- 1Department ofs Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, China
- 2School of Life Science and Technology, Computational Biology Research Center, Harbin Institute of Technology, Harbin, China
Background and Objective: Sentinel lymph node biopsy (SLNB) is used to assess the status of axillary lymph node (ALN), but it causes many adverse reactions. Considering the low rate of sentinel lymph node (SLN) metastasis in T1 breast cancer, this study aims to identify the characteristics of T1 breast cancer without SLN metastasis and to select T1 breast cancer patients who avoid SLNB through constructing a nomogram.
Methods: A total of 1,619 T1 breast cancer patients with SLNB in our hospital were enrolled in this study. Through univariate and multivariate logistic regression analysis, we analyzed the tumor anatomical and clinicopathological factors and constructed the Heilongjiang Medical University (HMU) nomogram. We selected the patients exempt from SLNB by using the nomogram.
Results: In the training cohort of 1,000 cases, the SLN metastasis rate was 23.8%. Tumor volume, swollen axillary lymph nodes, pathological types, and molecular subtypes were found to be independent predictors for SLN metastasis in multivariate regression analysis. Distance from nipple or surface and position of tumor have no effect on SLN metastasis. A regression model based on the results of the multivariate analysis was developed to predict the risk of SLN metastasis, indicating an AUC of 0.798. It showed excellent diagnostic performance (AUC = 0.773) in the validation cohort.
Conclusion: The HMU nomogram for predicting SLN metastasis incorporates four variables, including tumor volume, swollen axillary lymph nodes, pathological types, and molecular subtypes. The SLN metastasis rates of intraductal carcinoma and HER2 enriched are 2.05% and 6.67%. These patients could be included in trials investigating the SLNB exemption.
Introduction
Breast cancer has the highest incidence rate among female malignant tumors, accounting for 24.2% of all new cases each year (1). Breast cancer treatment drugs are constantly evolving, as is the concept of surgery. From the initial “expanded radical treatment” to “modified radical treatment,” and to the current “breast-conserving surgery,” all of them reflect that breast cancer surgery focuses not only on effective treatment, but also on maximizing aesthetics and minimizing trauma.
SLN is the first regional lymph node from the primary tumor metastasis and the first lymph node capable of receiving lymph fluid from a specific organ and region (2). It can be used as a treatment and prognostic factor for breast cancers (3–5). Therefore, SLNB can predict the metastasis status of ALNs with a low false-negative rate, allowing more patients to avoid upper limb pain, sensory loss, and lymphedema caused by axillary lymph node dissection (6, 7). However, approximately 65%–70% of patients have suffered from unnecessary invasive axilla surgery (8, 9). This raises the question of whether we can pinpoint who might avoid SLNB.
Several studies have found a strong association between the molecular subtypes and the axillary status in breast cancer patients (10, 11). Furthermore, whether SLNB should be performed for luminal A breast cancer is still controversial (12). At the same time, the reports verified that tumor size was positively correlated with the SLN metastasis rate (13). T1 patients with small tumors and lower SLN metastasis rates (14) are more likely to be exempt from SLNB. So we enrolled 1,619 T1 breast cancer patients in this study and identified predictors for SLN metastasis in T1 breast cancers, especially the relationship between SLN metastasis and molecular subtypes.
The goal of this retrospective study was to establish a predictive model that includes tumor volume, swollen axillary lymph nodes, pathological types, and risk subtypes for SLN metastasis in T1 breast cancers. In addition, patients with a low risk of SLN metastasis could be exempt from SLNB.
Patients and Methods
Patients
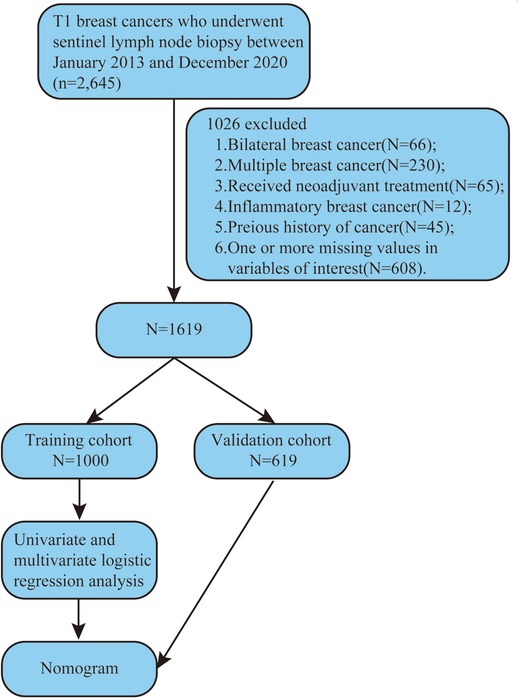
We reviewed the clinicopathologic data of breast cancer patients with SLN metastasis who underwent SLNB during surgery at Harbin Medical University Cancer Hospital between January 1, 2013 and December 31, 2020. Patients with SLN metastasis were examined by SLNB during surgery. Figure 1 depicts the selection of patients for model development.
