- 1Department of Neurosciences, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
- 2Department of Pathology, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
Background: Hemorrhage into optic pathway–hypothalamic glioma (OPHG) is rare. Variable clinical presentations and outcomes are associated with such pathology. We aim to present two infants presented with OPHG and a systematic review of the literature.
Methods: We describe two cases of infants presenting with sudden decreased vision, poor feeding, and irritability due to OPHG. Both patients underwent urgent craniotomy and subtotal resection followed by chemotherapy. We systematically reviewed the literature using PubMed, Google Scholar, and Embase. In addition, we included all English published reports for all ages discussing the optic pathway (optic nerve and optic chiasm) or hypothalamic glioma associated with hemorrhage from the year of the first reported case (1970) to January 2022.
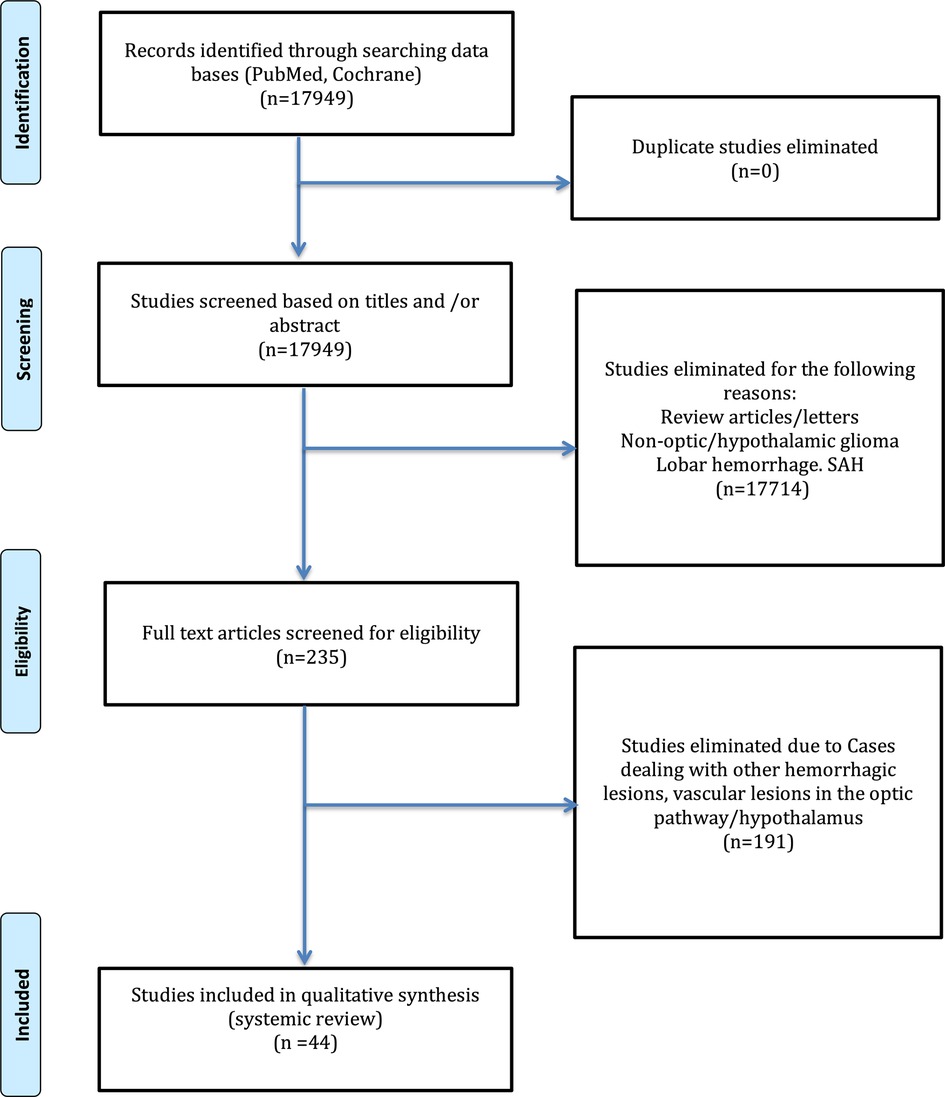
Results: Of 17,949, 44 articles met the inclusion criteria of this review. A total of 56 cases were described with a mean of 21.35 years (0.5–70), with the male gender 52% and the female gender 45%. The hemorrhage location was sellar/suprasellar in 43% cases. Histopathology of included cases was pilocytic astrocytoma in 41%, followed by pilomyxoid astrocytoma in 16% cases. The outcome was unfavorable; 37.5% cases showed improvement, whereas 18% cases resulted in death.
Conclusion: Apoplexy of the OPHG can be fatal and associated with poor outcomes. A systematic review of the literature has shown that younger age, pilocytic or pilomexyoid astrocytoma histopathology, and chiasmal/hypothalamic locations are associated with a higher risk of intertumoral hemorrhage and poor prognosis. Further genetic studies for OPHG may provide information for high-risk patients.
Introduction
Optic pathway–hypothalamic glioma (OPHG) can be found anywhere along the optic pathway, often in the chiasmatic-hypothalamic region (1). These tumors account for 3%–5% of all pediatric brain neoplasms and can also be diagnosed in adulthood (2–4). The most common histopathology encountered is World Health Organization (WHO) grade 1 pilocytic astrocytoma, followed by pilomyxoid astrocytoma (2, 3).
