- 1Department of Ultrasonography, Zhongshan People's Hospital (ZSPH), Zhongshan, China
- 2Department of Respiratory Medicine, Zhongshan People's Hospital (ZSPH), Zhongshan, China
By searching lliteratures till January 5, 2022, we evaluated the role of the mediastinal nodal staging of endobronchial ultrasound-guided fine-needle aspiration (EBUS) and endoscopic ultrasound-guided fine-needle aspiration (EUS) in lung cancer. A total of 20 studies with 2,961 patients were included in this study. The pooled sensitivity, specificity, PLR, and NLR for EBUS were 0.79, 0.97, 27.29, and 0.25, respectively. EUS showed staging performance similar to EBUS. The staging performance was significantly improved when combining EBUS + EUS.
Introduction
Around 1.8 million new cases and 1.59 million deaths are recorded every year, making lung carcinoma one of the commonest cancer and the main causes of cancer death among men (1–3). Except for abandon smoking, the best method in decreasing lung cancer incidence and mortality is deemed as the early-stage diagnosis, followed by surgical resection (4).
Accurate staging is the critical step for manageing cases with lung carcinoma. Since the prognosis of cases with lung cancer are affected by the presence of mediastinal lymph node metastasis, lymph node staging is crucial for physicians for clinical diagnosis. In recent years, chest computed tomography (CT) and integrated positron emission tomography (PET)-CT have been extensivly used for nodal staging, and mediastinoscopy is deemed as a “gold standard” for lymph node staging (4–7). Endobronchial ultrasound-guided fine-needle aspiration (EBUS) was implemented since 2004 and was reported to be a less invasive method in diagnosing mediastinal and hilar lymph node metastasis (8). It is compatible with the convex-probe EBUS scope with a diagnostic yield similar to that of mediastinoscopy (9, 10). Similarly, endoscopic ultrasound-guided fine-needle aspiration (EUS) showed mediastinal restaging sensitivity from 75%–92% (11–13). Moreover, aimed to obtain enhanced sensitivity in the initial mediastinal staging of lung cancer, EBUS and EUS are combined due to their complimentary access to mediastinal lymph gland.
In the current study, we aimed to quantitatively analyze the mediastinal nodal staging performance of EBUS and EUS in lung cancer cases.
Methods
Our current study were done following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (14).
Literature search
MEDLINE, Cochrane Library, and EMBASE were used for searching targeted studies published up to January 5, 2022. The individual and combined were used to search relevant studies: “mediastinal staging”, “non-small-cell lung cancer”, endoscopic echography”, “fine-needle aspiration”, “EUS”, “EBUS”, “endobronchial ultra-sonography”. The search strategies are described in Supplementary file. The bibliography of on the topic were browsed for obtaining more potential studies.
Eligibility criteria
Literatures that fulfilled the inclusion criteria were included:
(1) reseachers employed EBUS and EUS(-B) to stage mediastinal lymph nodes in patients with non-small cell lung cancer (NSCLC);
(2) studies reported true positive (TP), true negative (TN), false positive (FP), and false negative (FN);
(3) mediastinal lymph nodes were confirmed by mediastinoscopy, surgical lymph node dissection, or radiological follow-up;
(4) studies published in English;
(5) when the population was reported in duplicate, studies that provided detailed information or were newly published articles were taken into consideration.
Studies were excluded if they were:
(1) short reports, commens, communications, reviews;
(2) papers focused on diagnosing primary lung tumors;
(3) studies included cases after induction therapy;
(4) studies published in other language than English.
Data extraction and definitions
Needed information were extracted by two authors independently. Each disagreement was settled by consensus. For all included studies, we extracte: name of the first author, the year of publication, countries, study types, sample size, characteristics of cases, characteristics of EBUS and EUS(-B), diagnostic parameters, i.e. TP, TN, FP, and FN. The results were appraised as high/low risk or unclear risk. Moreover, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (15) was applied to evaluate the study evidence quality.
Quality of studies assessment
We usedthe Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (16) to assesse the quality of the included studies independently by the two authors. The assessement of QUADAS-2 was based on four items: (1) how the cases were selected; (2) index test, it was the descriptions of how the studies were implented and how the results were interpretatd; (3) reference standard, it includes descriptions standards of the the references; (4) flow and timing, it includeshow the cases were included and excluded.
Statistical analysis
For each study, a 2 × 2 table was used to assess the test accuracy: sensitivity, specificity, positive like likelihood ratio (PLR), and negative likelihood ratio (NLR). The summary receiver operating characteristic (SROC) curves, the area under the curves (AUCs), and associated standard errors (SEs) were deduced. The sensitivity and specificity from the included studies were pooled by random-effects models and the AUCs of the SROC curves were determined using the DerSimonian-Laird random-effects method. The 95% confidence intervals (CIs) of the merged parameters were used to compare and assess the relative performances of these techniques.
The I2 was empolyed to evaluate the consistency of the effect size, which assess the variability by percentages. Heterogeneity was described as low, moderate, and high according to the values of I2 as 25%, 50%, and 75%, respectively (17). We also included QUADAS-2 score, study design, type of confirmation, and the country as covariates of univariate meta-regression analysis (weighted inverse variance). The relative DOR (RDOR) was calculated. Publication biases were calculated by Begg’s rank correlation (18) and Egger’s weighted regression methods (19). We used Meta-Disc software programs (version 1.4, Ramón y Cajal Hospital, Madrid, Spain) and R programme (version 4.2.0) for the data analyses. Publication biases were carried out using STATA 15.0. p < 0.05 indicated statistical significance for all analyses.
Role of the funding source
No external funding was received for this study. The corresponding author had full access to all the data and made the final decision of publication.