Molecular Typing
Estrogen receptor (ER), progesterone receptor (PR) and ki67 were determined using immunohistochemistry and HER2 by immunohistochemistry or fluorescent in situ hybridization (FISH). Based on ER, PR, and HER2 status, patients were categorized into five molecular subtypes: luminal A[ER(+) and/or PR(+), HER2(−), ki67 ≤ 14%]; luminal B HER2(−)[ER(+) and/or PR(+), HER2(−), ki67>14%]; luminal B HER2(+)[ER(+) and/or PR(+), HER2(+)]; HER2 enriched [ER(−) and PR(−), HER2(+)] and triple negative[ER(−) and PR(−), HER2(−)]. Based on univariate analysis results, we regrouped molecular subtypes, and defined them as risk subtypes: low-risk subtype[HER2 enriched]; median risk subtype[Luminal B HER(+) and TNBC]; high-risk subtype[Luminal A and Luminal B HER(−) ].
Statistical Analysis
Univariate analysis was performed to detect predictors for SLN metastasis. Then, multivariate analysis, including all variables from the univariate analysis that were related to SLN status, was performed to test the factors’ independence. Statistical significance was defined as p < 0.05; odds ratio (OR) and 95% confidence intervals (CI) were also calculated. Statistical tests were two-sided, and analyses were performed using SPSS v.19.0 Software (SPSS, Chicago, IL, http://www.spss.com).
Results
Clinicopathological and Tumor Anatomical Factors of the Study Population
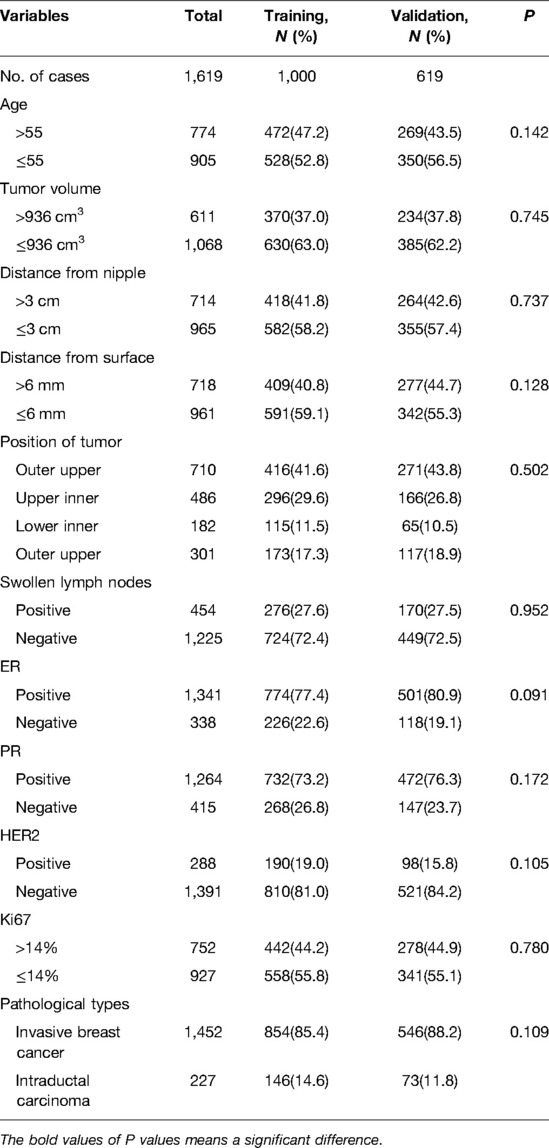
1,619 female patients with T1 breast cancer were enrolled. 1,000 patients between January 1, 2013 and April 10, 2018 were classified as a training cohort. The remaining 619 patients from April 10, 2018 to December 31, 2020 were classified as a validation cohort. The training cohort and the validation cohort were comparable in clinicopathological and tumor anatomical factors (Table 1). The median patient age was 55 years. The median tumor volume (length × width × width × 0.5) was 936 cm3. The SLN metastasis rate of the training cohort was 23.8% (n = 1,000), and that of validation was 24.4% (n = 619).
