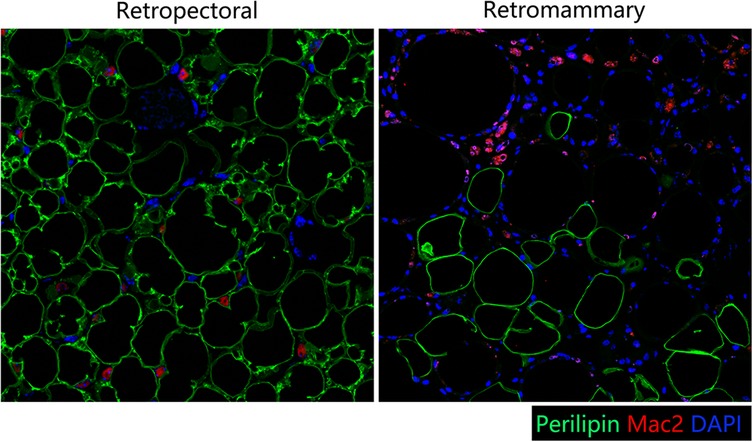
Figure 7. Immunofluorescence staining of perilipin and MAC-2. (Left) In the retropectoral transferred fat sample, several multilocular adipocytes and few MAC-2 positive (+) macrophages were found. (Right) In the retromammary transferred fat sample, less adipocytes and more MAC-2+ macrophages were found.
Despite the increasingly widespread use offat transfer in plastic surgery, there is no superior anatomical plane in the breast for fat grafting that is accepted in the literature. This study aims to evaluate the clinical outcome of fat grafting into different injection planes for breast augmentation. The three spaces (subcutaneous, retromammary, and retropectoral) in breasts were selected as the injection planes in our study. Subcutaneous space is a suitable fat grafting plane because it is where adipose tissue naturally grows; however, this space has too little room to contain enough lipoaspirates for augmentation. The retromammary and retropectoral spaces have moreroom and are commonlywhere implants are placed for breast augmentation (18–20). In implant-based breast augmentation, the retromammary space was the first plane described and still frequently used (21). The retropectoral pocket, introduced in 1968, was refined by the division of the costochondral origins of pectoralis major and has become increasingly popular (22, 23). Evidence from implant surgery indicates that fat grafts should ideally be placed in either of these two spaces to allow enough room for augmentation.
Our study first divided the patients into two groups. In both groups, lipoaspirates were injected into the three layers (the subcutaneous, retromammary, and retropectoral spaces), but were distributed in different proportions. The retromammary group received 2/3 lipoaspirate into the retromammary space, while only 1/6 was injected into the retropectoral plane. The retropectoral group received 2/3 lipoaspirate into the retropectoral space, while only 1/6 was injected into the retromammary plane. The 3D laser scanner was used to evaluate fat graft retention rate. The results suggested that the graft retention rate in the retropectoral group was slightly higher than in the retromammary group, but no statistical difference was observed (p > 0.05). MRI results also clearly showed that the fat graft could survive well in the retropectoral plane. It is believed that blood circulation at this site is less pronounced than in the intraparenchymal space, and that movement of muscle would compromise the retention rate of fat grafts. Therefore, the retropectoral plane was not recommended for fat grafting by most surgeons. However, our results suggested that retropectoral fat graft retention was not compromised, and was even slightly higher than in the retromammary group, though no statistic difference was found.
Few studies have provided data on fat graft retention in the retropectoral plane. One study showed that the mean fat graft retention rate in the retropectoral plane was 23.4% in the late post-operative period (24). The mean retention rate in our study was 39.28% and thus higher than their result. Indeed, these two results are not comparable, because of uncontrolled, potentially interfering factors, including fat processing methods, injection volume and volume distribution in different injection planes.
Although the two patient groups shared similar retention rates, the retropectoral group had significantly fewer complications than the retromammary group. Large oil cysts were not observed in any patient in the retropectoral grafting group after surgery. By contrast, two patients in the retromammary group developed palpable nodules, and two patients developed oil cysts up to 5 cm in size. The occurrence of large oil cysts was significantly lower in the retropectoral than in the retromammary group, indicating that fat grafting into the retropectoralplane reduced the development of oil cysts, while fat grafting into the retromammary plane had a higher risk for oil cyst formation. Ultrasound scanning (ACUSON Sequoia, Siemens) was used to assesse the possible complications, including post-operative hematoma, infection, calcification and oil cysts in all patients. No distinct changes were observed in the BIRADS classification in 6 months post-operation. Masaaki et al. analyzed the ultrasound results of 256 patients after fat grafting and found that 77% of the breasts had lumps located beneath the mammary glands, 17% beneath the skin, and 6% beneath the pectoralis major muscle (25, 26).