Results
Study selection
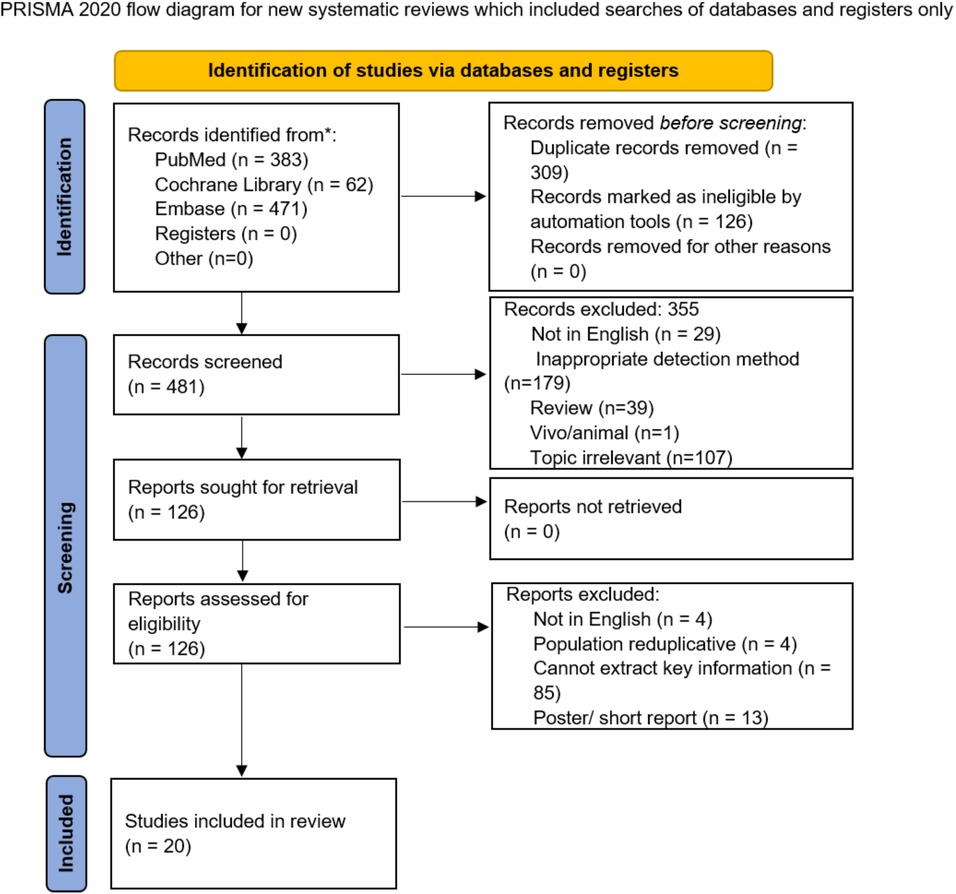
The search strategy obtained 916 potentially relevant studies and 309 of them were excluded due to overlap, while 355 were excluded after screening the titles or abstracts due to topic relevance or improper study design. Finally, 20 articles (20–39) were included. The study selection is illustrated in Figure 1.
Study characteristics
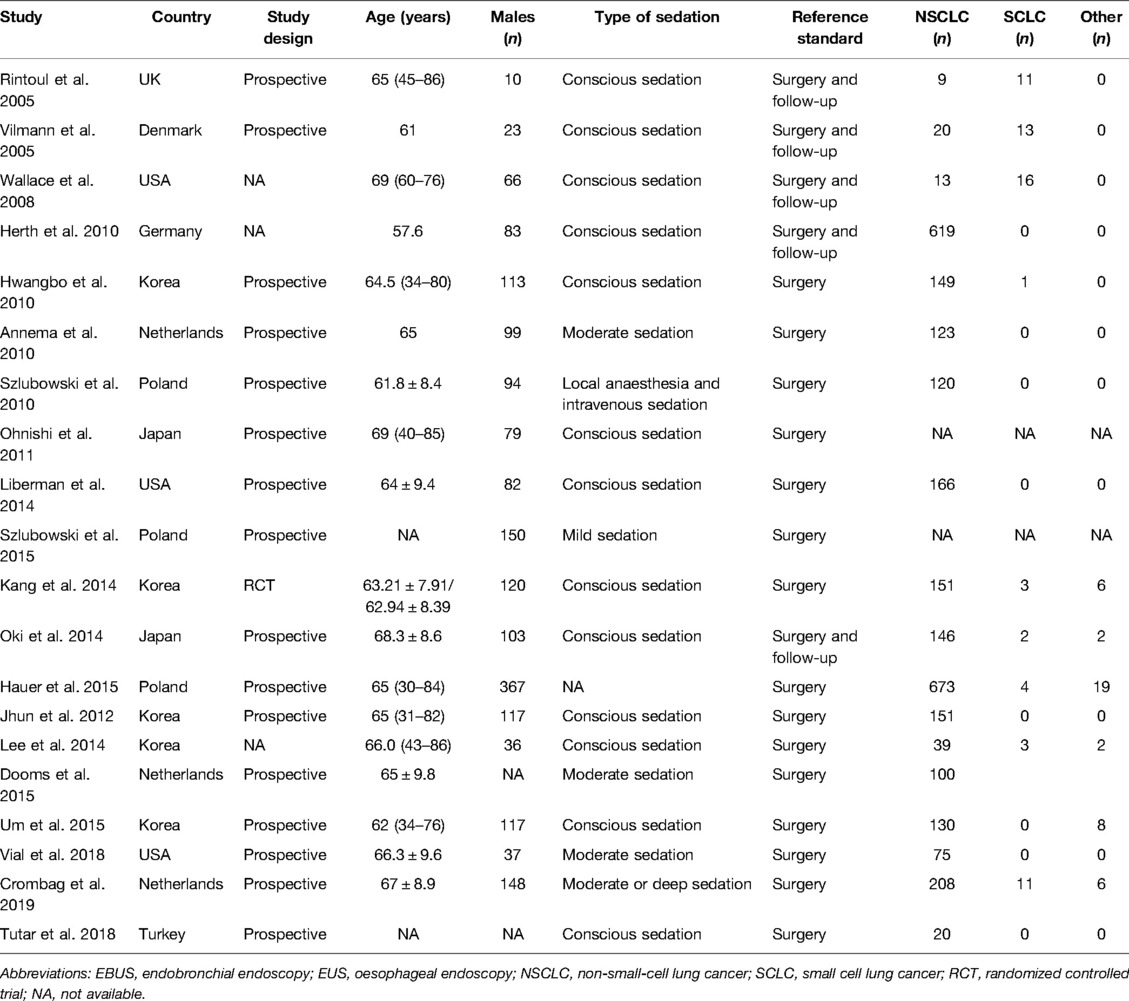
Table 1 presents the characteristics of study participants. A total of 20 studies with 2,961 participants were included in this meta-analysis. The sample size of the studies ranged from 20 to 696, and the studies were published between 2005 and 2019. Among these, 5 studies were conducted in South Korea, 3 each in the USA, Netherlands, and Poland, 2 in Japan, and 1 each in the UK, Denmark, Germany, and Turkey. Moreover, 8 studies assessed the diagnostic performance using contrast-enhanced (CE)-CT while 4 used CE-magnetic resonance imaging (MRI). The majority of the studies were prospective, and one was a randomized controlled trial. The data of each study are presented in Table 2.