The Identification of Independent Prognostic Factors for SLN Metastasis
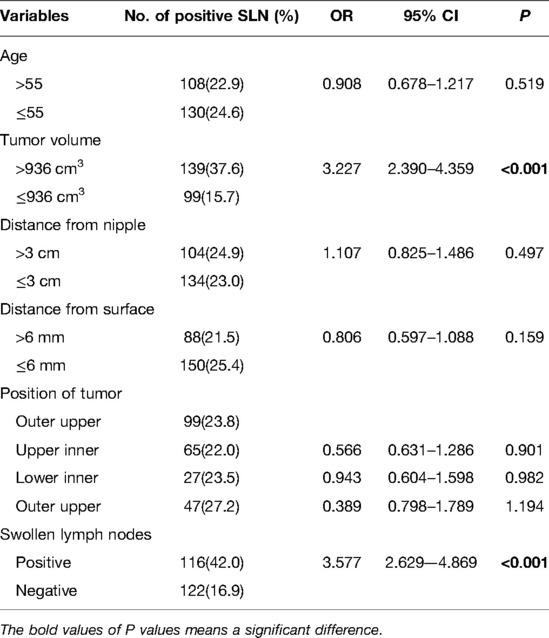
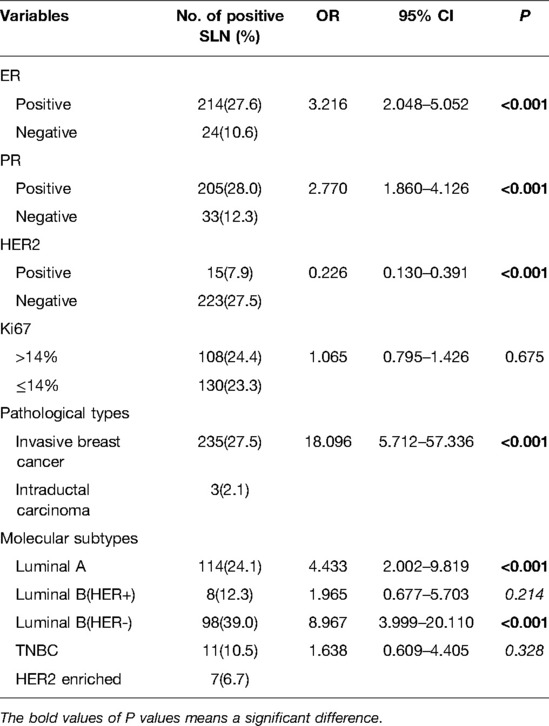
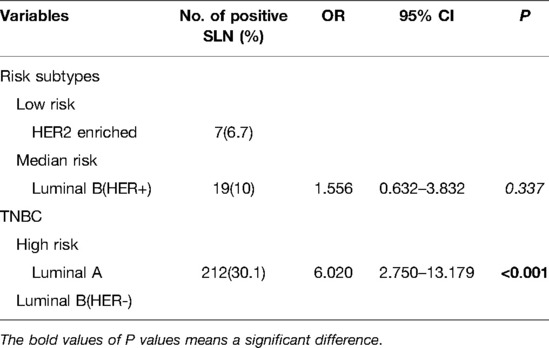
To determine the independent predictors for SLN metastasis in the training cohort, a univariate analysis was first performed. Only tumor volume and swollen axillary lymph nodes, among tumor anatomical factors, were significantly associated with SLN metastasis (Table 2). Among clinicopathological factors, ER, PR, HER2, pathological types and molecular subtypes were significantly associated with SLN metastasis (Table 3). Therefore, breast cancer patients of ER positive, PR positive, and HER2 negative are more likely to develop SLN metastasis.
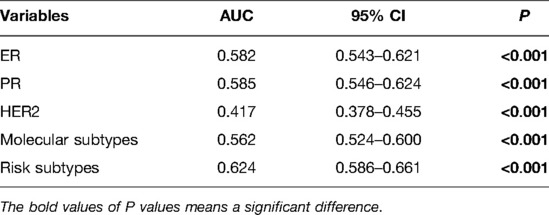
Before performing multivariate analysis, we analyzed the value of ER, PR, HER2, and molecular subtypes and compared their AUC values through Receiver-operating characteristic (ROC) analysis. The results are shown in Figure 2A and Table 4. The four variables have low AUC values. To improve their AUC, we retyped breast cancer based on the status of ER, PR, and HER2 and defined them as risk subtypes. The AUC value was 0.624 (Figure 2B and Table 4). Furthermore, the univariate analysis also showed that risk subtypes were related to SLN metastasis (Table 5).
