- 1Department of Gastroenterology, The Second Affiliated Hospital of Shandong First Medical University, Taian, China.
- 2Department of Thoracic Surgery, The Second Affiliated Hospital of Shandong First Medical University, Taian, China.
Background: Neoadjuvant anti-programmed death receptor-1 (PD-1) blockade has been reported to improve the prognosis of locally advanced esophageal squamous cell carcinoma (ESCC). This study was aimed to evaluate the efficacy and safety of neoadjuvant camrelizumab plus chemoradiotherapy in locally advanced ESCC.
Methods: We retrospectively enrolled ESCC patients who received camrelizumab plus chemoradiotherapy as neoadjuvant therapy before surgery from May 2019 to September 2021.
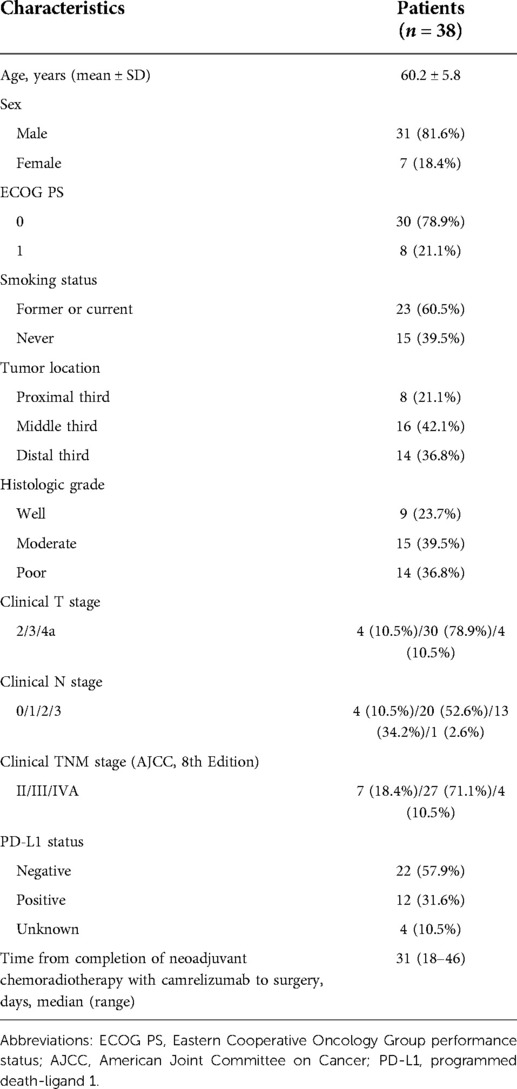
Results: A total of 38 eligible patients were enrolled. The neoadjuvant treatment was well tolerated with no serious treatment-related adverse events. 36 (94.7%) patients achieved a R0 resection without hospital mortality or any other serious intraoperative complications. The objective response rate (ORR) was 63.2% and the disease control rate (DCR) was 100.0%. The major pathological response (MPR) was 50.0% and the complete pathological response (pCR) was 39.5%. With a median follow-up of 18.5 months, 6 (15.8%) patients had died. The overall survival (OS) and disease-free survival (DFS) at 12 months were 87.6% and 78.7%, respectively. Subgroup analysis demonstrated that patients who got MPR or pCR achieved improved survival, while PD-L1 expression did not reach statistically difference in predicting survival.
Conclusions: Neoadjuvant camrelizumab plus chemoradiotherapy is safe and efficacious in treating patients with locally advanced ESCC.
Introduction
With over 500,000 newly diagnosed cases and 509,000 annual deaths, esophageal carcinoma ranks as the seventh most common cancer and the sixth leading cause of cancer death worldwide in 2018 (1). Esophageal squamous cell carcinoma (ESCC) is the predominant histologic subtype, accounting for 87% of all esophageal cancers (2). Surgery remains as the mainstay treatment for patients with early-stage ESCC; however, a great proportion of patients shave developed into locally advanced stage, and surgery alone is not satisfactory due to high recurrence and metastasis rate (3). With the advent of neoadjuvant therapy, preoperative chemoradiotherapy followed by surgery has developed into the standard-of-care treatment for locally advanced ESCC (4–6).
