
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 08 August 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.890116
This article is part of the Research TopicBariatric Surgery: Its influence on the development, diagnosis and treatment of tumorsView all 5 articles
Background: Bariatric surgery is an effective method for severe obesity and its related comorbidities, in which inflammation plays a crucial role. The aim of this study was to investigate the changes of Neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein (CRP) in patients undergoing laparoscopic sleeve gastrectomy (LSG) and to explore the related factors including gender.
Methods: We retrospectively included 72 patients undergoing LSG in our hospital from 2017 to 2020. Clinical information, laboratory investigations as well as parameters derived from traditional and 2D strain echocardiography were collected. Univariate logistic model was used in myocardial performance index (MPI) and E/E′ analysis. Univariate and Multivariate logistic model were used in NLR analysis.
Results: At baseline, all patients had normal left ventricular ejection fraction (LVEF). The myocardial performance index (MPI) (OR = 1.218 (95%CI 1.040, 1.426); p = 0.0142) and E/E′ (OR = 1.364 (95%CI 1.124, 1.655); p = 0.0017) were independently associated with CRP. LSG led to a significant decrease in inflammatory markers (NLR, 2.4 ± 1.59 vs.1.7 ± 0.86; CRP, 5.6 ± 3.17 vs. 2.1 ± 2.35 mg/L, respectively, both p < 0.001),which was more in NLR among female than male (OR = 3.14 (95%CI 1.112, 8.870); p = 0.031).
Conclusions: The present study indicated a significant correlation between subclinical cardiac dysfunction and CRP among obese patients. Furthermore, female patients might benefit more from bariatric surgery on inflammation.
Obesity is one of worldwide health problems, that has nearly tripled since 1975. More than 1.9 billion adults and 39 million children under the age of 5 were overweight or obese in 2020 (1). It has also become an epidemic in China in the latest three decades (2). Overweight and obesity are independent risk factors for hypertension, cardiovascular disease, diabetes, steatohepatitis and even cancer (3). Inflammation is considered to be involved in the occurrence and the development of both obesity and obesity-associated diseases (4).
Laparoscopic sleeve gastrectomy (LSG), one of the most commonly used procedures, is considered as an effective therapeutic option for morbidly obese patients (5). Previous studies indicated LSG could significantly reduced the serum C-reactive protein (CRP) level (6). As a traditional inflammatory marker, CRP is a simple and reliable indicator and has been widely used to assess the inflammation burden (7). The baseline level of CRP could also predict the short-term postoperative infectious complications as well as postoperative mortality (8, 9). The neutrophil-to-lymphocyte ratio (NLR) is a novel inflammatory marker and has been found to be associated with incidence, morbidity, and mortality in several systemic inflammatory diseases (10). It can be used as a prognostic predictor for metabolic syndrome in healthy population (11), diabetes in morbid obesity patients (12) and 30-day outcomes in bariatric surgery patients (13). The aim of the present study was to investigate the changes of NLR and CRP following bariatric surgery and to explore correlation between them and subclinical left ventricular myocardial function as well as the related factors including gender.
We retrospectively reviewed patients undergoing LSG in our hospital from 2017 to 2020 through the electronic medical record database. The inclusion criteria were: (1) patients with complete clinical information and laboratory data before and with follow up for around 6 months after surgery; (2) patients underwent standard echocardiography and 2D strain echocardiography before surgery. Patients with acute or chronic infection, chronic systemic inflammatory disease, secondary hypertension, acute stress, organs' failure and those who were receiving the medications affecting leukocytes were excluded.
We recorded baseline patient characteristics including sex, age, height (cm), weight (kg), body mass index (BMI, kg/m2), history of hypertension, diabetes and dyslipidemia, respectively. Biochemical measurements included leukocyte count, neutrophil count, lymphocyte count, C-reactive protein (CRP), alanine amino transferase (ALT), aspartate amino transferase (AST), serum creatinine (Scr), uric acid (UA), glucose, insulin, C peptide, cholesterol, triglycerides, low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C). All above were measured in the venous blood samples obtained in the morning after an 8-hour fasting. NLR was calculated by dividing the neutrophil count by the lymphocyte count. The average of NLR was around 2.0 in 20–30 years old (14).