The initial clinical presentation would be visual disturbance with cases that progress to blindness (5). Due to the proximity of these lesions to sellar and suprasellar structures, endocrine and hypothalamic dysfunction would be observed (5). Moreover, as these lesions are also close to the cerebrospinal fluid pathway, hydrocephalus can result from their compressive effect (5). This type of pathology poses verities of challenges as it has a wide spectrum of symptomatology, and it is close to many eloquent brain structures, with treatment tailored on a case-by-case basis (6)
Intertumoral hemorrhage has always been linked to malignant tumors (6). Pituitary macroadenoma is considered the most common low-grade tumor associated with hemorrhage (7). Bleeding into optic gliomas is considered rare, and the highest rate of intertumoral hemorrhage was associated with pilocytic astrocytoma according to multiple series (8–10).
Herein, we describe two cases of infants who presented with hemorrhage into optic glioma, with a systematic review of the current literature regarding such presentation and outcome.
Methods
Cases’ Descriptions
We describe two cases of infants presenting with sudden decreased vision, poor feeding, and irritability due to optic chiasmatic/hypothalamic glioma. Both patients underwent urgent craniotomy and subtotal resection followed by chemotherapy. This study was approved by the Institutional Research Board (IRB # 2022-CR-06).
Case 1
A 9-month-old baby boy presented with his parents to the emergency department with the inability to follow objects, repeated vomiting, and irritability for three days. He is an outcome of uneventful full-term gestation. His postnatal history was unremarkable and neurodevelopmental milestones were normal.
On examination, he was dehydrated and frequently cried. His head circumference was in the upper 90 percentiles for age, but the anterior fontanel was small with no evidence of increased intracranial pressure. There were no stigmata of neurofibromatosis type 1 (NF1). The neurological examination revealed adequate power and tone in all extremities. There is a poor pupillary response to light and no clear visual recognition of moving objects. There was no ophthalmoplegia or facial weakness, and the corneal and gag reflexes were brisk.
Urgent computed tomography (CT) scan revealed a large hyperdense suprasellar lesion and mild ventricular dilatation (Figure 1). A magnetic resonance imaging (MRI) scan revealed large sellar and suprasellar cystic and solid lesions containing subacute components of blood products. The lesion has marked heterogeneous enhancement following intravenous contrast administration, and there is marked mass effect and mild ventriculomegaly (Figure 2). Magnetic resonance angiography (MRA) revealed no vascular abnormality.

Figure 1. CT scan of the brain (case 1) revealed a large hyperdense suprasellar lesion and mild ventricular dilatation.

Figure 2. Sagittal (A) and axial (B) T2-WI and axial (C) T1-WI MRI scan sequences of the brain reveal large sellar and suprasellar cystic and solid lesions containing subacute components of blood products. The lesion is enhanced heterogeneously following intravenous contrast administration (D–F).
The baby had normal routine laboratory workup with particularly no coagulopathy or pituitary dysfunction on admission. He was admitted to the pediatric intensive care unit (PICU) to optimize her general condition. Ophthalmological consultation documented his poor visual response. He was started on dexamethasone (2 mg intravenous injection followed by 0.5 mg hourly). The surgery was performed the next day on the right frontotemporal craniotomy. The dura was open curvilinear and reflected anteriorly. There was no significant brain swelling, and there was no need to insert a ventricular drain. The optic-carotid cistern and sylvian fissure were widely opened, which made more brain relaxation. The tumor was yellowish firm, with areas of soft cystic consistencies. Intratumoral microscopic decompression of the suprachiasmatic and hypothalamic solid and cystic parts was achieved using an ultrasonic aspirator. There were mixed-blood products of recent and old components within cystic regions. After achieving adequate decompression, craniotomy closure was done in layers. The patient was transferred to PICU in stable condition with an estimated blood loss of around 120 ml. He received an intraoperative blood transfusion of 100 ml.
The patient had an uneventful postoperative period; he was extubated three days later. There was no postoperative endocrinopathy, and his visual status was the same. Postoperative brain MRI revealed adequate decompression of 50% of the tumor, and he was discharged in stable condition 12 days after surgery.
The histopathology examination was consistent with pilomyxoid astrocytoma with hemorrhagic background and necrosis (Figure 3).
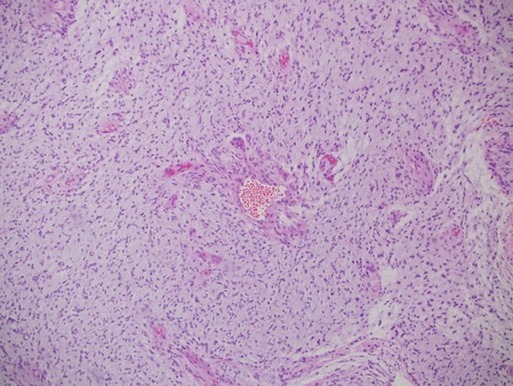
Figure 3. Histopathology examination showed astrocytoma with myxoid features suggestive of pilomyxoid astrocytoma with a background of hemorrhagic necrosis.
He was evaluated at outpatient clinics by pediatric oncology service and started on MOB chemotherapy protocol (including nitrogen mustard, vincristine, and procarbazine for one year). At the age of 5 years, his brain MRI scan revealed a good response to stable residual tumor treatment (Figure 4). His visual evaluation revealed a blind left eye, but he could see and recognize objects from the right eye.