Recently, anti-programmed death 1 (PD-1) antibodies have paved the way for a new era of cancer immunotherapy, and thus have been approved by the Food and Drug Administration as second-line treatment in unresectable ESCC patients (7, 8). Neoadjuvant administration of anti-PD-1 agents has demonstrated encouraging efficacy with favorable tolerability in other malignancies including lung cancer, melanoma and colorectal cancer (9–11). Moreover, anti-PD-1 agents combined with chemotherapy or chemoradiation has been exploited in a neoadjuvant treatment setting for locally advanced ESCC, the results of which produced an acceptable therapeutic response and a low-toxicity profile (12–15). For example, Park et al. reported that neoadjuvant pembrolizumab plus platinum-based chemoradiotherapy may not increase the operative risk or reduce the quality of radical dissection including lymphadenectomy (16).
Notably, camrelizumab, a novel IgG4-kappa PD-1 inhibitor developed in China, has been previously witnessed for adjuvant or second-line therapy of locally advanced ESCC (17, 18). However, the research on neoadjuvant camrelizumab plus chemoradiotherapy in treating locally advanced ESCC is limited. This retrospective study was aimed to evaluate the efficacy and safety of camrelizumab combined with chemoradiotherapy in neoadjuvant treatment of locally advanced ESCC.
Materials and methods
Patient selection
From May 2019 to September 2021, we retrospectively recruited ESCC patients who received camrelizumab plus chemoradiotherapy as neoadjuvant therapy before surgery at the Second Affiliated Hospital of Shandong First Medical University (Shandong, China). The key inclusion criteria were the following: (1) Patients with histologically confirmed locally advanced ESCC, which was defined as cT1N1-3M0 or cT2-4aN0-3M0 (AJCC, 8th Edition); (2) Patients were aged 18 years or older; (3) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; and (4) With adequate organ function for surgical resection. Patients were excluded when they had other anti-tumor treatments before or during the neoadjuvant treatment, immunodeficiency disease, and other significant concurrent malignant tumors. The study was approved by institutional review board of Second Affiliated Hospital of Shandong First Medical University, and carried out in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all patients.
Neoadjuvant therapy and surgical procedures
Camrelizumab was given 200 mg intravenously every 3 weeks (a cycle). Simultaneously, the paclitaxel was administered intravenously at a dose of 100 mg/m2 of body-surface area on days 1 and 8, and the carboplatin was administered intravenously at an area under the curve of 5 mg/ml per minute on day 1. The intensity-modulated radiotherapy was given according to Chinese treatment guidelines for esophageal carcinoma (19), and was prescribed to cover 95% of the planning gross tumor volume (PGTV), given at 2.0 Gy per fraction, five fractions per week. The surgery was performed at the surgeon's decision after completion of at least 2 cycles of neoadjuvant therapy. Patients were re-evaluated with contrast-enhanced CT within 1 week before surgery. Standard minimally invasive esophagectomy (MIE) was performed for all patients, and the upper tumor mainly was treated with three-incision McKeown surgery (three fields or two fields). The middle and lower segment tumors with two-incision Ivor-Lewis surgery. Besides, a gastric tube was applied to reconstruct the digestive tract after esophagectomy. The surgical indicators, including operative time, blood loss, blood transfusion, hospital stay, and resection margin were first recorded. The postoperative complications, including pneumonia, chylothorax, pleural effusion, wound infection, and recurrent nerve paralysis were also recorded. Patients were followed postoperatively with routine CT scans every 3 months in the first year following the treatment, and every 6 months thereafter.
Assessment
The primary outcome was overall survival (OS) and disease-free survival (DFS) at 12 months. OS was defined as the time from neoadjuvant treatment to date of death, and DFS was defined as the time from neoadjuvant treatment until disease recurrence or death. Secondary outcomes were radiologic response prior to surgery, pathological responses including major pathological response (MPR) and complete pathological response (pCR), and treatment-related adverse events (TRAEs). Radiologic responses were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. MPR was defined as residual tumor cells ≤10% at the time of surgery, and pCR was defined as tumors without any viable tumor cells. TRAEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, CA, USA) and was expressed as combined positive score (CPS) by dividing the number of PD-L1-stained tumor and immune cells with the total number of viable tumor cells and multiplying by 100. The tumor PD-L1 expression was defined positive when the CPS ≥1%.