Assessment of study quality and risk of bias
None of the included studies was judged as high risk, suggesting that the quality of all the eligible articles was acceptable. The quality assessment results are presented in Supplementary Table S1.
Staging accuracy of EBUS
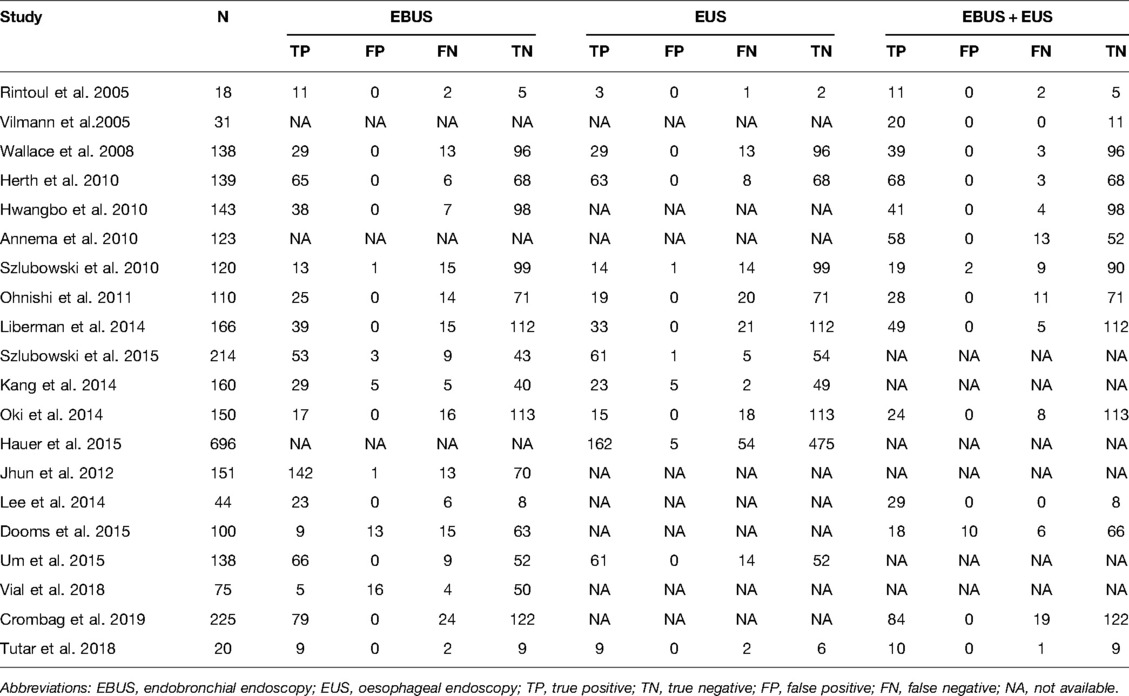
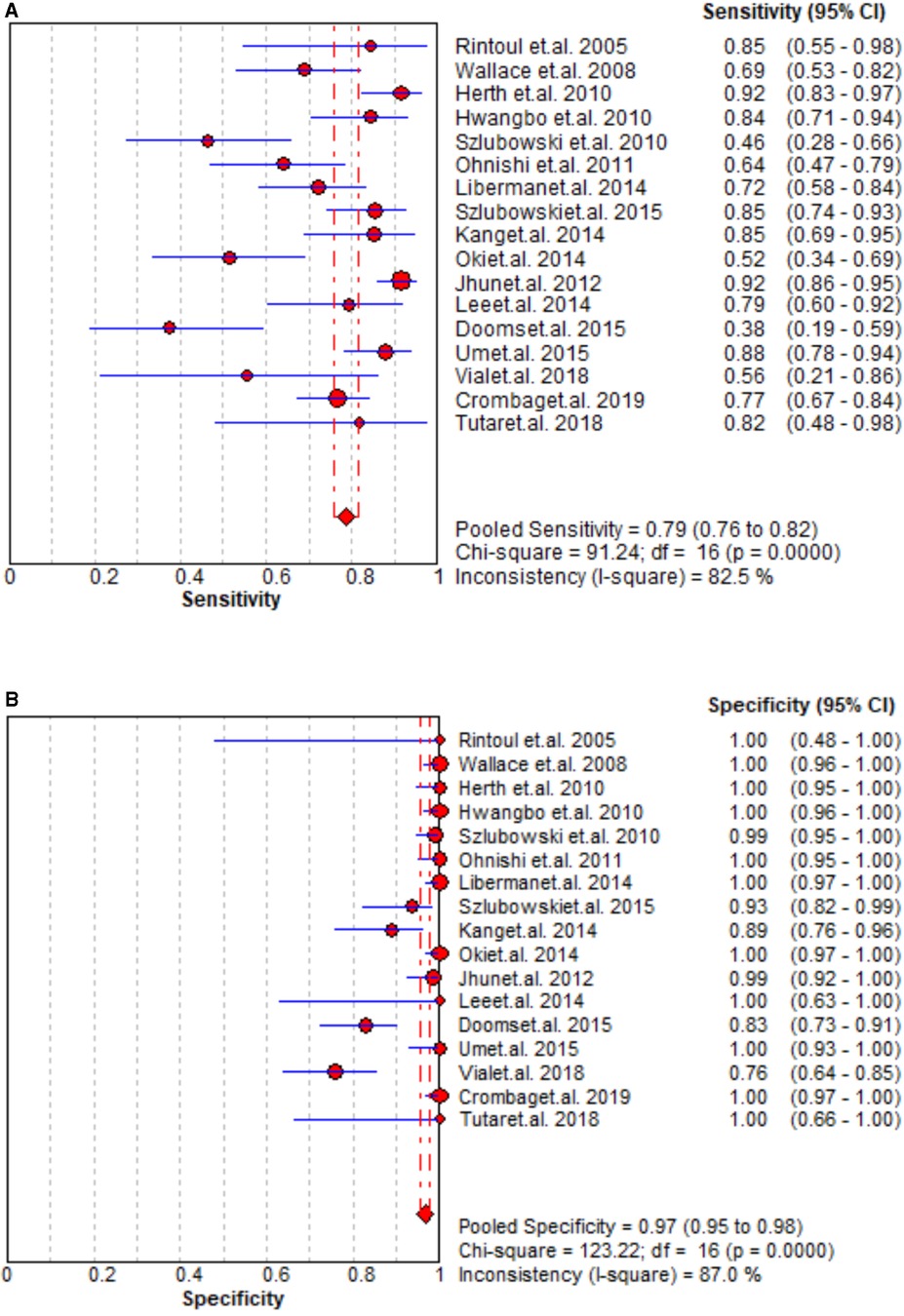
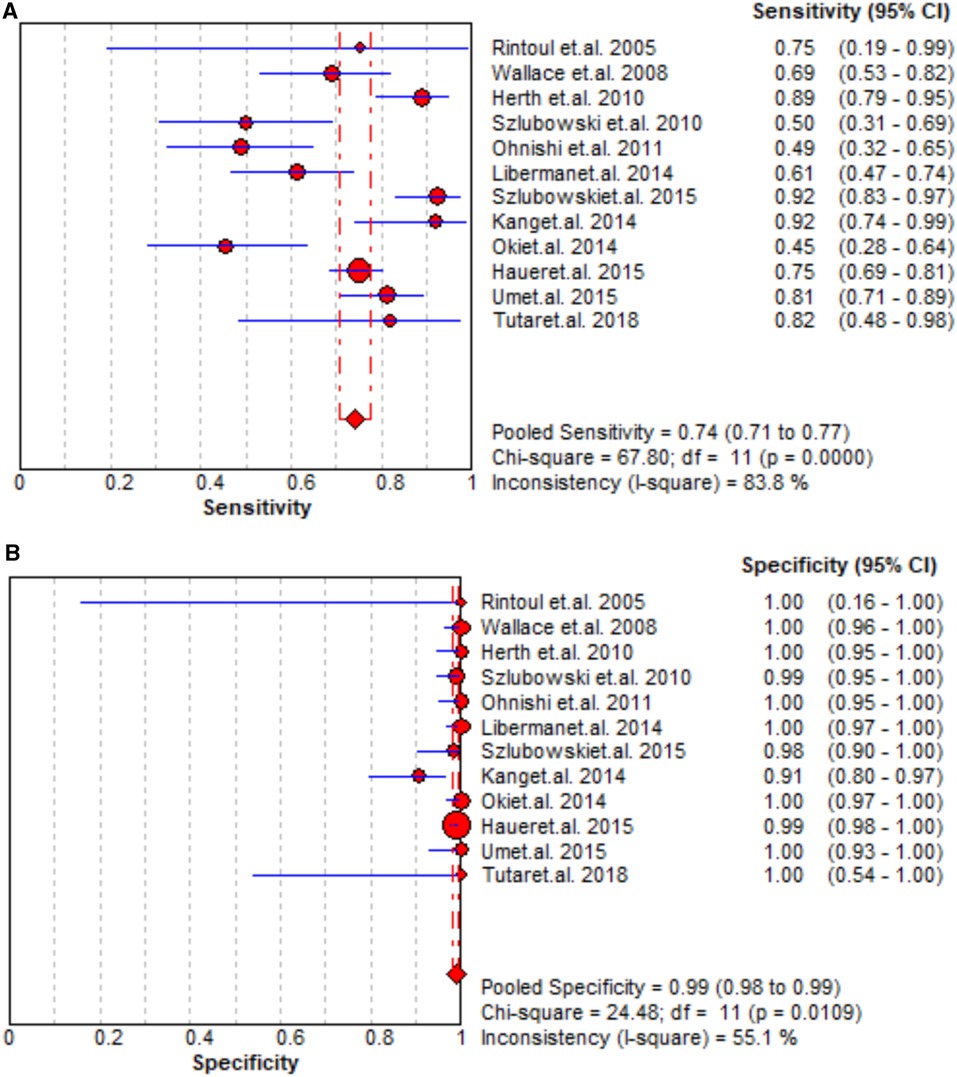
The staging performance of EBUS of 17 studies revealed sensitivity and specificity 0.46–0.92 and 0.76–1.00, respectively. The pooled sensitivity, specificity, PLR, and NLR for EBUS for mediastinal nodal staging in lung cancer were 0.79 (95% CI = 0.76–0.82), 0.97 (95% CI = 0.95–0.98), 27.29 (95% CI = 9.82–75.83), and 0.25 (95% CI = 0.18–0.36), respectively. The results are presented in Figure 2 and Supplementary Figure S1. As shown in Supplementary Figure S2, the pooled AUC for EBUS was 0.895 ± 0.0594 SE).