Currently, most researchers use animal models to investigate the regenerative mode of fat grafts due to difficultly in accessing the human samples. In this study, one patient who underwent implant breast augmentation 6 months after autologous fat grafting allowed us to access a human sample of fat grafts. Because there is no fat on the surface of (retromammary) or beneath (retropectoral) the pectoralismajor under normal conditions (27, 28), the retromammary and retropectoral spaces were selected to be sampled in this study. Therefore, we harvested the surviving faton the surface of and beneath the pectoralis major. The histological results showed that the retropectoral fat graft had a healthy adipose tissue structure. Few oil cysts or MAC-2+ macrophages were observed in the retropectoral fat graft.
In theory, muscular tissue, as it is more vascularized, may be a protective factor for AFG and increase fat intake when compared to AFG in the subcutaneous/subglandular space (29). In the immunofluorescence results, numerous multilocular adipocytes were observed in the retropectoral fat graft. These may be newly-born adipocytes or beige adipocytes. Since the sample was harvested at 6 months post-fat-grafting and regeneration should have finished, we assumethat these adipocytes were of the beige type. Recently, screening of muscle cells over-expressing PGC-1α has led to the identification of PGC-1α-dependent myokines involved in promoting beige fat thermogenesis, includingirisin, β-aminoisobutyric acid (BAIBA), myostatin, and FGF-21 (30). Therefore, the pectoralis major muscle may induce browning of the retropectoral fat graft. Previously, we showed that tamoxifen-induced browning of adipose tissue could improve fat graft quality and retention rate (31). Zhu et al. also reported that supplementation with extracellular vesicles derived from adipose-derived stem cells promote beige adipose regenerationand increases fat graft survival (32). The browning of transferred fat induced by the pectoralis major muscle would probably contribute to the fat graft retention improvement.
The limitation of this study is that it is an exploratory study with a small sample size and the results obtained are not robust. In addition, this study lacks long-term follow-up data, which makes this study unable to provide information on other complications, such as embolism, calcification and long-term retention rate. Moreover, the volumetric method applied in our study is the use of a laser scanning instrument and 3D analysis. Though it is an economical and reproducible method, measurements of volume changes with the 3D analysis might not be as accurate as CT and MRI. Later, we plan to conduct a larger sample size and more scientific and rigorous confirmatory study to demonstrate the conclusions of this study.
This exploratory study indicates that compared with retromammary fat grafting, retropectoral fat grafting had a similar fat graft retention rate and significantly lower complication rate. The morphology of the adipocytes in the retropectoral fat is uniform, and few oil cysts were observed. The retromammary fathad more oil cysts and macrophage infiltration. The retropectoral space show great potential to become a favorable recipient site, but further study is required to verify.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the institutional review board of Nanfang Hospital, Southern Medical University. The patients/participants provided their written informed consent to participate in this study.
BL and YQ contributed equally to this study and should be considered co-first authors. *JC and YL contributed equally to this study and should be considered co-corresponding authors. All authors contributed to the article and approved the submitted version.