Statistical analyses
All statistical analyses were done using SPSS 20.0 (IBM SPSS Inc., Chicago, IL, USA). Continuous variables were presented with as a mean ± standard deviation when the normality was verified by Shapiron-Wilk test (P > 0.1), otherwise median and range. The categorical variables were expressed as counts and percentages. The Kaplan-Meier method was used to estimate DFS and OS and corresponding 95% CIs. Differences were considered to be significant when P < 0.05.
Results
Patient characteristics
As shown in Table 1, a total of 38 patients with ESCC were included (31 males, 7 females; median age 59 years). 30 (78.9%) patients had ECOG PS 0 and 8 (21.1%) had ECOG PS 1. More than half of the patients (60.5%) were smokers. Tumors were located in the proximal third of the esophagus in 8 (21.1%) patients, the middle third in 16 (42.1%) patients, and the distal third in 14 (36.8%) patients. There were 7 (18.4%) patients at TNM stage II, 27 (71.1%) patients at stage III, and 4 (10.5%) patients at stage IVA. Moreover, 22 (57.9%) patients had negative PD-L1 expression and 12 (31.6%) patients had positive PD-L1 expression. Time from completion of neoadjuvant therapy to surgery was 31 days (18–46).
Safety
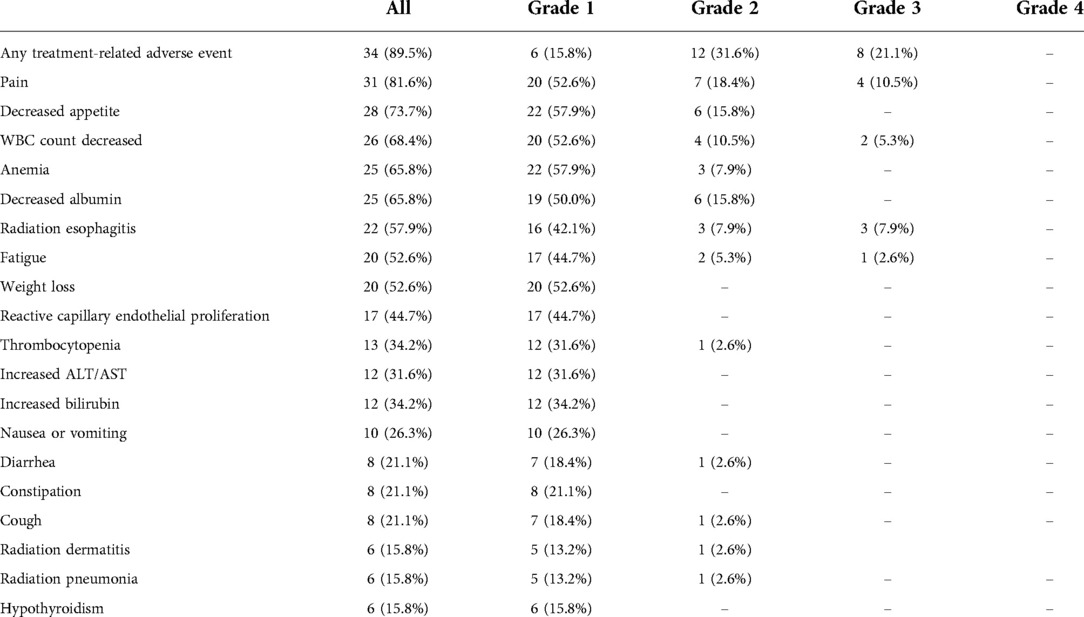
The TRAEs during neoadjuvant treatment are shown in the Table 2. A total of 34 (89.5%) patients experienced some form of TRAEs. Grade 3 adverse events occurred in 8 (21.1%) patients, and no grade 4 or 5 adverse events were observed. Grade 3 radiation esophagitis occurred in 3 (7.9%) patients. Other grade 3 adverse events included pain in 4 (10.5%) patients, decreased WBC count in 2 (5.3%) patients, and fatigue in 1 (2.6%) patient. No treatment-related deaths were reported. Immune-related adverse events occurred in 18 (47.4%) patients. The most common immune-related adverse events were reactive capillary endothelial proliferation in 17 (44.7%) patients, and hypothyroidism in 6 (15.8%) patients, all in grade 1. Other immune-related adverse events including myocarditis, hepatitis, and nephritis were not observed.