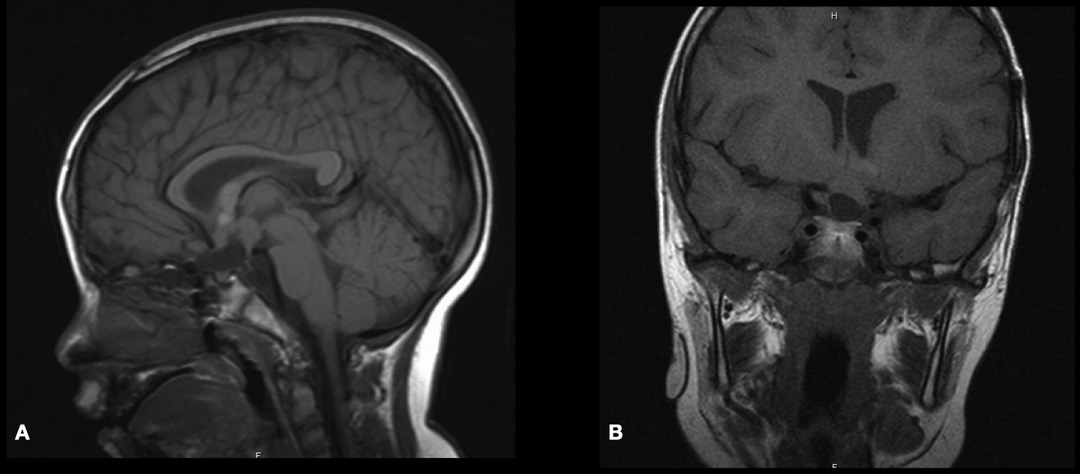
Figure 4. Postoperative sagittal and coronal T1-WI MRI scan demonstrating stable residual tumor at 5-year follow-up.
Case 2
A one-year-old baby girl, with a normal state of health until two weeks from admission, presented with decreased activity and oral intake and repeated vomiting 4–5 times a day. No seizures, trauma, or loss of consciousness. She was delivered at 35-week gestation by cesarean section (CS) due to uncontrolled gestational diabetes mellitus. Her postnatal history was unremarkable and neurodevelopmental milestones were within normal.
On examination, she was awake but irritable. General physical examination revealed head circumference within the 90 percentiles for age with normal small anterior fontanel. There were multiple cafe au lait spots (more than six) in the trunk and extremities, measuring greater than 5 mm. Neurological examination revealed normal tone and power in all extremities. Her pupils were sluggish to light with decreased response to external stimuli and moving objects, but further tests could not be assessed due to the baby’s cooperation. The optical coherence tomography test was difficult to perform due to the infants’ clinical condition. However, there was no ophthalmoplegia, and her corneal response, facial movement, and gag reflex were within normal.
On admission, her laboratory workup was normal, with no evidence of coagulopathy. A brain CT scan was performed in the emergency department, revealing a sizeable suprasellar lesion with an internal hemorrhagic component: mild ventriculomegaly (Figure 5). A brain MRI scan demonstrated a large suprasellar mass with mixed solid and cystic components representing subacute hemorrhages, a heterogeneous enhancement following intravenous contrast administration. The ventricular systems were mildly increased in size, and MRA revealed no vascular abnormalities (Figure 6).
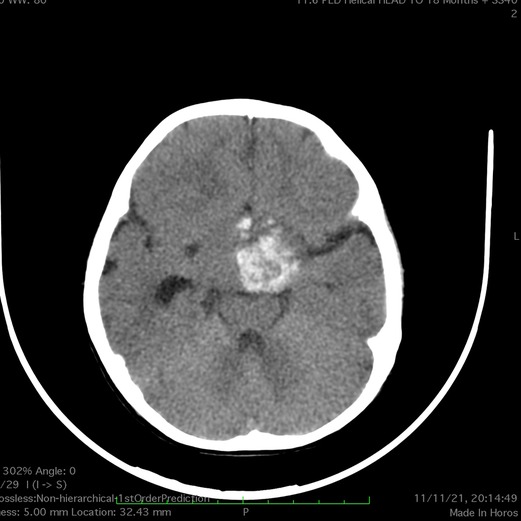
Figure 5. CT scan of the brain (case 2) reveals a large hyperdense suprasellar lesion and mild ventricular dilatation.

Figure 6. Sagittal (A), axial (B), and coronal (C) T2-WI and sagittal (D), axial (E) T1-WI MRI scan sequences of the brain demonstrating a large suprasellar mass with mixed solid and cystic components representing subacute hemorrhages. The T2-GRE sequence (F) revealed marked hypointensity of the tumor representing acute hemorrhage.
The patient was admitted to PICU well hydrated and started on dexamethasone (2 mg intravenous injection followed by 0.5 mg hourly). She underwent right frontotemporal craniotomy the following day. The dura was open curvilinear and reflected anteriorly. There was no significant brain swelling or edema. The sylvian fissure and optic-carotid cistern were opened, which made more brain relaxation. The tumor was yellowish soft, with areas of firm and areas of soft cystic consistencies. Intratumoral microscopic decompression of the suprachiasmatic and hypothalamic solid and cystic parts was achieved using an ultrasonic aspirator. There were old and new blood products within the large cystic component. After achieving adequate decompression, craniotomy closure was done in layers, and the patient was transferred to PICU in stable condition with an estimated blood loss of around 200 ml. She received an intraoperative blood transfusion of 150 ml.
The patient remained ventilated in PICU for two days after surgery. Her postoperative course revealed transient diabetic insipidus, and she received two doses of desmopressin for two days. Her neurological examination was unchanged. A postoperative brain MRI scan (within 24 h) revealed 60%–70% adequate decompression. The patient was evaluated by pediatric oncology and endocrinology services and discharged on Day 10. She was up to date on her neurodevelopmental milestones: able to cruise on furniture, sit without support, has a good pincer grasp in both hands, able to say 2–3 words, recognize faces and follow objects, and interact with her parents and her elder sister. Pediatric neurology discharged her on clobazam 2.5 mg at bedtime for insomnia and myoclonic seizures and levothyroxine 0.25 mg as per pediatric endocrinology advice.
Histopathology examination of the specimen showed astrocytoma with myxoid features suggestive of pilomyxoid astrocytoma with a background of hemorrhage and necrosis (Figure 7). Therefore, genetics was done and a WES study was recommended to rule out NF1.