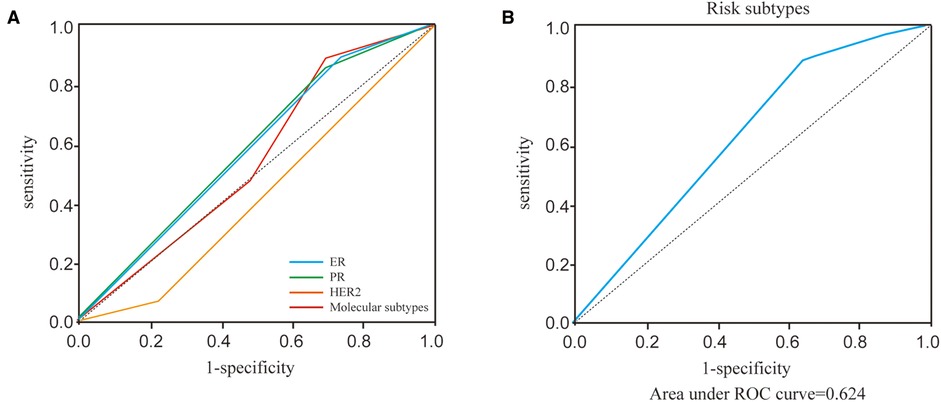
Figure 2. ROC curves of ER, PR, HER2, molecular subtypes and risk subtypes. (A) ROC curves of ER, PR, HER2, molecular subtypes. (B) ROC curves of risk subtypes.
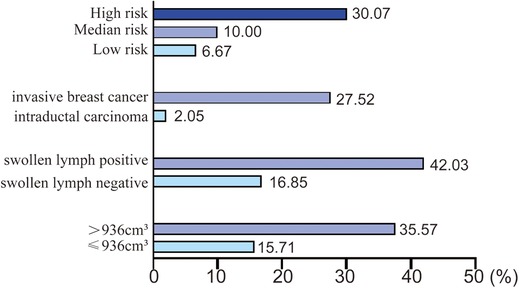
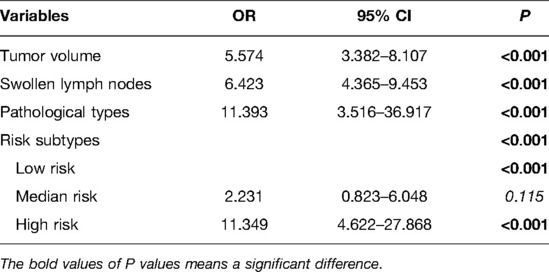
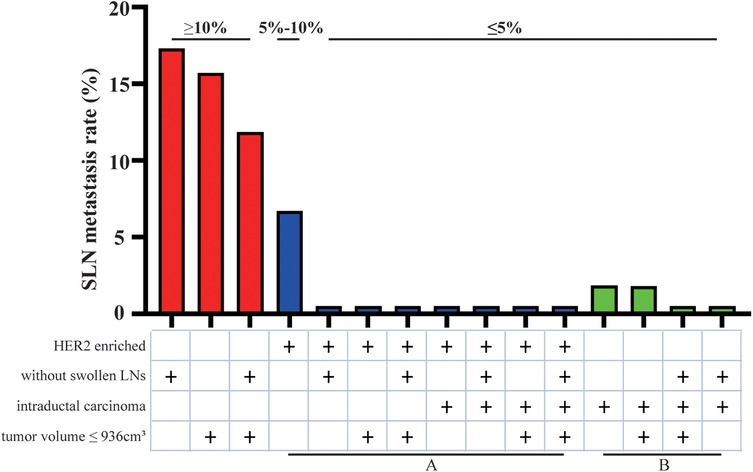
Then multivariate analysis indicated that tumor volume, swollen axillary lymph nodes and pathological types were independent statistically significant predictors for SLN metastasis (Table 6). Furthermore, luminal A and luminal B HER2 (−), as the high-risk subtypes, were also independent statistically predictors for SLN metastasis. The SLN metastasis rates of these four variables are shown in Figure 3.