Surgical treatment
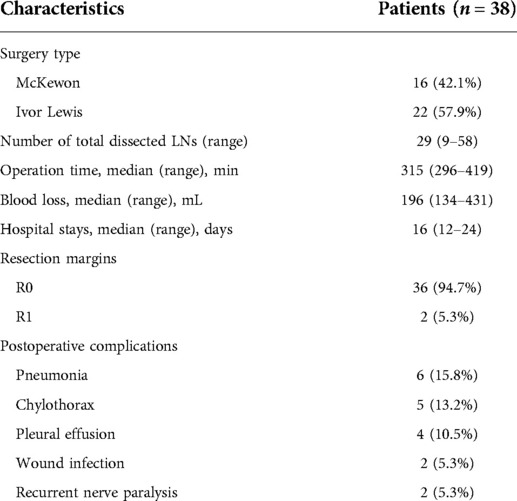
The surgical details are presented in the Table 3. 16 (42.1%) patients had the McKewon surgery and 22 (57.9%) patients had Ivor Lewis surgery. The median operation time was 315 min (296–419), the median amount of blood loss was 196 mL (134–431), and the median hospital stay was 16 days (12–24). 36 (94.7%) patients achieved a R0 resection and 2 (5.3%) patients had a R1 resection. During the postoperative periods, 6 (15.8%) patients had pneumonia, 5 (13.2%) had chylothorax, 4 (10.5%) had pleural effusion, 2 (5.3%) had wound infection, and 2 (5.3%) had recurrent nerve paralysis. There was no death in hospital or any other serious intraoperative complications.
Clinical treatment response
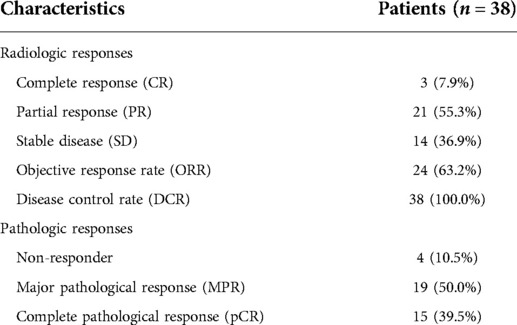
The radiologic and pathologic responses are summarized in Table 4. Of all 38 patients, 3 (7.9%) patients had a complete response (CR), 21 (55.3%) patients had a partial response (PR), and 14 (36.9%) had a stable disease (SD). The objective response rate (ORR) was 63.2% and the disease control rate (DCR) was 100.0%. According to postoperative pathological results, 19 (50.0%) patients had a MPR, 15 (39.5%) patients had a pCR, and 4 (10.5%) patients were non-responders.
Survival profiles
By January 1, 2022, the median follow-up period was 18.5 months (range 3.4–28.5) and 6 (15.8%) patients had died. As demonstrated in Figure 1, the median OS and DFS was not reached in all patients; the OS rate was 87.6% at 12 months, 77.2% at 18 months, and 77.2% at 24 months; the DFS rate was 78.7% at 12 months, 73.5% at 18 months, and 73.5% at 24 months. In subgroup analyses, patients with a MPR or pCR achieved better OS (all P < 0.001, Figure 2A) and DFS (all P < 0.001, Figure 2B) than non-responders. Despite no significant statistical difference, patients with positive tumor PD-L1 expression tended to be associated with longer OS (P = 0.170, Figure 3A) and DFS (P = 0.118, Figure 3B).