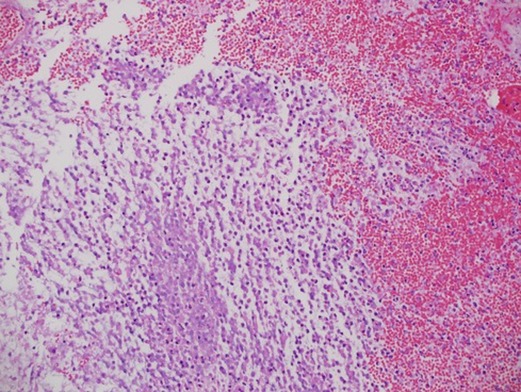
Figure 7. Histopathology examination of the specimen showed astrocytoma with myxoid features suggestive of pilomyxoid astrocytoma with a background of hemorrhagic necrosis.
A pediatric oncology service followed the patient. She was labeled NF1 as she met the following two criteria: cafe au lait and positive family history (her father and elder sister had cutaneous stigmata of NF1). The patient started on chemotherapy protocol Cog a9952, Carboplatin Plus Vincristine for one year. At six months, an early brain MRI scan revealed a good response to the treatment (Figure 8).
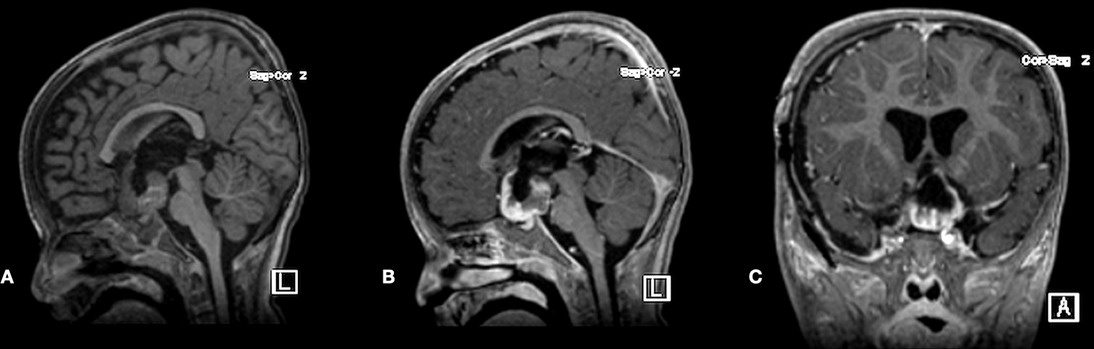
Figure 8. Postoperative plain sagittal T1-WI MRI scan and post-contrast T1-WI sagittal and coronal MRI scans demonstrated adequate decompression and stable residual tumor at 6-month follow-up.
Qualitative Systematic Review
This study was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search Strategy
An extensive literature search was conducted to include English-published case reports discussing optic pathway/hypothalamic glioma hemorrhage, covering the time from January 1970 (date of first reported case) up to January 2022. Several databases were utilized, including PubMed, Google Scholar, and Embase. Several related keywords were used, such as “optic glioma,” “chiasm,” “hypothalamus,” “hemorrhage,” “apoplexy,” and “bleeding.”
Inclusion Criteria
All case reports or case series of the optic pathway (optic nerve and optic chiasm) or hypothalamic glioma associated with hemorrhage for pediatric and adult populations were included from the first reported case (1970) to January 2022. Cases dealing with other hemorrhagic lesions and vascular lesions in the optic pathway/hypothalamus were excluded.
Data Extraction
Abstracts were reviewed by two authors (RM and YM), inclusion criteria were applied, and any disagreement was resolved by discussion and review with the senior author (SB). Full-text abstracts that met the inclusion criteria were accessed and reviewed. For all included cases, the following variables were collected: author/year, age in years, gender, history of neurofibromatosis type 1 (NF1), pertinent medical history, clinical features, location of the hemorrhage, treatment received, histological diagnosis, and outcome. All variables were collected using a Microsoft Excel Sheet.
Results
Our search strategy yielded 17,949 articles, out of which 235 were included for full-text review. Only 44 articles were included in the final qualitative analysis (Figure 9). The summary of included articles of the 56 cases is shown in Table 1. All included articles were of low level of evidence as the majority were either case reports or case series. There were around 56 optic pathway/hypothalamic glioma-associated hemorrhage cases from the included articles, including our reported cases.