Staging accuracy of EUS
A total of 12 studies reported that the staging performance of EUS was similar to that of EBUS with sensitivity and specificity 0.49–0.92 and 0.98–1.00, respectively. When pooling the results, the combined sensitivity, specificity, PLR, and NLR were 0.74 (95% CI = 0.71–0.77), 0.99 (95% CI = 0.98–0.99), 36.91 (95% CI = 16.73–81.40), and 0.28 (95% CI = 0.20–0.39), respectively, but the AUC was slightly high as 0.9682 ± 0.0143. The data of sensitivity and specificity, PLR and NLR, and AUCs are presented in Figures 3, Supplementary Figures S3 and S4, respectively.
Staging accuracy of EBUS + EUS
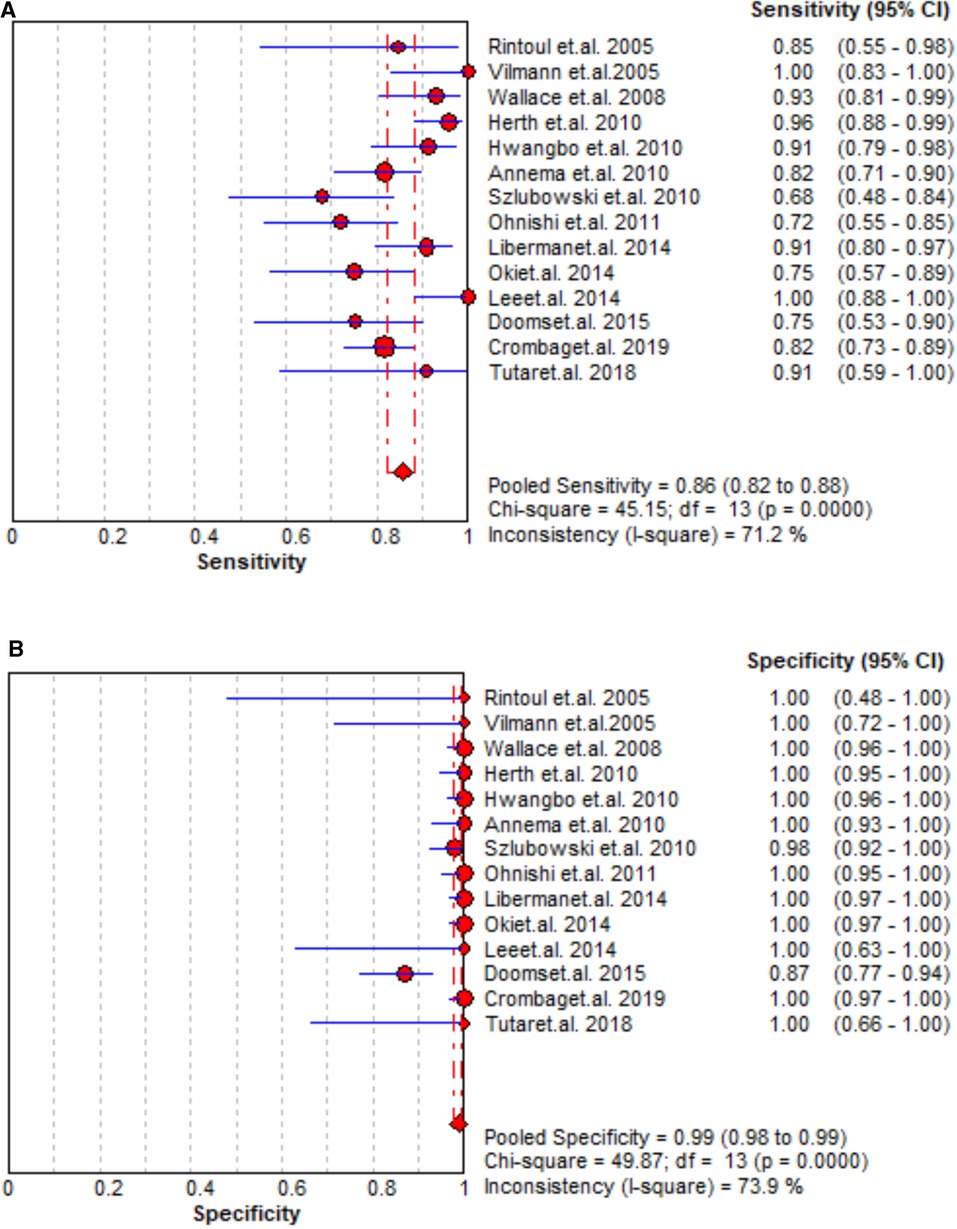
A total of 14 studies assessed the staging accuracy of EBUS + EUS, and the majority of them reported significantly high sensitivity (0.72–0.96). A significantly improved staging accuracy of EBUS + EUS was observed with pooled sensitivity, specificity, PLR, and NLR as 0.86 (95% CI = 0.82–0.88, Figure 4), 0.99 (95% CI = 0.98–0.99, Figure 4), 49.48 (95% CI = 15.17–161.35, Supplementary Figure S5), and 0.17 (95% CI = 0.12–0.23, Supplementary Figure S5), respectively, and AUC was also high (0.0.9722 ± 0.0194; Supplementary Figure S6).
Multiple regression analysis
As shown in Table 3, studies with high-quality QUADAS-2 score, varied design, conducted in various countries, and confirmed by different methods indicated that these factors do not affect the staging accuracy substantially.