Construction and Validation of the SLN Metastasis Nomogram
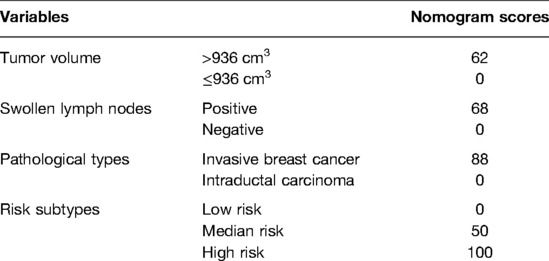
The four independent variables, including tumor volume, swollen axillary lymph nodes, pathological types, and risk subtypes, were incorporated to construct the HMU nomogram for estimating the SLN metastasis (Figure 4A). Each factor could be assigned a score by the HMU nomogram (Table 7). By summing the score of each factor together, the total score corresponded to an estimated SLN metastasis rate (Figure 4A).
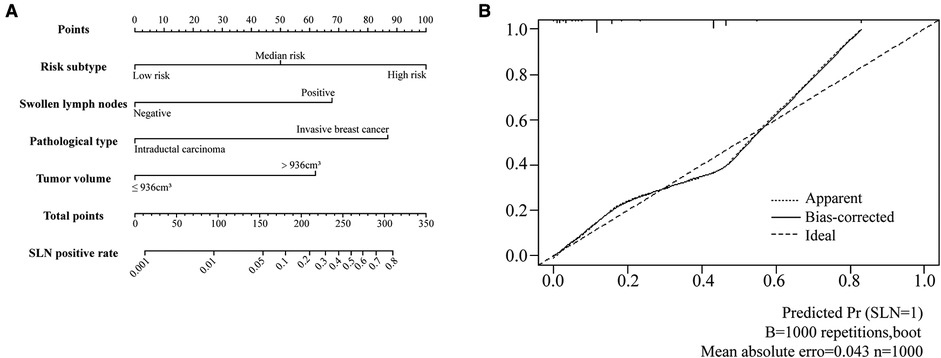
Figure 4. Nomogram to predict the probability of SLN metastases in T1 breast cancer patients and calibration plot. (A) The nomogram of SLN metastases rate. (B) The calibration plot of nomogram.
The constructed HMU nomogram was then validated internally and externally. In the training cohort, ROC analysis showed that the AUC was 0.798 (Figure 5A). When fitted into the validation cohort, the AUC of the prediction model derived from the training cohort was 0.773 (Figure 5B). The calibration curves also revealed that the predictive model could accurately match the SLN metastasis rate (Figure 4B). These results demonstrated that the predictive model performs well in SLN metastasis. For example, the SLN metastasis rate in HER-type intraductal carcinoma, with tumor volume ≤936 cm3 and without swollen axillary lymph nodes, is less than 0.1%. We believe that such patients do not require SLNB. If the tumor volume of HER2-invasive breast cancer is ≤936 cm3, there is no swollen axillary lymph node. If the SLN metastasis rate is less than 1%, the clinician may not perform SLNB after considering the patient’s wishes and clinical experience. Therefore, by calculating the patient’s SLN metastasis rate according to the above four variables incorporated into the nomogram, we could provide a reference for the patient to decide whether to perform SLNB.
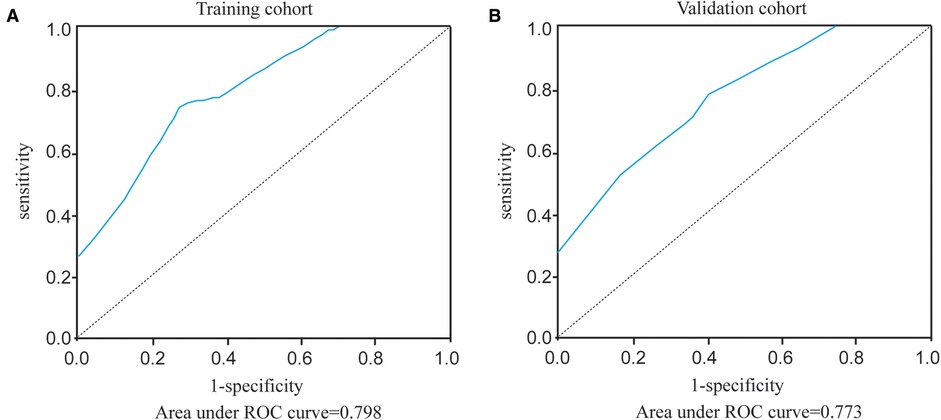
Figure 5. ROC curves of our prediction model in the training cohort and validation cohort. (A) Area under ROC curve of training cohort. (B) Area under ROC curve of validation cohort.