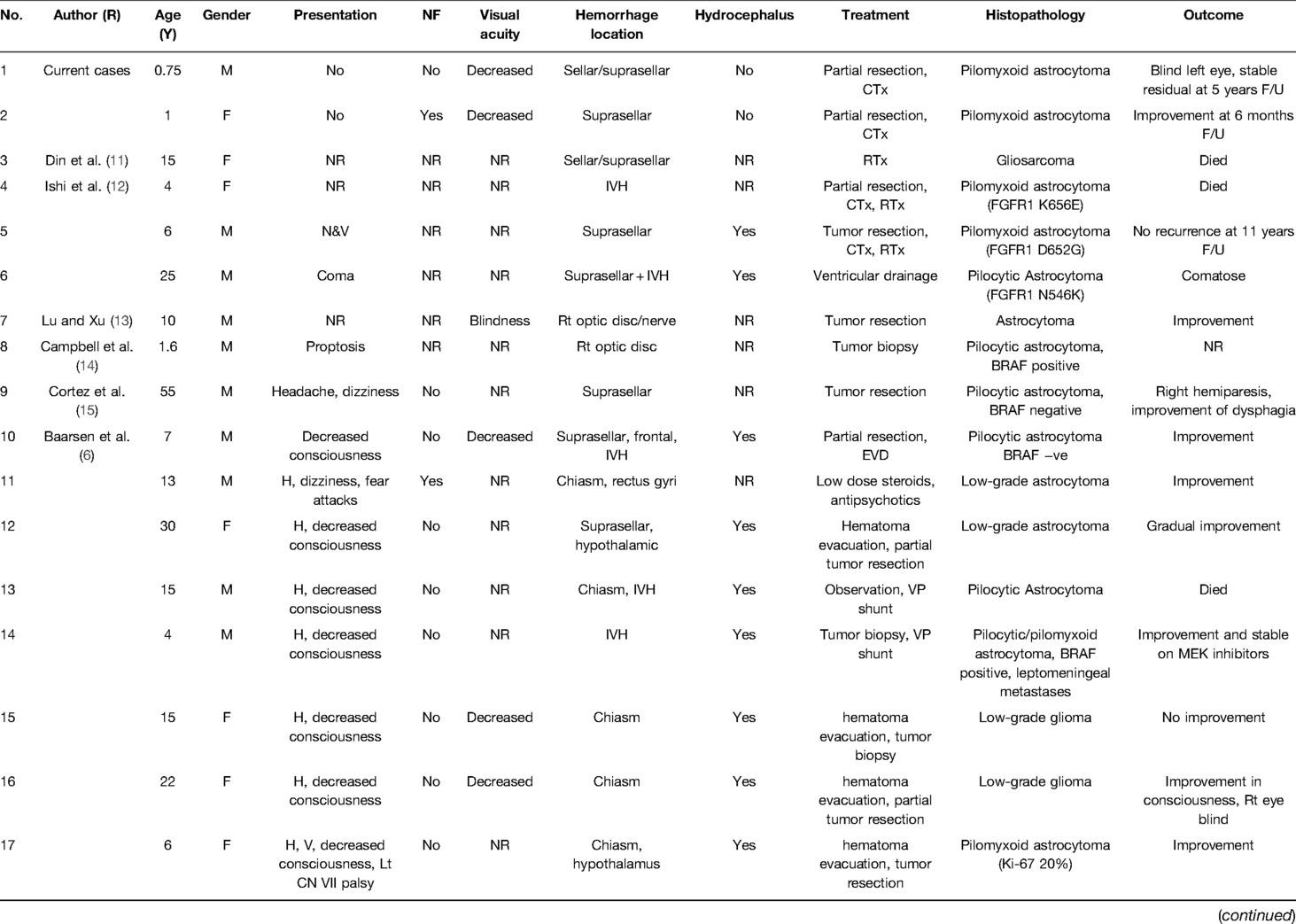
The mean age of included case was 21.35 years (0.5–70), with the male gender comprising 52% and the female gender comprising 45%. The presence of neurofibromatosis stigmata was mentioned in five cases (9%), absent in 52%, and not reported in 39.3% cases.
Clinically, in 37.5% cases, patients experienced decreased visual acuity and even vision loss, while 62.5% failed to mention that. Visual field deficits were reported in only 11 cases (20%), while it was not possible to examine due to the young age of the patients in 9% cases. Some patients, especially those with sellar/suprasellar hemorrhage, presented with high ICP symptoms (29%).
The hemorrhage location was sellar/suprasellar in 43% cases, followed by intraventricular hemorrhage in 20%. In 14% cases, hemorrhage was confined to the orbit.
Histopathology of included cases was pilocytic astrocytoma in 41% cases, pilomyxoid astrocytoma in 16% cases, followed by others (43%), including cases of glioblastoma, gliosarcoma, high-grade diffuse astrocytoma, and ganglioglioma.
Regarding outcome, 37.5% cases showed improvement, whereas 18% resulted in death. Three were highly associated with highly malignant types of gliomas (glioblastoma and gliosarcoma). Others were related to the chiasmal/hypothalamic hemorrhage location. It has also been observed that in chiasmatic/hypothalamic glioma cases, the persistence of multiple endocrinopathies and diabetes insipidus in 4% patients.
Discussion
It has been discussed in the literature that intertumoral hemorrhage into optic pathway/hypothalamic glioma is rare (6). Our cases above add to the reported cases of such rare occurrences.
In regard to risk factors of intertumoral hemorrhage into gliomas, it has been speculated that a young age can be associated with a higher risk of hemorrhage (7, 10, 47). Although low-grade gliomas such as pilocytic astrocytoma are frequent in the younger population, some might argue that intertumoral hemorrhage in optic gliomas is rather related to tumor histopathology than age (6). Even though reported cases did not show any meaningful gender difference, pregnancy would be one of the risk factors that lead to the development of intertumoral hemorrhage, as hypothesized by Czyzyk and associates (51).
From our systematic review and with the inclusion of our own described cases, we found that the histopathology of 42.9% of included cases was pilocytic astrocytoma, followed by pilomyxoid astrocytoma. This would support the hypothesis that these tumors have a higher risk of associated hemorrhage, as these tumors might be more vascular with vessel proliferation and thinner vessel walls (21, 29). In addition, Ishi and colleagues have speculated that low-grade gliomas with FGFR1 mutation are associated with spontaneous hemorrhage in adult and pediatric populations, as evidenced by their retrospective review of 66 patients (12).
Concerning NF stigmata, most of the cases did not mention the absence of such occurrence. Shibahara et al. argued that NF1 is associated with a higher rate of intertumoral hemorrhage in gliomas (10). However, still not enough evidence supporting this hypothesis.
Clinical presentation varies between cases and depending on the age, as evident in the included cases. Most of the symptoms and signs are related to either visual deterioration or elevated intracranial pressure. Furthermore, patients may complain of decreased visual acuity in the affected eye/s and visual field cut depending on the site of hemorrhage and proptosis (Table 1).