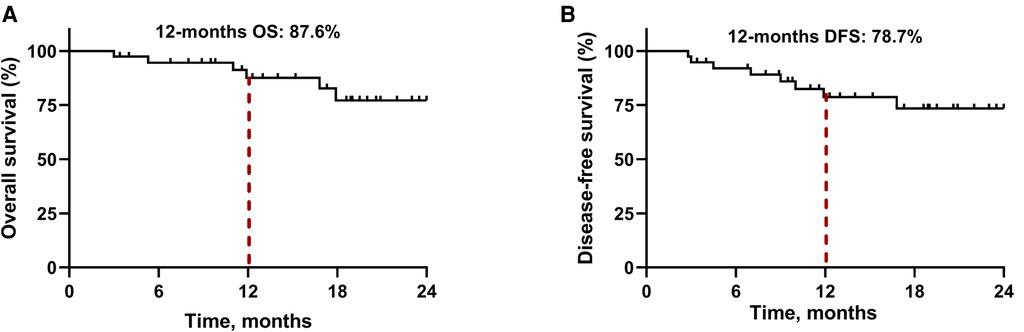
Figure 1. Kaplan-Meier survival curves in all patients. (A) Overall survival. (B) Disease-free survival.
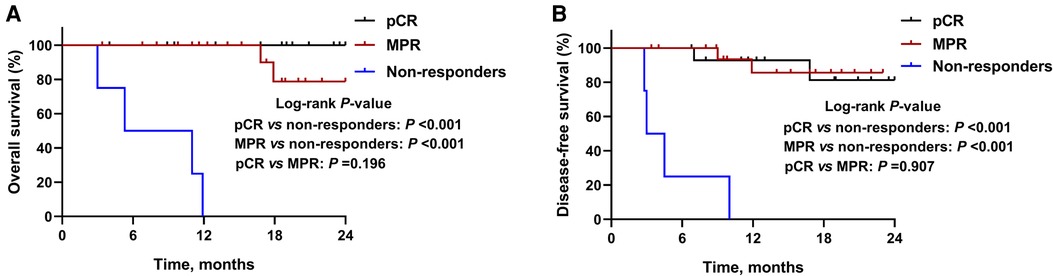
Figure 2. Kaplan-Meier survival curves stratified by pathological responses. (A) Overall survival and (B) disease-free survival for patients with major pathological response (MPR), complete pathological response (pCR), or non-responders.
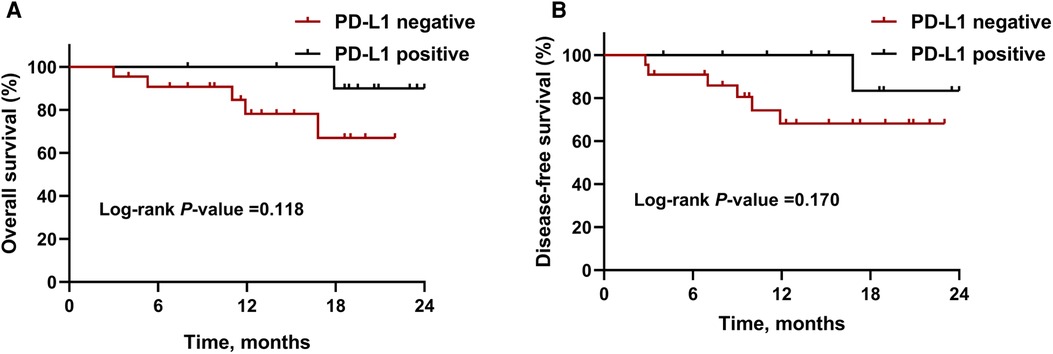
Figure 3. Kaplan-Meier survival curves stratified by PD-L1 expression. (A) Overall survival and (B) disease-free survival for patients with PD-L1 negative or positive expression. Abbreviations: PD-L1, programmed death-ligand 1.
Discussion
This is a retrospective study to assess neoadjuvant camrelizumab plus chemoradiotherapy for locally advanced ESCC patients, which achieved a promising MPR rate of 50.0%, a pCR rate of 39.5%, and the R0 resection rate of 94.7%. This neoadjuvant therapy regimen showed optimal survival outcomes with an OS of 87.6% and a DFS of 78.7% at 12 months. This neoadjuvant treatment was well tolerated with a manageable safety profile.