Patients Exempted from Sentinel Lymph Node Biopsy
According to the four variables in the HMU nomogram, we presented the SLN metastasis rate of patients with different characteristics (Figure 6). Patients with low metastasis rates are characterized by intraductal carcinoma (2.05%), low risk (6.67%), and median risk subtypes (10.00%). Therefore, those with HER2 enriched (group A) and intraductal carcinoma (group B) could be included in trials investigating the SLNB exemption. Patients with other characteristics would have lower metastasis rates, such as those with HER2 enriched associated tumor volume smaller than 936 cm3 or without axillary lymphadenopathy, so they also could be included in this study.
Discussion
The SLN metastasis is the gold standard for assessing ALN metastasis, but SLNB still has the following problems: positive SLN exemption, false negative rate, and complications after SLNB (6–9). Therefore, patients could avoid SLNB if some screening criteria can be defined to correctly assess the sentinel metastasis.
To fully evaluate the tumor size in this study, we adopted the concept of tumor volume, which took into account the tumor’s long diameter and short diameter. When the tumor volume is less than or equal to 936 cm3, the SLN metastasis rate is low (15.71%). This is consistent with previous studies that large tumors increase the risk of SLN metastasis (15–17). Swollen axillary lymph nodes are also highly suggestive of SLN metastasis (42.03%). However, some lymph node enlargement without SLN metastasis may be caused by congenital development of inflammation (18).
There is still controversy about whether SLNB should be performed in ductal carcinoma of breast cancer (19, 20). According to a meta-analysis, the incidence of SLN metastasis was 7.4 in patients with a preoperative diagnosis of intraductal carcinoma (21). Another study suggested that the only criterion for recommending SLNB in intraductal carcinoma should be any uncertainty about the presence of invasive lesions (22). Therefore, considering the risk of missed detection of microinvasion in some intraductal carcinomas and the high risk of intraductal carcinomas, we included intraductal carcinomas in the study. Intraductal carcinoma of the high-risk subtype has a tumor volume greater than 936 cm3, accompanied by swollen axillary lymph nodes, and the SLN metastasis rate is as high as 30%, so SLNB should be performed. Studies have shown that the positive rate of SLNB in patients diagnosed with intraductal carcinoma by preoperative core needle biopsy is significantly higher than that in patients diagnosed with intraductal carcinoma after surgery (23, 24). If the preoperative diagnosis of intraductal carcinoma with swollen axillary lymph nodes is associated with undetected microinvasion, core needle biopsy should be performed to confirm the status of the swollen axillary lymph nodes (25–27).
Moreover, among the five molecular types of breast cancer, luminal A and luminal B HER2(−) have the highest SLN metastasis rate (30.07%). In other words, patients with ER(+)/PR(+)/HER2(−) T1 breast cancer are more likely to develop SLN metastasis. This is also consistent with previous studies, which confirm that triple-positive breast cancer is more prone to SLN metastasis (28), and that triple-negative breast cancer has a lower SLN metastasis rate (29). Our study demonstrated that ki67 has no effect on SLN metastasis of T1 breast cancer, which is consistent with Fabinshy's finding (30). However, another study found that ki67 was positively correlated with SLN metastasis (31). T1 breast cancer may be smaller, on the other hand, so ki67 is more likely to reflect the proliferation state rather than metastasis.
According to the study on an American breast cancer patient conducted by Memorial Sloan Kettering Cancer Center (MSKCC), age, tumor size, tumor type, lymphovascular invasion, tumor location, multifocality, ER and PR were all associated with SLN metastasis (32). The nomogram’s AUC is 0.754. The Fudan University Shanghai Cancer Center in China, with an AUC value of 0.7649, included age, tumor size, tumor location, tumor type, and lymphovascular invasion (33). Two studies predicted the risk factors of SLN metastasis, but they ignored the impact of molecular subtypes on SLN metastasis. More importantly, our study focused on patients with low SLN metastasis rate. We thought that T1 breast cancer patients reduced the implementation of SLNB with less risk. The AUC value is 0.798 in the HMU nomogram, indicating that SLNB could be avoided more safely and effectively.