As these hemorrhages can present with signs of high ICP requiring urgent intervention, a CT scan is the modality of choice to determine the site and extent of hemorrhage (6). On the other hand, MRI is useful for differentiating between optic pathway tumors, pituitary tumors, and vascular anomalies (32). Vascular imaging is required in cases of bleeding into the optic pathway as it can result from cavernous or arteriovenous malformations in such locations (52, 53).
Our systematic review has shown how variable treatment options for such pathology range from observation to extensive tumor resection. Most cases have been managed by evacuating the resultant hematoma with decompression of the tumor. However, some reports have carried enucleation of the affected optic nerve, as reported by Yanoff et al. and Dawan et al. (17, 48).
Ten deaths have been reported in the included literature, most of which were related to intraventricular hemorrhage or hemorrhage into the hypothalamus. Out of these 10 deaths, three cases were due to high-grade gliomas (glioblastoma and gliosarcoma) (11, 24, 35). On the other hand, van Baarsen and colleagues suggested that hemorrhage into glioma of the optic nerves carries a more benign course than hypothalamic and chiasmal ones since this type of hemorrhage is confined to the orbit and does not cause intracerebral damage (6).
As mentioned earlier, management decisions should be tailored based on the case since, in some cases, surgical intervention could lead to more deterioration (6). In cases of hydrocephalus, cerebrospinal fluid diversion needs to be done on an urgent basis (6). In other instances, disturbance of the level of consciousness might be due to a compressive effect on the hypothalamus, where surgical decompression and hematoma evacuation are indicated (6). In other cases, surgery is indicated to prevent further deterioration of vision and for histopathological diagnosis (6).
Conclusion
Intertumoral hemorrhage into optic pathway/hypothalamic gliomas is a rare occurrence. We described two infants presenting with sudden decreased vision, poor feeding, and irritability. Our systematic review has shown that intratumoral hemorrhage in OPHG was prevalent at a younger age, pilocytic or pilomexyoid astrocytoma histopathology, and associated with poor prognosis. Further studies with genetic analysis of OPHG may help identify the population at a higher risk of developing such a devastating hemorrhagic complication.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author Contributions
Conception and design: SB, YM. Acquisition of data: RM, YM. Analysis and interpretation of data: YM. Drafting the article: YM, RM. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Study supervision: SB. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aquilina K, Daniels DJ, Spoudeas H, Phipps K, Gan HW, Boop FA. Optic pathway glioma in children: does visual deficit correlate with radiology in focal exophytic lesions? Childs Nerv Syst. (2015) 31(11):2041–9. doi: 10.1007/s00381-015-2855-7
2. Binning MJ, Liu JK, Kestle JR, Brockmeyer DL, Walker ML. Optic pathway gliomas: a review. Neurosurg Focus. (2007) 23(5):E2. doi: 10.3171/FOC-07/11/E2
3. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
4. Shofty B, Constantini S, Bokstein F, Ram Z, Ben-Sira L, Freedman S, et al. Optic pathway gliomas in adults. Neurosurgery. (2014) 74(3):273–9; discussion 9–80. doi: 10.1227/NEU.0000000000000257
5. Hill CS, Khan M, Phipps K, Green K, Hargrave D, Aquilina K. Neurosurgical experience of managing optic pathway gliomas. Childs Nerv Syst. (2021) 37(6):1917–29. doi: 10.1007/s00381-021-05060-8
6. van Baarsen K, Roth J, Serova N, Packer RJ, Shofty B, Thomale UW, et al. Optic pathway-hypothalamic glioma hemorrhage: a series of 9 patients and review of the literature. J Neurosurg. (2018) 129(6):1407–15. doi: 10.3171/2017.8.JNS163085
7. Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. (1982) 10(4):437–44. doi: 10.1227/00006123-198204000-00004
8. Licata B, Turazzi S. Bleeding cerebral neoplasms with symptomatic hematoma. J Neurosurg Sci. (2003) 47(4):201–10; discussion 10. PMID: 14978474
9. Lieu AS, Hwang SL, Howng SL, Chai CY. Brain tumors with hemorrhage. J Formos Med Assoc. (1999) 98(5):365–7. PMID: 10420706
10. Shibahara I, Kanamori M, Kumabe T, Endo H, Sonoda Y, Ogawa Y, et al. Hemorrhagic onset of pilocytic astrocytoma and pilomyxoid astrocytoma. Brain Tumor Pathol. (2009) 26(1):1–5. doi: 10.1007/s10014-008-0243-7
11. Din NU, Ishtiaq H, Rahim S, Abdul-Ghafar J, Ahmad Z. Gliosarcoma in patients under 20 years of age. A clinicopathologic study of 11 cases and a detailed review of the literature. BMC Pediatr. (2021) 21(1):101. doi: 10.1186/s12887-021-02556-9