Transthoracic echocardiograms were performed with a frame rate of 85 Hz. Standard echocardiographic parameters, including left ventricular (LV) dimension and LV ejection fraction (LVEF), peak early (E) and late (A) mitral inflow velocities, deceleration time of early mitral flow velocity (DT), early mitral annular velocity at the septal annular site (e1′) and at the lateral annular site (e2′). E/E′ = E/((e1′ + e2′)/2). LV mass was calculated with the following formula: LV mass (g) = 0.8 × {1.04 × ((LVID + PWT + SWT)3 − (LVID)3)} + 0.6. LV internal dimension (LVID), septal (SWT) and posterior wall thickness (PWT) were measured at end diastole according to American Society of Echocardiography recommendations (15). LVMI was corrected for the body surface. In addition, LV myocardial global longitudinal strain (LV-GLS) was measured by 2D speckle tracking (Vivid 7, GE Healthcare) which through the software semi-automatically tracked the myocardial motion during the cardiac cycle. LV-GLS was averaged from apical 4-, 2- and 3-chamber views. Abnormal myocardial function was defined as LV-GLS < 18% (16). The myocardial performance index (MPI) was measured by flow Doppler images (17). The cardiac time intervals included the isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT), and ejection time (ET). MPI was defined as ((IVCT + IVRT)/ET).
Date analysis was performed using SPSS 23.0 statistical software. Continuous variables were expressed as mean ± standard deviation (normal distribution, mean ± SD) or as median (nonparametric distribution, P25, P75). Categorical variables were expressed as numbers and percentages. We checked skewness and kurtosis for normality of our data. We assessed the changes of the parameters after bariatric surgery using a paired t test for normally distributed datasets or a Wilcoxon signed- rank test for non-normally distributed datasets where applicable. Univariate and multivariate logistic analysis were performed for MPI and E/E′, as well as NLR/CRP changes from baseline. Risk associations were expressed as Odds Ratio (OR) (95% CI). For all tests, a p value <0.05 was considered to be significant. Delta values were obtained by subtracting value measured 6 months after LSG from the value at baseline.
78 patients undergoing LSG met the inclusion criteria, 6 cases were excluded due to uninterpretable echocardiographic data. The follow-up interval was 6.7 ± 1.7months. Thus, totally 72 patients were evaluated in the final study with mean age of 29 ± 9.43 years (Table 1). 47% of all patients were male (n = 34). Average BMI was 39.6 ± 6.30 kg/m2, which was higher among males (41.5 ± 6.27 vs. 37.8 ± 5.86 kg/m2, p = 0.02). Preoperative comorbidity included hypertension in 18 of the patients, diabetes in 19 and dyslipidemia in 38. Patients with hypertension or diabetes had a higher CRP level (6.95 ± 2.89 vs. 5.27 ± 3.2 mg/L, p = 0.05; 6.83 ± 2.85 vs. 5.28 ± 3.2 mg/L, p = 0.05, respectively).

Table 1. Demographic characteristics and echocardiographic variables of study population before surgery.
Echocardiographic indices were also shown in Table 1. All patients had normal LVEF prior to LSG. Median LVIDd and LVMI were 49.0 ± 3.63 mm and 75.8 ± 14.47 g/m2, respectively. 17 patients were found with MPI > 0.34 and 48 patients were found with decreased GLS, indicating subclinical impaired LV function. Male patients had a larger LV diameter, thicker LV walls and lower LV-GLS than the females (all p < 0.05). In logistic regression models, those patients with higher CRP were more likely to have higher MPI (OR = 1.218 (95%CI 1.040, 1.426); p = 0.0142) and higher E/E′ (OR = 1.364 (95%CI 1.124, 1.655); p = 0.0017) (shown in Figure 1, Supplementary Tables S1, S2).
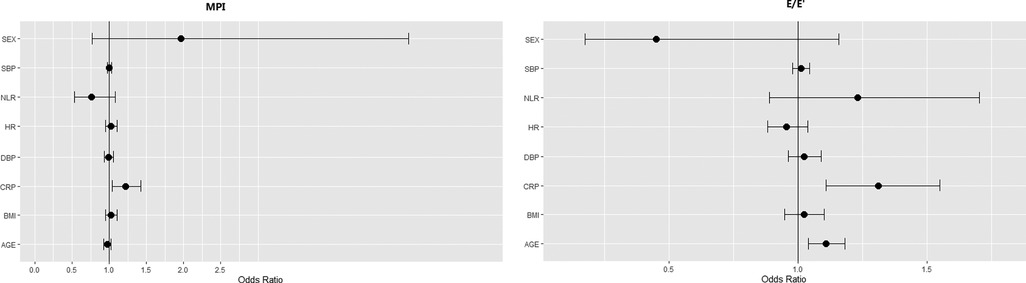
Figure 1. Effect of clinical parameters and inflammatory factors on MPI and E/E′: results of univariate analysis. In univariate logistic regression models, baseline MPI and E/E′ were used as the outcome and medians of the outcomes (MPI > 0.305 (n = 36), E/E′ > 6.5 (n = 35)) were used as the events. Risk associations were expressed as Odds Ratio (OR) (95% CI). p-values < 0.05 was considered significant. SBP, systolic blood pressure; NLR, neutrophil-to-lymphocyte ratio; HR, heart rate; DBP, diastolic blood pressure; CRP, C-reactive protein; BMI, body mass index.