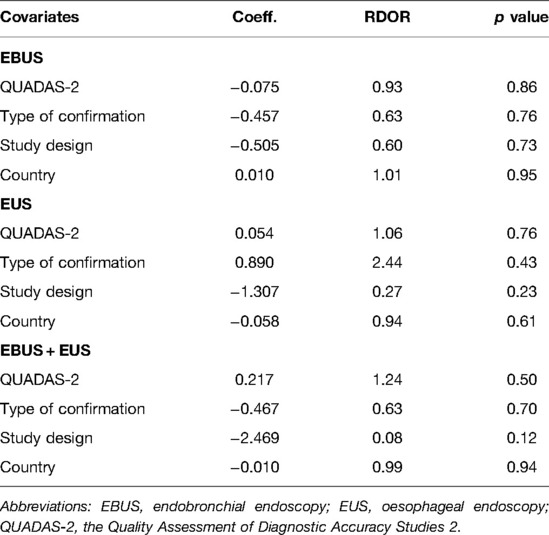
Table 3. Weighted meta-regression of the effects of methodologic al characteristics, study design, country, and type of confirmation.
Certainty of the evidence
Overall, the certainty of the evidence was low when assessed according to GRADE criteria.
Publication bias
No significant publication bias was seen with P-values more than 0.05of Begg’s rank correlation analysis and Egger’s weighted regression analysis (Supplementary Table S2).
Discussion
In our current meta-analysis, the accuracy of EBUS and EUS was investigated for the mediastinal nodal staging in lung cancer cases. 20 studies with 2,961 participants were finally included and pooled. Both EBUS and EUS provide accurate performance in the mediastinal nodal staging in lung cancer cases. When combining EBUS and EUS, the staging performance was improved further.
The staging of lung cancer started with radiology (19). F-Fluorodeoxyglucose PET or PET-CT reported a high diagnostic accuracy for mediastinal staging with sensitivity and specificity as 0.85 and 0.90, respectively (40). A previous meta-analysis reported that the sensitivity and specificity of PET-CT for detecting metastatic lymph nodes was 0.78 and 1.00, respectively (41). Similar results were also reported by previous meta-analyses (42–45). In the current study, with inclusion of more revelant studies, the sensitivity and specificity was 0.79 and 0.97 for EBUS and 0.74 and 0.99 for EUS, respectively; the staging performance in detecting metastatic lymph nodes was similar to that of PET-CT. Although EBUS provides access to mediastinal lymph nodes commonly involved in lung cancer, and EUS complements this by accessing nodes beyond the reach of EBUS in the inferior mediastinum, the majority of the lymph node stations in the mediastinum are accessed by endosonographic guidance (46, 47). Therefore, the enhanced staging performance of the combination of EBUS and EUS is reasonable and undisputed.
However, the finding in the current study showed that EBUS and EUS were similar to PET, but it does not propagate that it should be adopted as the preferred choice for the staging of mediastinal lymph nodes in patients with known or suspected lung cancer (48, 49). Unlike CT or PET-CT, EBUS or EUS acquires invasive tissue samples. However, EBUS has the advantage of assessing the lymph node stations that are in close proximity to the airways, such as paratracheal and subcarinal stations (50, 51). The non-invasive restaging technique, i.e., CT or PET-CT, suggested persistent mediastinal lymph node involvement post-induction therapy that requires tissue confirmation (52, 53), and EBUS or EUS provided the best access to those lymph node stations. In previous years, the obstruction for endosonography-guided needle sampling techniques, the EBUS or EUS, has limited accuracy in the mediastinal restaging of lung cancer (54, 55). These findings highlighted the clinical meaning of EBUS or EUS. Nevertheless, due to the performance of EBUS or EUS is highly effected by clinical experiences of operators. Establishing EBUS or EUS as a diagnostic and therapeutic method is therefore still a challengd (56). Given the cost of interventional EBUS or EUS is high and EBUS or EUS is deficient in majority of regions, tsherefore, when these two methods are implemented, several concerns arise, such as they are likely to be more operator-dependent and have a steeper learning curve compared to the initial mediastinal staging for lung cancer cases (57, 58).
Nevertheless, the current study has some limitations when interpreting the results. First, 20 studies with limited lung cancer cases were included in the current meta-analysis. Due to liminted included cases, we canot conduct the subgroup or sensitivity analyses according to the stages of lung cancer and the type of carcinoma. Second, the mean age and sex ratio of the participants in each study varied largely, which in turn caused heterogeneity and decreased the stability of the results. Third, the stages of lung cancer differed among the included studies, which might decrease the comparability of the included studies. Fourth, the heterogeneity of the imaging technique was moderate, and the results require further validation in the future. However, due to the limited number of included studies, subgroup analysis on the imaging technique could not be performed. However, we cannot ensure that all parameters are constant. Fifth, the paper was registered in retrospectives in the PROSPERO (ID: 323791), which decrease transparency of the meta-analyses. Sixth, potential language bias might exist as articles not published in English were excluded.
Conclusions
In conclusion, both EBUS and EUS provide accurate and comparable staging performance. The combination EBUS and EUS provide enhanced accurate results on the staging of lung cancer cases. However, additional studies with large sample sizes are warranted to further confirm our findings.