In conclusion, we developed and validated a nomogram for predicting SLN metastasis by adopting clinicopathological and tumor anatomical factors location from 1,000 T1 breast cancer patients. The remaining 619 T1 breast cancer patients were classified as validation cohort for external validation. The HMU nomogram provides comprehensive SLN metastasis information to optimize surgical procedures and benefit breast cancer patients. We focused on patients included in the SLNB exemption study, including intraductal carcinoma, HER2-enriched. Those with HER2-enriched and other low-risk factors may also be included in the study.
The potential limitations should be considered. First, more patients’ information from other hospitals will be more useful for validating HMU nomograms. Second, the SLNB exemption only applies to T1 breast cancer patients, and additional and refined HMU nomograms should be further studied for various types of breast cancer patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Changhong Zhao Harbin Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SX and YZ provided direction and guidance throughout the preparation of this manuscript. GL, XM analyzed and interpreted the patient, tumor, and risk factor data as well as drafted the manuscript. JZ, XZ, YC generated the figures and made significant revisions to the manuscript. HL, LZ and XZ provided patient data and clinical information. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (Grant Nos. 81872149 and 8207101096).
Conflict of Interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Nieweg OE, Tanis PJ, Kroon BB. The definition of a sentinel node. Ann Surg Oncol. (2001) 8(6):538–41. doi: 10.1007/s10434-001-0538-y
3. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. (1989) 63(1):181–7. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h
4. Silverstein MJ, Skinner KA, Lomis TJ. Predicting axillary nodal positivity in 2282 patients with breast carcinoma. World J Surg. (2001) 25(6):767–72. doi: 10.1007/s00268-001-0003-x
5. Fowble B, Solin LJ, Schultz DJ, Frequency GR, Relapse So. And outcome of regional node failures following conservative surgery and radiation for early breast cancer. Int J Radiat Oncol Biol Phys. (1989) 17(4):703–10. doi: 10.1016/0360-3016(89)90055-2
6. Cody HS 3rd. Sentinel lymph node mapping in breast cancer. Breast Cancer. (1999) 6(1):13–22. doi: 10.1007/BF02966901
7. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the nsabp B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. (2010) 102(2):111–8. doi: 10.1002/jso.21535
8. Viale G, Zurrida S, Maiorano E, Mazzarol G, Pruneri G, Paganelli G, et al. Predicting the Status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. (2005) 103(3):492–500. doi: 10.1002/cncr.20809
9. Nos C, Harding-MacKean C, Freneaux P, Trie A, Falcou MC, Sastre-Garau X, et al. Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. (2003) 90(11):1354–60. doi: 10.1002/bjs.4325
10. Nakagawa T, Huang SK, Martinez SR, Tran AN, Elashoff D, Ye X, et al. Proteomic profiling of primary breast cancer predicts axillary lymph node metastasis. Cancer Res. (2006) 66(24):11825–30. doi: 10.1158/0008-5472.CAN-06-2337
11. Zhang J, Pei J, Liu H. Clinical risk analysis of non-visualized sentinel lymph node in breast cancer. Cancer Biomark. (2018) 23(2):179–83. doi: 10.3233/CBM-170958
12. Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. (2018) 27(1):95–120. doi: 10.1016/j.soc.2017.08.005
13. Chua B, Ung O, Taylor R, Boyages J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg. (2001) 71(12):723–8. doi: 10.1046/j.1445-1433.2001.02266.x
14. Ding J, Jiang L, Wu W. Predictive value of clinicopathological characteristics for sentinel lymph node metastasis in early breast cancer. Med Sci Monit. (2017) 23:4102–8. doi: 10.12659/msm.902795
15. Atkinson EN, Brown BW, Montague ED. Tumor volume, nodal status, and metastasis in breast cancer in women. J Natl Cancer Inst. (1986) 76(2):171–8.3456058
16. Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat. (2018) 170(3):647–56. doi: 10.1007/s10549-018-4796-9
17. Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. (2005) 200(4):516–26. doi: 10.1016/j.jamcollsurg.2004.11.012
18. Dimcic M, Stevanovic O, Jakovljevic B. [Metastasis in axillary lymph nodes in breast carcinoma–possibilities of mammographic diagnosis]. Srp Arh Celok Lek. (1997) 125(3–4):124–6. Epub 1997/03/01.9221520
19. Garganese G, Fragomeni SM, Bove S, Fabbri C, D'Alba P, Chiesa F, et al. Current controversies in the treatment of ductal carcinoma in situ of the breast. Transl Cancer Res. (2017):S307–18.