Previous concern with neoadjuvant treatment remains the delay of surgery due to disease progression or serious TRAEs during neoadjuvant treatment. In our study, no patient progressed during the neoadjuvant treatment duration. This neoadjuvant treatment regimen resulted in no more than Grade 3 adverse events and the toxicity was manageable. All TRAEs were similar to either modality given alone in ESCC as previously reported (19–23). The incidence of radiation pneumonitis was relatively lower in our study compared with concurrent chemoradiotherapy (2.6% vs. 9.6–20.3% in grade 2; 0% vs. 1.8–7.4% in grade 3). Moreover, the incidence of radiation esophagitis did not increase compared with concurrent chemoradiotherapy (7.9% vs. 16.9–32.7% in grade 2; 7.9% vs. 3.2–5.6% in grade 3). Reactive capillary endothelial proliferation was the most common immune-related adverse event reported in camrelizumab monotherapy (75%–79%) (21, 24). The results of this study showed a much lower frequency (44.7%) of reactive capillary endothelial proliferation, and was all in grade 1 without special treatment. In addition, the surgical procedure was safe and controllable, as blood loss was minimal, operative time and hospital stay were reasonable, and the incidence of surgical complications was low. All the results indicated that the quality of our neoadjuvant treatment did not increase the risk of surgery and decrease the quality of surgery.
To data, several studies have evaluated the therapeutic efficacy of neoadjuvant PD-1blockade combined with chemotherapy or chemoradiotherapy for patients with ESCC. For instance, Yang et al. reported a pCR rate of 33.3% and a MPR of 41.7% in patients with locally advanced ESCC who have received neoadjuvant camrelizumab plus chemotherapy (12). Moreover, another retrospective study demonstrated that an R0 resection rate of 96.3%, a pCR rate of 33.3%, and an ORR of 88.9% were achieved in patients with locally advanced ESCC treating neoadjuvant nivolumab or pembrolizumab plus chemotherapy (13). In one retrospective study evaluating locally advanced ESCC patients receiving neoadjuvant immunotherapy (camrelizumab, pembrolizumab, or sintilimab) plus chemotherapy, the pCR rate was 34.21%, the MPR rate was 42.1%, and the R0 resection rate was 92.11% (14). In another study including 16 cases conducted by Yang et al., the ORR was 81.3%, the DCR was 100%, the pCR rate was 31.3%, and the R0 resection rate was 93.8% in locally advanced ESCC patients receiving neoadjuvant camrelizumab plus chemotherapy (15). They also reported the survival profile including a 1-year PFS of 83% and OS of 90.9%. In this study, 19 (50.0%) patients had a MPR, 15 (39.5%) patients had a pCR, and the survival was favorable with an OS of 87.6% and a DFS of 78.7% at 12 months. Overall, much more impressive results of pathological responses and survival outcomes were determined in the present study compared with the above studies. Moreover, this study adopted a rather homogenous PD-1 inhibitor regimen (camrelizumab) with a relatively larger number of patients.
Data of subgroup analysis demonstrated that patients who got MPR or pCR were associated with improved survival, which further reinforce the widely usage of pathological response as surrogate clinical endpoints for long-term survival (25–27). In this study, despite no statistically significant difference, patients with higher PD-L1 expression tended to have longer survival. PD-L1 expression remains the commonly explored biomarker for predicting the response to anti-PD1 therapy in several cancers including lung cancer (28), melanoma(29), and gastric cancer (30), whereas biomarker role of PD-L1 expression was disputable when analyzing the association between PD-L1 expression and the response to a PD-1 blockade (31). In ESCORT and ATTRACTION-3 studies, tumor PD-L1 expression was not a robust biomarker of the survival benefit for patients with advanced ESCC (7, 24). The correlation of PD-L1 expression and clinical outcomes are warranted for further investigation in ESCC.
There are sone limitations to this study. First, this is a retrospective study with inherent selection bias, which needs to be clarified in further prospective research. Second, the sample size of eligible patients in this study is relatively small. Third, the follow-up period was relatively short, and further survival analysis including 3- or 5-year survival rates should be performed in the future.