Author Contributions
XZL and SZL carried out the studies, participated in collecting data, and drafted the manuscript. MQY and KY performed the statistical analysis and participated in its design. XZL and WHG participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors contributed to the article and approved the submitted version.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fsurg.2022.890993/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mao Y, Yang D, He J, Krasna MJ. Epidemiology of Lung Cancer. Surg Oncol Clin N Am. (2016) 25(3):439–45. doi: 10.1016/j.soc.2016.02.001
2. Schwartz AG, Cote ML. Epidemiology of Lung Cancer. Adv Exp Med Biol. (2016) 893:21–41. doi: 10.1007/978-3-319-24223-1_2
3. Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. (2019) 85(1):8, 1–16. doi: 10.5334/aogh.2419
4. Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer Control. (2014) 21(1):9–14. doi: 10.1177/107327481402100102
5. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. (2007) 75(1):56–63. doi: 10.1186/1471-2296-8-1
6. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
7. Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. (2020) 41(1):1–24. doi: 10.1016/j.ccm.2019.10.001
8. Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, LIizasa T, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. (2004) 126(1):122–8. doi: 10.1378/chest.126.1.122
9. Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax. (2009) 64(9):757–62. doi: 10.1136/thx.2008.109868
10. Khoo KL. Mediastinal re-staging of non small-cell lung cancer. Thorac Cancer. (2012) 3(2):145–9. doi: 10.1111/j.1759-7714.2011.00097.x
11. Stigt JA, Oostdijk AH, Timmer PR, Shahin GM, Boers JE, Groen HJ. Comparison of EUS-guided fine needle aspiration and integrated PET-CT in restaging after treatment for locally advanced non-small cell lung cancer. Lung Cancer. (2009) 66(2):198–204. doi: 10.1016/j.lungcan.2009.01.013
12. Madan K, Madan M, Iyer H, Mittal S, Madan NK, Rathi V, et al. Utility of Elastography for Differentiating Malignant and Benign Lymph Nodes During EBUS-TBNA: A Systematic Review and Meta-analysis. J Bronchology Interv Pulmonol. (2022) 29(1):18–33. doi: 10.1097/LBR.0000000000000781
13. Pausawasdi N, Maipang K, Sriprayoon T, Charatchareonwitthaya P. Role of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in the Evaluation of Abdominal Lymphadenopathy of Unknown Etiology. Clin Endosc. (2022) 55(2):279–86. doi: 10.5946/ce.2021.218-IDEN
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
16. Schueler S, Walther S, Schuetz GM, Schlattmann P, Dewey M. Methodological quality of diagnostic accuracy studies on non-invasive coronary CT angiography: influence of QUADAS (Quality Assessment of Diagnostic Accuracy Studies included in systematic reviews) items on sensitivity and specificity. Eur Radiol. (2013) 23(6):1603–22. doi: 10.1007/s00330-012-2763-0
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50(4):1088–1101. doi: 10.2307/2533446
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
20. Rintoul RC, Skwarski KM, Murchison JT, Wallace WA, Walker WS, Penman ID. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J. (2005) 25(3):416–21. doi: 10.1183/09031936.05.00095404
21. Vilmann P, Krasnik M, Larsen SS, Jacobsen GK, Clementsen P. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy. (2005) 37(9):833–9. doi: 10.1055/s-2005-870276
22. Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. (2008) 299(5):540–6. doi: 10.1001/jama.299.5.540
23. Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. (2010) 304(20):2245–52. doi: 10.1001/jama.2010.1705
24. Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. (2010) 138(4):790–4. doi: 10.1378/chest.09-2149
25. Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. (2010) 138(4):795–802. doi: 10.1378/chest.09-2100
26. Szlubowski A, Zieliński M, Soja J, Annema JT, Sośnicki W, Jakubiak M, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging–a prospective trial. Eur J Cardiothorac Surg. (2010) 37(5):1175–79. doi: 10.1016/j.ejcts.2009.11.015
27. Ohnishi R, Yasuda I, Kato T, Tanaka T, Kaneko Y, Suzuki T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy. (2011) 43(12):1082–9. doi: 10.1055/s-0030-1256766
28. Liberman M, Sampalis J, Duranceau A, Thiffault V, Hadjeres R, Ferraro P. Endosonographic mediastinal lymph node staging of lung cancer. Chest. (2014) 146(2):389–97. doi: 10.1378/chest.13-2349
29. Szlubowski A, Soja J, Kocon P, Talar P, Czajkowski W, Rudnicka-Sosin L, et al. A comparison of the combined ultrasound of the mediastinum by use of a single ultrasound bronchoscope versus ultrasound bronchoscope plus ultrasound gastroscope in lung cancer staging: a prospective trial. Interact Cardiovasc Thorac Surg. (2012) 15(3):442–6; discussion 446. doi: 10.1093/icvts/ivs161
30. Kang HJ, Hwangbo B, Lee GK, Nam BH, Lee HS, Kim MS, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax. (2014) 69(3):261–8. doi: 10.1136/thoraxjnl-2013-203881
31. Oki M, Saka H, Ando M, Kitagawa C, Kogure Y, Seki Y. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg. (2014) 148(4):1169–77. doi: 10.1016/j.jtcvs.2014.05.023
32. Hauer J, Szlubowski A, Żanowska K, Rudnicka-Sosin L, Trybalski Ł, Grochowski Z, et al. Minimally invasive strategy for mediastinal staging of patients with lung cancer. Pol Arch Med Wewn. (2015) 125(12):910–3. doi: 10.20452/pamw.3209
33. Jhun BW, Park HY, Jeon K, Koh WJ, Suh GY, Chung MP, et al. Nodal stations and diagnostic performances of endobronchial ultrasound-guided transbronchial needle aspiration in patients with non-small cell lung cancer. J Korean Med Sci. (2012) 27(1):46–51. doi: 10.3346/jkms.2012.27.1.46