The pre- and postoperative evaluation of biochemical measurements were summarized in Table 2. After LSG, all patients managed to achieve significant reduction on BMI (39.6 ± 6.30 vs. 28.9 ± 6.44 kg/m2, p < 0.0001). The blood pressure and heart rate got significantly lower. NLR and CRP had both significantly decreased (NLR: 2.4 ± 1.59 vs. 1.7 ± 0.86, CRP: 5.6 ± 3.17 vs. 2.1 ± 2.35 mg/L, respective, both p < 0.0001). There were also notable reductions in ALT, AST, UA, glucose, insulin, C peptide (all p < 0.0001). Moreover, LSG could significantly improve the lipid profile. Cholesterol, triglycerides and LDL-C significantly decreased while HDL-C significantly increased (all p < 0.05). The univariate analysis demonstrated that female might gain more reduction in NLR than male (OR = 2.81 (95%CI 1.078, 7.327); p = 0.0345). Adjusting for age, delta BMI and prevalence of hypertension, the result was similar (OR = 3.14 (95%CI 1.112, 8.870); p = 0.031) (shown in Table 3, 4). Unfortunately, there were no statistical correlations between the reduction of inflammatory marks and delta BMI, delta SBP, delta TC, delta TG and delta LDL-C. Only delta HDL was shown to be significantly correlated with CRP decrease in multivariate logistic regression analysis. (OR 0.086, (95%CI 0.008, 0.96); p = 0.046)).
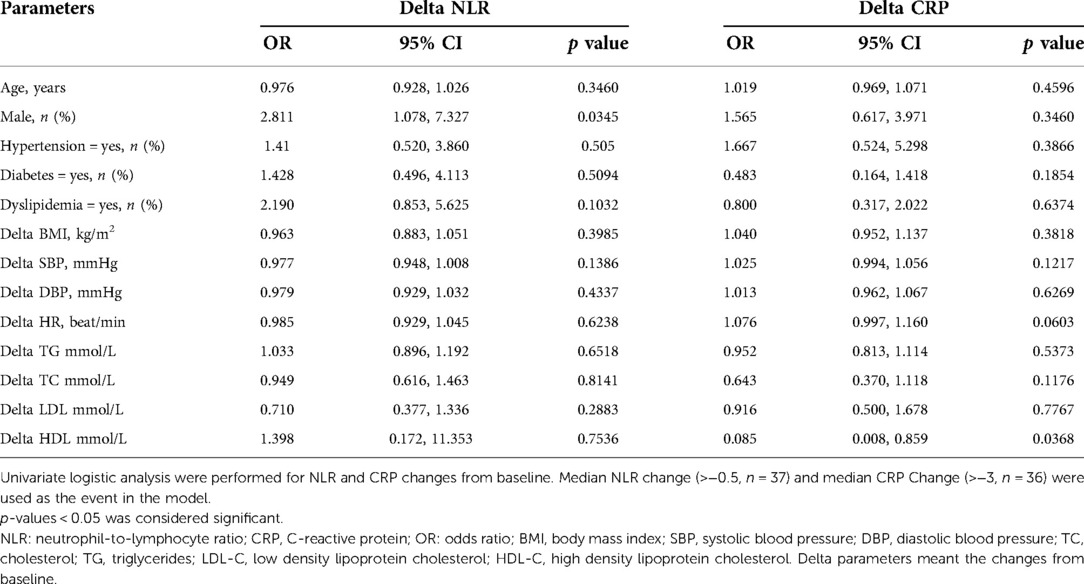
Table 3. Univariate logistic regression analysis of the association of the association between delta NLR, delta CRP and the influencing factors.
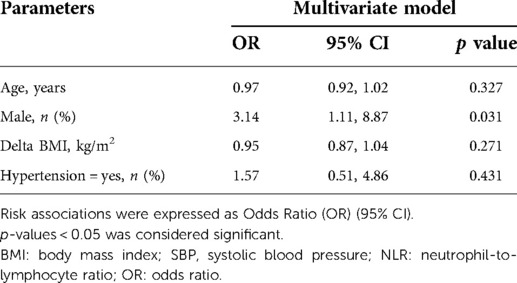
Table 4. Association between delta NLR and the related factors including gender using multivariate logistic analysis.