This work was supported by the National Nature Science Foundation of China (81971852, 81801932, 81901976, 82002052), the Natural Science Foundation of Guangdong Province of China (2019A1515010641, 2021A1515011721, 2019A1515110112).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Khouri RJ, Khouri RK. Current clinical applications of fat grafting. Plast Reconstr Surg. (2017) 140(3):466e–86e. doi: 10.1097/PRS.0000000000003648
2. Coleman K, Coleman WR. Update on nonfacial fat transplantation. Dermatol Surg. (2020) 46(Suppl 1):S38–S45. doi: 10.1097/DSS.0000000000002636
3. Hanson SE. The future of fat grafting. Aesthet Surg J. (2021) 41(Supplement_1):S69–S74. doi: 10.1093/asj/sjab130
4. Salibian AA, Frey JD, Bekisz JM, Choi M, Karp NS. Fat grafting and breast augmentation: a systematic review of primary composite augmentation. Plast Reconstr Surg Glob Open. (2019) 7(7):e2340. doi: 10.1097/GOX.0000000000002340
5. Maximiliano J, Munhoz AM, Pedron M, Oliveira ACP, Duarte DW, Neto R, et al. Hybrid breast augmentation: a reliable formula for preoperative assessment of fat graft volume based on implant volume and projection. Aesthet Surg J. (2020) 40(8):NP438–52. doi: 10.1093/asj/sjaa017
6. Munhoz AM, de Azevedo Marques Neto A, Maximiliano J. Subfascial ergonomic axillary hybrid (SEAH) breast augmentation: a surgical approach combining the advantages of incision, pocket, silicone gel, and fat grafting in primary and revision breast augmentation surgery. Aesthet Surg J. (2021) 41(6):NP364–84. doi: 10.1093/asj/sjab029
7. Nava MB, Blondeel P, Botti G, Casabona F, Catanuto G, Clemens MW, et al. International expert panel consensus on fat grafting of the breast. Plast Reconstr Surg Glob Open. (2019) 7(10):e2426. doi: 10.1097/GOX.0000000000002426
8. Chopan M, White JA, Sayadi LR, Buchanan PJ, Katz AJ. Autogenous fat grafting to the breast and gluteal regions: safety profile including risks and complications. Plast Reconstr Surg. (2019) 143(6):1625–32. doi: 10.1097/PRS.0000000000005617
9. Orholt M, Larsen A, Hemmingsen MN, Mirian C, Zocchi ML, Vester-Glowinski PV, et al. Complications after breast augmentation with fat grafting: a systematic review. Plast Reconstr Surg. (2020) 145(3):530e–7e. doi: 10.1097/PRS.0000000000006569
10. Kaoutzanis C, Xin M, Ballard TNS, Welch KB, Momoh AO, Kozlow JH, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. (2016) 76(3):270–5. doi: 10.1097/SAP.0000000000000561
11. Groen JW, Negenborn VL, Twisk JW, Ket JC, Mullender MG, Smit JM. Autologous fat grafting in cosmetic breast augmentation: a systematic review on radiological safety, complications, volume retention, and patient/surgeon satisfaction. Aesthet Surg J. (2016) 36(9):993–1007. doi: 10.1093/asj/sjw105
12. Wang C, Panayi AC, Luan J. How to objectively evaluate nodule complications and volume changes after fat grafting in breast augmentation. Aesthet Surg J. (2018) 38(8):NP127. doi: 10.1093/asj/sjy089
13. Sarfati I, Parra RF, Terem-Rapoport CA, Benyahi D, Nos C, Clough KB. A prospective randomized study comparing centrifugation and sedimentation for fat grafting in breast reconstruction. J Plast Reconstr Aesthet Surg. (2017) 70(9):1218–28. doi: 10.1016/j.bjps.2017.06.010
14. Garza RM, Rennert RC, Paik KJ, Atashroo D, Chung MT, Duschr D, et al. Studies in fat grafting: Part IV. adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg. (2015) 135(4):1045–55. doi: 10.1097/PRS.0000000000001104
15. Strong AL, Cederna PS, Tubin JP, Coleman SR, Levi B. The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast Reconstr Surg. (2015) 136(4):897–912. doi: 10.1097/PRS.0000000000001590
16. Yang J, Zhang R, Shen J, Hu Y, Lv Q. The Three-Dimensional Techniques in the Objective Measurement of Breast Aesthetics. Aesthetic Plast Surg. (2015) 39(6):910–5. doi: 10.1007/s00266-015-0560-2
17. Pu LL, Yoshimura K, Coleman SR. Fat grafting: current concept, clinical application, and regenerative potential, Part 2. preface. Clin Plast Surg. (2015) 42(3):xiii–xiv. doi: 10.1016/j.cps.2015.05.001