34. Lee KJ, Suh GY, Chung MP, Kim H, Kwon OJ, Han J, et al. Combined endobronchial and transesophageal approach of an ultrasound bronchoscope for mediastinal staging of lung cancer. PLoS One. (2014) 9(3):e91893. doi: 10.1371/journal.pone.0091893
35. Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest. (2015) 147(1):209–15. doi: 10.1378/chest.14-0534
36. Kim HK, Jung SH, Han J, Lee KJ, Park HY, Choi YS, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol. (2015) 10(2):331–7. doi: 10.1097/JTO.0000000000000388
37. Tutar N, Yurci A, Güneş I, Gülmez İ, Gürsoy Ş, Önal Ö, et al. The role of endobronchial and endoscopic ultrasound guided fine needle aspiration for mediastinal nodal staging of non-small-cell lung cancer. Tuberk Toraks. (2018) 66(2):85–92. doi: 10.5578/tt.66866
38. Vial MR, O'Connell OJ, Grosu HB, Hernandez M, Noor L, Casal RF, et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology. (2018) 23(1):76–81. doi: 10.1111/resp.13162
39. Crombag LMM, Dooms C, Stigt JA, Tournoy KG, Schuurbiers OCJ, Ninaber MK, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J. (2019) 53(2):1800800. doi: 10.1183/13993003.00800-2018
40. Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. (2003) 139(11):879–92. doi: 10.7326/0003-4819-139-11-200311180-00013
41. Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143(5 Suppl):e211S–e250S. doi: 10.1378/chest.12-2355
42. Zhang R, Ying K, Shi L, Zhang L, Zhou L. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer. (2013) 49(8):1860–7. doi: 10.1016/j.ejca.2013.02.008
43. Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. (2016) 4(12):960–8. doi: 10.1016/S2213-2600(16)30317-4
44. Labarca G, Aravena C, Ortega F, Arenas A, Majid A, Folch E, et al. Minimally Invasive Methods for Staging in Lung Cancer: Systematic Review and Meta-Analysis. Pulm Med. (2016) 2016:1024709. doi: 10.1155/2016/1024709
45. Shen Y, Qin S, Jiang H. Endobronchial ultrasound-guided transbronchial needle aspiration combined with either endoscopic ultrasound-guided fine-needle aspiration or endoscopic ultrasound using the EBUS scope-guided fine-needle aspiration for diagnosing and staging mediastinal diseases: a systematic review and meta-analysis. Clinics (Sao Paulo). (2020) 75:e1759. doi: 10.6061/clinics/2020/e1759
46. Hwangbo B, Park EY, Yang B, Lee GK, Kim TS, Kim HY, et al. Long-Term Survival According to N Stage Diagnosed by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Non-Small Cell Lung Cancer. Chest. (2021) S0012-3692(21):04434-2. doi: 10.1016/j.chest.2021.11.032
47. Ito T, Kimura T, Kataoka K, Okachi S, Wakahara K, Hashimoto N, et al. A Pilot Study of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in the Diagnosis of Peripheral Pulmonary Lesions in Patients with Interstitial Lung Disease. Diagnostics (Basel). (2021) 11(12):2269–76. doi: 10.3390/diagnostics11122269
48. Nakajima T, Yasufuku K. The techniques of endobronchial ultrasound-guided transbronchial needle aspiration. Innovations (Phila). (2011) 6(1):57–64. doi: 10.1097/imi.0b013e31820c91a7
49. Inage T, Nakajima T, Yoshino I. Staging lung cancer: role of endobronchial ultrasound. Lung Cancer (Auckl). (2014) 5:67–72. doi: 10.2147/LCTT.S46195
50. Nakajima T, Yasufuku K, Fujiwara T, Yoshino I. Recent advances in endobronchial ultrasound-guided transbronchial needle aspiration. Respir Investig. (2016) 54(4):230–6. doi: 10.1016/j.resinv.2016.02.002
51. Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis. (2017) 9(Suppl 2):S83–S97. doi: 10.21037/jtd.2017.03.102
52. Nagano H, Takumi K, Nakajo M, Fukukura Y, Kumagae Y, Jinguji M, et al. Dual-Energy CT-Derived Electron Density for Diagnosing Metastatic Mediastinal Lymph Nodes in Non-Small Cell Lung Cancer: Comparison With Conventional CT and FDG PET/CT Findings. AJR Am J Roentgenol. (2022) 218(1):66–74. doi: 10.2214/AJR.21.26208
53. Shang Q, Zhao L, Pang Y, Meng T, Chen H. Differentiation of Reactive Lymph Nodes and Tumor Metastatic Lymph Nodes With 68Ga-FAPI PET/CT in a Patient With Squamous Cell Lung Cancer. Clin Nucl Med. (2022) 47(5):458–61. doi: 10.1097/RLU.0000000000003998
54. Darling GE. Lymph node assessment in early stage non-small cell lung cancer lymph node dissection or sampling? Gen Thorac Cardiovasc Surg. (2020) 68(7):716–24. doi: 10.1007/s11748-020-01345-y
55. Murthi M, Donna E, Arias S, Villamizar NR, Nguyen DM, Holt GE, et al. Diagnostic Accuracy of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) in Real Life. Front Med (Lausanne). (2020) 7:118. doi: 10.3389/fmed.2020.00118
56. Lesmana CRA, Paramitha MS, Gani RA. The Role of Interventional Endoscopic Ultrasound in Liver Diseases: What Have We Learnt? Can J Gastroenterol Hepatol. (2021) 2021:9948979. doi: 10.1155/2021/9948979
57. Zhang R, Ma Y, Xu G, Gao X, Luo G, Lin Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration and cervical mediastinoscopy for mediastinal staging of non-small cell lung cancer: a retrospective comparison study. J Thorac Dis. (2018) 10(3):1951–9. doi: 10.21037/jtd.2018.01.51
Keywords: EBUS, EUS, lung cancer, mediastinal nodal staging, meta-analysis
Citation: Liu X, Yang K, Guo W, Ye M and Liu S (2022) Mediastinal Nodal Staging Performance of Combined Endobronchial and Esophageal Endosonography in Lung Cancer Cases: A Systematic Review and Meta-Analysis. Front. Surg. 9:890993. doi: 10.3389/fsurg.2022.890993
Received: 7 March 2022; Accepted: 27 April 2022;
Published: 23 May 2022.
Edited by:
Gonzalo Labarca, Harvard Medical School, United StatesReviewed by:
Juan Uribe, Beth Israel Deaconess Medical Center, Harvard Medical School, United StatesDuilio Divisi, University of L'Aquila, Italy
Copyright © 2022 Liu, Yang, Guo, Ye and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhen Liu c3Vtc21pY2hhZWxAMTM5LmNvbQ==
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Xiaozhen Liu
Xiaozhen Liu Kun Yang1
Kun Yang1 Shaozhong Liu
Shaozhong Liu