12. Ishi Y, Yamaguchi S, Hatanaka KC, Okamoto M, Motegi H, Kobayashi H, et al. Association of the FGFR1 mutation with spontaneous hemorrhage in low-grade gliomas in pediatric and young adult patients. J Neurosurg. (2020) 134(3):733–41. doi: 10.3171/2019.12.JNS192155
13. Lu Y, Xu Y. Optic disk astrocytoma unassociated with tuberous sclerosis complex managed with surgical excision and a 7-year follow-up. Retin Cases Brief Rep. (2021) 15(4):462–7. doi: 10.1097/ICB.0000000000000821
14. Campbell AA, Gartrell-Corrado RD, Mansukhani M, Zanazzi G, Canoll P, Garvin JH, et al. SETD2 mutation in an aggressive optic nerve glioma. JAMA Ophthalmol. (2020) 138(1):102–4. doi: 10.1001/jamaophthalmol.2019.4511
15. Cortez GM, Monteiro A, Ludwig B, Hanel R. Reappraisal of haemorrhagic suprasellar pilocytic astrocytoma during adulthood. BMJ Case Rep. (2020) 13(10). doi: 10.1136/bcr-2020-235662
16. Motoyama HL, Yamada S, Nakada S, Kurose N, Tanimoto A. Intraorbital ancient pilocytic astrocytoma of the optic nerve in neurofibromatosis type 1 patient presenting with sudden ocular pain. SAGE Open Med Case Rep. (2018) 6:2050313X18761310. doi: 10.1177/2050313X18761310
17. Dewan A, Saran RK, Gupta SN, Arya D, Goel R. Intraocular ependymoma with blood-filled spaces: neoplasm or a reactive process with ependymal differentiation—a dilemma. Int J Surg Pathol. (2017) 25(4):368–73. doi: 10.1177/1066896917692098
18. Mathew DJ, Selvin SST, Kuruvilla SE, Kuriakose T. Ocular myiasis in a glioma: a case report. Nepal J Ophthalmol. (2016) 8(16):167–70. doi: 10.3126/nepjoph.v8i2.17007
19. Serova NK, Konovalov AN, Eliava SS, Tropinskaya OF, Kuchina OB, Eliseeva NM, et al. [Chiasm and optic nerve glioma manifested as hemorrhage (two clinical cases and a literature review)]. Zh Vopr Neirokhir Im N N Burdenko. (2016) 80(5):90–7. doi: 10.17116/neiro201680590-97
20. Wang Z, Yan HM, Zhou XR, Liu JK, Chang JY, Wang YT. Spontaneous intratumoural and intraventricular haemorrhage associated with a pilomyxoid astrocytoma in the hypothalamic/chiasmatic region. J Clin Neurosci. (2016) 33:217–20. doi: 10.1016/j.jocn.2016.03.033
21. Kapoor A, Savardekar A, Tewari MK, Chatterjee D, Radotra BD. Spontaneous hemorrhages in pediatric supratentorial pilocytic astrocytomas. Malignant presentation of a benign entity. Childs Nerv Syst. (2015) 31(9):1617–20. doi: 10.1007/s00381-015-2749-8
22. Arrese I, Sarabia R, Zamora T. Chiasmal haemorrhage secondary to glioma with unusual MRI appearance. Neurocirugia (Astur). (2014) 25(3):136–9. doi: 10.1016/j.neucir.2013.11.001
23. Della Puppa A, Rustemi O, Gioffre G. The rare event of optic-chiasmatic hemorrhagic low grade glioma in adulthood. Considerations on treatment strategy. Neurol Sci. (2014) 35(4):623–5. doi: 10.1007/s10072-013-1624-1
24. Ashur-Fabian O, Blumenthal DT, Bakon M, Nass D, Davis PJ, Hercbergs A. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: a case report and review of the literature. Anticancer Drugs. (2013) 24(3):315–23. doi: 10.1097/CAD.0b013e32835c7a47
25. Faraji AH, Engh JA, Horowitz M, Lunsford LD, Park DM. Multiple discrete aneurysmal subarachnoid hemorrhages during multimodality management of a hypothalamic glioma—case report. Clin Neurol Neurosurg. (2013) 115(5):632–5. doi: 10.1016/j.clineuro.2012.06.015
26. Liu Y, Zhang-Nunes S, Zhu X, Xu X, Sun X, Wu Y. Late adult onset optic pathway astrocytoma. J Clin Neurosci. (2013) 20(11):1610–2. doi: 10.1016/j.jocn.2012.09.044
27. Ball BG, Wetmore C, Giannini C, Wetjen NM, Meyer FB. Opticochiasmatic apoplexy in a five-year-old. Pediatr Neurosurg. (2011) 47(4):279–83. doi: 10.1159/000334309
28. Vogel TD, Kulwin CG, DeNardo AJ, Payner TD, Boaz JC, Fulkerson DH. Tumor bleeding from a de novo aneurysm associated with optic glioma. J Neurosurg Pediatr. (2011) 7(6):633–6. doi: 10.3171/2011.3.PEDS10562
29. Hamada H, Kurimoto M, Hayashi N, Nagai S, Kurosaki K, Nomoto K, et al. Pilomyxoid astrocytoma in a patient presenting with fatal hemorrhage. Case report. J Neurosurg Pediatr. (2008) 1(3):244–6. doi: 10.3171/PED/2008/1/3/244
30. White JB, Piepgras DG, Scheithauer BW, Parisi JE. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg. (2008) 108(2):223–6. doi: 10.3171/JNS/2008/108/2/0223
31. Garg A, Chugh M, Gaikwad SB, Chandra SP, Gupta V, Mishra NK, et al. Juvenile pilocytic astrocytoma presenting with subarachnoid hemorrhage. case report and review of the literature. J Neurosurg. (2004) 100(5 Suppl Pediatrics):525–9. doi: 10.3171/ped.2004.100.5.0525
32. Yokoyama S, Takayama K, Sueda M, Ishikawa Y, Hirano H. Optic nerve glioma manifesting as intratumoral hemorrhage in a pregnant woman–case report. Neurol Med Chir (Tokyo). (2003) 43(11):559–62. doi: 10.2176/nmc.43.559