18. Berry MG, Cucchiara V, Davies DM. Breast augmentation: Part III–preoperative considerations and planning. J Plast Reconstr Aesthet Surg. (2011) 64(11):1401–9. doi: 10.1016/j.bjps.2011.03.028
19. Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. (2013) 66(9):1165–72. doi: 10.1016/j.bjps.2013.04.046
20. Strasser EJ. Results of subglandular versus subpectoral augmentation over time: one surgeon’s observations. Aesthet Surg J. (2006) 26(1):45–50. doi: 10.1016/j.asj.2005.11.007
21. Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. (2005) 116(7):2005–16. PMID: 16327616
22. Dempsey WC, Latham WD. Subpectoral implants in augmentation mammaplasty. preliminary report. Plast Reconstr Surg. (1968) 42(6):515–21. doi: 10.1097/00006534-196812000-00001
23. Watanabe K, Tsurukiyi K, Fugii Y. Subpectoral-transaxillary method of breast augmentation in orientals. Aesthetic Plast Surg. (1982) 6(4):231–6. doi: 10.1007/BF01570653
24. Guimaraes P, Sabino Neto M, Lage FC, Guirado FF, de Mello GGN, Ferreira LM. Evaluation of retropectoral fat grafting in breast reduction by magnetic resonance imaging: a pilot study. Aesthet Surg J. (2019) 39(5):518–23. doi: 10.1093/asj/sjy147
25. Shida M, Chiba A, Ohashi M, Yamakawa M. Ultrasound diagnosis and treatment of breast lumps after breast augmentation with autologous fat grafting. Plast Reconstr Surg Glob Open. (2017) 5(12):e1603. doi: 10.1097/GOX.0000000000001603
26. Munhoz AM, de Azevedo Marques Neto A, Maximiliano J. Optimizing surgical outcomes with small-volume silicone implants associated with autogenous fat grafting in primary and revision breast augmentation surgery: soft weight hybrid (SWEH) concept. Aesthetic Plast Surg. (2021). doi: 10.1007/s00266-021-02653-1. Online ahead of print.
27. Lee YK, Skalski MR, White EA, Tomasian A, Phan DD, Patel DB, et al. US and MR imaging of pectoralis major injuries. Radiographics. (2017) 37(1):176–89. doi: 10.1148/rg.2017160070
28. Maione L, Caviggioli F, Vinci V, Lisa A, Barbera F, Siliprandi M, et al. Fat graft in composite breast augmentation with round implant: a new concept for breast reshaping. Aesthetic Plast Surg. (2019) 43(2):552–3. doi: 10.1007/s00266-018-1282-z
29. de Arruda EGP, Munhoz AM, Matsumoto W, Ueda T, Montag E, Okada A, et al. Impact of fat graft thickness and harvesting technique on adipocyte viability in a new porcine experimental model: an immunohistochemical analysis. Aesthet Surg J. (2021) 41(6):NP616–30. doi: 10.1093/asj/sjaa256
30. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. (2012) 481(7382):463–8. doi: 10.1038/nature10777
31. Cai J, Li B, Wang J, Liu K, Zhang Y, Liao Y, et al. Tamoxifen-prefabricated beige adipose tissue improves fat graft survival in mice. Plast Reconstr Surg. (2018) 141(4):930–40. doi: 10.1097/PRS.0000000000004220
32. Zhu YZ, Zhang J, Hu X, Wang ZH, Wu S, Yi YY. Supplementation with extracellular vesicles derived from adipose-derived stem cells increases fat graft survival and browning in mice: a cell-free approach to construct beige fat from white fat grafting. Plast Reconstr Surg. (2020) 145(5):1183–95. doi: 10.1097/PRS.0000000000006740
Keywords: autologous fat transfer, breast augmentation, pectoralis major, oil cysts, inflammation, macrophage
Citation: Li B, Quan Y, He Y, He Y, Lu F, Liao Y and Cai J (2022) A Preliminary Exploratory Study of Autologous Fat Transplantation in Breast Augmentation With Different Fat Transplantation Planes. Front. Surg. 9:895674. doi: 10.3389/fsurg.2022.895674
Received: 14 March 2022; Accepted: 20 May 2022;
Published: 10 June 2022.
Edited by:
Fabio Medas, Faculty of Medicine and Surgery, University of Cagliari, ItalyReviewed by:
Andrea Lisa, Humanitas Cancer Center, Humanitas Research Hospital, ItalyCopyright © 2022 Li, Quan, He, He, Lu, Liao and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunjun Liao eXVuanVuMTAwMEBzaW5hLmNvbQ== Junrong Cai ZHJqdW5yb25nY2FpQG91dGxvb2suY29t
Specialty section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.