Conclusions
Neoadjuvant camrelizumab plus chemoradiotherapy exhibits good feasibility and safety in treating patients with locally advanced ESCC. More prospective studies are needed to validate the expected efficacy of this neoadjuvant therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Second Affiliated Hospital of Shandong First Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors made substantial contributions to the conception of the study. FC, QW, LQ, and YM contributed to patient recruitment, data collection and clinical investigation. SC, YY, PS, and BJ contributed to data analysis and interpretation of data. All authors drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Thrift AP. The epidemic of oesophageal carcinoma: where are we now? Cancer Epidemiol. (2016) 41:88–95. doi: 10.1016/j.canep.2016.01.013
3. Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding esophageal cancer: the challenges and opportunities for the next decade. Front Oncol. (2020) 10:1727. doi: 10.3389/fonc.2020.01727
4. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
5. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19(1):68–74. doi: 10.1245/s10434-011-2049-9
6. de Gouw D, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Fütterer JJ, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. (2019) 14(7):1156–71. doi: 10.1016/j.jtho.2019.04.004
7. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
8. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
9. Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res. (2020) 9(6):2696–715. doi: 10.21037/tlcr-2020-63
10. Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. (2021) 108(12):1417–25. doi: 10.1093/bjs/znab342
11. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. (2018) 24(11):1649–54. doi: 10.1038/s41591-018-0197-1
12. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death-1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med. (2021) 9(15):1254. doi: 10.21037/atm-21-3352
13. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. (2021) 12(1):1–10. doi: 10.21037/jgo-20-599
14. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. (2021) 13(6):3518–28. doi: 10.21037/jtd-21-340
15. Yang P, Zhou X, Yang X, Wang Y, Sun T, Feng S, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. (2021) 19(1):333. doi: 10.1186/s12957-021-02446-5
16. Park SY, Hong MH, Kim HR, Lee CG, Cho JH, Cho BC, et al. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis. (2020) 12(11):6426–34. doi: 10.21037/jtd-20-1088
17. Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. (2020) 40(12):711–20. doi: 10.1002/cac2.12119
18. Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. (2021) 26(7):e1110–e24. doi: 10.1002/onco.13797
19. Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J Clin Oncol. (2019) 37(20):1695–703. doi: 10.1200/JCO.18.02122
20. Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol. (2017) 3(11):1520–8. doi: 10.1001/jamaoncol.2017.1598
21. Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. (2018) 24(6):1296–304. doi: 10.1158/1078-0432.CCR-17-2439
22. de Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, de Araújo Lima França B, et al. A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer. (2018) 88:21–30. doi: 10.1016/j.ejca.2017.10.005
23. Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology. (2021) 10(1):1971418. doi: 10.1080/2162402X.2021.1971418
24. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
25. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. (2012) 30(15):1796–804. doi: 10.1200/JCO.2011.38.8595
26. Weissferdt A, Pataer A, Vaporciyan AA, Correa AM, Sepesi B, Moran CA, et al. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer. (2020) 21(4):341–8. doi: 10.1016/j.cllc.2019.11.003
27. Shibata H, Saito S, Uppaluri R. Immunotherapy for head and neck cancer: a paradigm shift from induction chemotherapy to neoadjuvant immunotherapy. Front Oncol. (2021) 11:727433. doi: 10.3389/fonc.2021.727433
28. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
29. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. (2018) 36(4):383–90. doi: 10.1200/JCO.2016.71.8023
30. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. (2018) 24(9):1449–58. doi: 10.1038/s41591-018-0101-z
Keywords: esophageal squamous cell carcinoma, neoadjuvant therapy, camrelizumab, chemoradiotherapy, survival
Citation: Chen F, Qiu L, Mu Y, Sun S, Yuan Y, Shang P, Ji B and Wang Q (2022) Neoadjuvant chemoradiotherapy with camrelizumab in patients with locally advanced esophageal squamous cell carcinoma. Front. Surg. 9:893372. doi: 10.3389/fsurg.2022.893372
Received: 10 March 2022; Accepted: 11 July 2022;
Published: 2 August 2022.
Edited by:
Alessandro Gonfiotti, University of Florence, ItalyReviewed by:
Zhihua Yao, Affiliated Cancer Hospital of Zhengzhou University, ChinaStefano Bongiolatti, Careggi University Hospital, Italy
Francesco Zaraca, Ospedale di Bolzano, Italy
© 2022 Chen, Qiu, Mu, Sun, Yuan, Shang, Ji and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifei Wang Y29uZ2x1c3VpZG9jQDE2My5jb20=
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Fei Chen1
Fei Chen1 Bo Ji
Bo Ji