33. Aichholzer M, Gruber A, Haberler C, Bertalanffy A, Slavc I, Czech T. Intracranial hemorrhage from an aneurysm encased in a pilocytic astrocytoma—case report and review of the literature. Childs Nerv Syst. (2001) 17(3):173–8. doi: 10.1007/s003810000364
34. Devi BI, Shukla D, Bhat D, Santosh V. Hypothalamic tumour with haemorrhage. Childs Nerv Syst. (2001) 17(9):567–9. doi: 10.1007/s003810100449
35. Wright M, Kamal A, Whittle IR, Vaughan GT. Chiasmal apoplexy, an unusual complication of cerebral glioblastoma. Eye (Lond). (1999) 13(Pt 1):120–1. doi: 10.1038/eye.1999.27
36. Golash A, Thorne J, West CG. Low grade pilocytic astrocytoma presenting as a spontaneous intracerebral haemorrhage in a child. Br J Neurosurg. (1998) 12(1):59–62. doi: 10.1080/02688699845564
37. Hwang SL, Huang TY, Chai CY, Howng SL. Hypothalamic juvenile pilocytic astrocytoma presenting with intracerebral hemorrhage. J Formos Med Assoc. (1998) 97(11):784–7. PMID: 9872037
38. Matsumoto K, Akagi K, Abekura M, Maeda Y, Kitagawa M, Ryujin H, et al. Hypothalamic pilocytic astrocytoma presenting with intratumoral and subarachnoid hemorrhage. Neurol Med Chir (Tokyo). (1997) 37(11):849–51. doi: 10.2176/nmc.37.849
39. Hasegawa H, Bitoh S, Koshino K, Obashi J, Kobayashi Y, Kobayashi M, et al. Mixed cavernous angioma and glioma (angioglioma) in the hypothalamus–case report. Neurol Med Chir (Tokyo). (1995) 35(4):238–42. doi: 10.2176/nmc.35.238
40. Sorenson EJ, Silbert PL, Benarroch EE, Jack CR, Parisi JE. Transient amnestic syndrome after spontaneous haemorrhage into a hypothalamic pilocytic astrocytoma. J Neurol Neurosurg Psychiatry. (1995) 58(6):761–3. doi: 10.1136/jnnp.58.6.761
41. Byard RW, Bourne AJ, Hanieh A. Sudden and unexpected death due to hemorrhage from occult central nervous system lesions. A pediatric autopsy study. Pediatr Neurosurg. (1991) 17(2):88–94. doi: 10.1159/000120573
42. Applegate LJ, Pribram HF. Hematoma of optic nerve glioma–a cause for sudden proptosis. Magnetic resonance imaging findings. J Clin Neuroophthalmol. (1989) 9(1):15–9. PMID: 2522939
43. Jordan DR, Anderson RL, White GL Jr., Mamalis N. Acute visual loss due to a calcified optic nerve glioma. Can J Ophthalmol. (1989) 24(7):335–9. PMID: 2624916
44. Yokota A, Kajiwara H, Matsuoka S, Kohchi M, Matsukado Y. Subarachnoid hemorrhage from brain tumors in childhood. Childs Nerv Syst. (1987) 3(2):65–9. doi: 10.1007/BF00271124
45. Maitland CG, Abiko S, Hoyt WF, Wilson CB, Okamura T. Chiasmal apoplexy. Report of four cases. J Neurosurg. (1982) 56(1):118–22. doi: 10.3171/jns.1982.56.1.0118
46. Waga S, Shimizu T, Sakakura M. Diencephalic syndrome of emaciation (Russell’s syndrome). Surg Neurol. (1982) 17(2):141–6. doi: 10.1016/S0090-3019(82)80043-8
47. Charles NC, Nelson L, Brookner AR, Lieberman N, Breinin GM. Pilocytic astrocytoma of the optic nerve with hemorrhage and extreme cystic degeneration. Am J Ophthalmol. (1981) 92(5):691–5. doi: 10.1016/S0002-9394(14)74663-X
48. Yanoff MDR, Zimmerman L. Juvenile pilocytic astrocytoma (‘glioma’) of the optic nerve. In: Jakobiec FA, editors. Ocular and adnexal tumors. Aescluapius: Birmingham (1978).
49. Glew WB. Simulated pituitary apoplexy: report of an unusual case due to hemorrhage into hypothalamic astrocytoma. Ann Ophthalmol. (1977) 9(2):139–42. PMID: 843014
50. Schneider RC, Kriss FC, Falls HF. Prechiasmal infarction associated with intrachiasmal and suprasellar tumors. J Neurosurg. (1970) 32(2):197–208. doi: 10.3171/jns.1970.32.2.0197
51. Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. (2003) 18(7):471–8. doi: 10.1177/08830738030180070401
52. Crocker M, Desouza R, King A, Connor S, Thomas N. Cavernous hemangioma of the optic chiasm: a surgical review. Skull Base. (2008) 18(3):201–12. doi: 10.1055/s-2007-1023231
Keywords: optic glioma, hypothalamic glioma, hemorrhage, apoplexy, optic chiasm
Citation: Baeesa S, Maghrabi Y, Moshref R and Al-Maghrabi J (2022) Optic Pathway–Hypothalamic Glioma Apoplexy: A Report of Two Cases and Systematic Review of the Literature. Front. Surg. 9:891556. doi: 10.3389/fsurg.2022.891556
Received: 7 March 2022; Accepted: 6 May 2022;
Published: 30 May 2022.
Edited by:
Zohreh Habibi, Tehran University of Medical Sciences, IranReviewed by:
Sandip Chatterjee, Vivekananda Institute of Medical Sciences, IndiaMehdi Zeinalizadeh, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Iran
Copyright © 2022 Baeesa, Maghrabi, Moshref and Almaghrabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saleh Baeesa c2JhZWVzYUBrZnNocmMuZWR1LnNh
Specialty section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Saleh Baeesa
Saleh Baeesa Yazid Maghrabi
Yazid Maghrabi Rana Moshref
Rana Moshref Jaudah Al-Maghrabi2
Jaudah Al-Maghrabi2