20. Terribile DA, Accetta C, d’Archi S, Paris I, Di Giorgio D, Garganese G, et al. Axillary lymph node surgical treatment. Transl Cancer Res. (2017):S390–S404.
21. Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, Thompson AM. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. (2008) 95(5):547–54. doi: 10.1002/bjs.6162
22. Moore KH, Sweeney KJ, Wilson ME, Goldberg JI, Buchanan CL, Tan LK, et al. Outcomes for women with ductal carcinoma-in-situ and a positive sentinel node: a multi-institutional audit. Ann Surg Oncol. (2007) 14(10):2911–7. doi: 10.1245/s10434-007-9414-8
23. Schneider C, Trocha S, Mckinley B, Shaw J, Bielby S, Blackhurst D, et al. The use of sentinel lymph node biopsy in ductal carcinoma in situ. Am Surg. (2010) 76(9):943–6. doi: 10.1177/000313481007600925
24. van la Parra RF, Ernst MF, Barneveld PC, Broekman JM, Rutten MJ, Bosscha K. The value of sentinel lymph node biopsy in ductal carcinoma in situ (DCIS) and DCIS with microinvasion of the breast. Eur J Surg Oncol. (2008) 34(6):631–5. doi: 10.1016/j.ejso.2007.08.003
25. Klauber-Demore N, Tan LK, Liberman L, Kaptain S, Fey J, Borgen P, et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol. (2000) 7(9):636–42. doi: 10.1007/s10434-000-0636-2
26. Pendas S, Dauway E, Giuliano R, Ku N, Cox CE, Reintgen DS. Sentinel node biopsy in ductal carcinoma in situ patients. Ann Surg Oncol. (2000) 7(1):15–20. doi: 10.1007/s10434-000-0015-z
27. Kelly TA, Kim JA, Patrick R, Grundfest S, Crowe JP. Axillary lymph node metastases in patients with a final diagnosis of ductal carcinoma in situ. Am J Surg. (2003) 186(4):368–70. doi: 10.1016/S0002-9610(03)00276-9
28. Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. (2008) 8(3):249–56. doi: 10.3816/CBC.2008.n.028
29. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and Her-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. (2008) 26(14):2373–8. doi: 10.1200/JCO.2007.14.4287
30. Thangarajah F, Malter W, Hamacher S, Schmidt M, Kramer S, Mallmann P, et al. Predictors of sentinel lymph node metastases in breast cancer-radioactivity and Ki-67. Breast. (2016) 30:87–91. doi: 10.1016/j.breast.2016.09.003
31. Ozemir IA, Orhun K, Eren T, Baysal H, Sagiroglu J, Leblebici M, et al. Factors affecting sentinel lymph node metastasis in Turkish breast cancer patients: predictive value of Ki-67 and the size of lymph node. Bratisl Lek Listy. (2016) 117(8):436–41. doi: 10.4149/bll_2016_085
32. Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. (2007) 25(24):3670–9. doi: 10.1200/JCO.2006.08.8013
Keywords: T1 breast cancer, SLNB, exempting, axillary surgery, molecular subtypes
Citation: Li G, Zhao J, Zhang X, Ma X, Li H, Chen Y, Zhang L, Zhang X, Wu J, Wang X, Zhang Y and Xu S (2022) Toward Exempting from Sentinel Lymph Node Biopsy in T1 Breast Cancer Patients: A Retrospective Study. Front. Surg. 9:890554. doi: 10.3389/fsurg.2022.890554
Received: 6 March 2022; Accepted: 7 June 2022;
Published: 28 June 2022.
Edited by:
Giuseppe Campagna, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Gianni Lazzarin, Abano Terme Hospital, ItalySimona Maria Fragomeni, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Li, Zhao, Zhang, Ma, Li, Chen, Zhang, Zhang, Wu, Wang, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shouping Xu U2hvdXBpbmd4dUBocmJtdS5lZHUuY24= Yan Zhang emhhbmd0eW9AaGl0LmVkdS5jbg==
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
†These authors have contributed equally to this work and share first authorship
 Guozheng Li1†
Guozheng Li1† Jiyun Zhao
Jiyun Zhao Xingda Zhang
Xingda Zhang Hui Li
Hui Li Yihai Chen
Yihai Chen Xin Zhang
Xin Zhang Yan Zhang
Yan Zhang Shouping Xu
Shouping Xu