The present study confirmed that inflammation played a critical role in severe obesity. A great proportion of individuals with preserved LV ejection fraction had abnormal subclinical myocardial function as shown by increased MPI and decreased GLS. We indicated, for the first time, a significant correlation between MPI and CRP among obese patients. Furthermore, at 6 months post-surgery, the majority of individuals' inflammatory burden significantly improved, which was in agreement with previous studies (6). Female patients might benefit more from bariatric surgery on subclinical inflammation, that was first reported, to the best of our knowledge.
In the current study, a high proportion of patients were found with elevated NLR (44.4%) and CRP (75%) before surgery, which was consistent with an underlying inflammatory process (4). Similar to the findings of Bulur O et al. and Randell EW et al., we also found that the levels of NLR and CRP significantly reduced after LSG surgery (6, 18). As non-specific inflammatory markers, those reductions indicated the improvement of systemic inflammatory response. It may be correlated with the significant reduction of adipose tissue inflammation (19), macrophage infiltration (20) and insulin resistance (21), which could partly explain the surgery-induced remission of diabetes and hypertension (22, 23). It may also be interrelated with the resolution of nonalcoholic fatty liver disease (24). Our study confirmed many benefits of LSG towards weight loss. Blood pressure, hepatic function, glucose and all the lipid profile significantly improved after LSG. Those indicated that the role of LSG on inflammatory burden involved multiple tissues and organs.
A novel finding of our study was that the bariatric surgery-induced benefits on inflammation was gender-related. Although there were higher levels of BMI, ALT, AST as well as LV diameter and LV mass in male patients, females gained a greater reduction after surgery. This indicated that the improvement of the inflammatory state was greater in females than in males. It might contribute to the fact that the overall mortality rate and the 30-day mortality were found significantly lower in females compared to males (25). However, the mechanisms underlying the gender-related difference have not yet been clearly elucidated. As we all know, the distribution of adipose tissue differed by gender. Females tended to accrue more fat in subcutaneous prior to menopause, while males accrued more fat in visceral (26). Studies reported that bariatric surgery as well as other weight loss strategies leaded to greater loss of adipose tissue in subcutaneous than in visceral (27). The reduction of adipose tissue could decrease the secretion of inflammatory adipokines and lower the infiltration of bone marrow-derived immune cells which produce cytokines and chemokines (28). Besides, sex hormones might be one possible factor influencing inflammation (29). Before menopause, females had a stronger inflammatory response with a significant peak level of NLR in their 30s (14, 30). Bariatric surgery could mediate sex hormones (31), and thus improve the regression of polycystic ovary syndrome and promote successful pregnancy (32), which might be related to the additional reduction of inflammatory burden in females. Certainly, there was still much to learn about the role of gender and sex hormones on inflammation (33).
MPI was a reliable and reproducible doppler-derived index that reflected both LV systolic and diastolic function, which was first introduced by Tei in 1995 (17). GLS, measured by speckle tracking echocardiography, was a novel parameter to assess the subtle change in LV systolic function (34). The present study indicated, for the first time, a significant correlation between MPI, E/E′ and CRP among obese patients. Whereas no association was found between decreased LV-GLS and inflammatory markers. This result indicated that obesity-induced inflammation, which was known as a persistent and low-grade inflammatory response, was related to myocardial dysfunction, especially the diastolic dysfunction. Besides, there was accumulating evidence indicating the correlation between increased epicardial adipose tissue (EAT) and LV diastolic dysfunction (35, 36). EAT could not only mediate and induce the adverse consequences of obesity or systemic inflammation on the heart, but also was a metabolic activator which could secrete some proinflammatory adipokines resulting in atrial and ventricular fibrosis (37). Myocardial reactive fibrosis provoked by chronic inflammation was the key factor for diastolic dysfunction (38). Recent studies demonstrated that CRP was not only a inflammation marker, but also a direct participant in inflammation (39). Bock C et al. found that CRP could modulate the intracellular calcium concentration with dose-dependent (40). Cytosolic and mitochondrial calcium handling was a significant contributor to myocardial function (41). However, further research is needed to identify the predictive inflammatory markers associated with the improvement of cardiac function post-surgery.
This study inevitably had some limitations. A major limitation is its retrospective design which could not allow us to draw causal relationship between inflammation markers and cardiac function in obesity. And this was a single-center study with small sample size. Those may limit the interpretation of our results, though an effort was made to include as many perioperative variables as possible and extensively adjust for confounding factors. Only a small proportion of patients underwent echocardiography at follow-up, which couldn't be included in the dataset. Therefore, multicenter studies with larger sample size are needed to further observe and confirm the dynamic changes of inflammation markers and cardiac function.
The present study indicated a significant correlation between subclinical cardiac dysfunction and CRP among obese patients. Bariatric surgery was effective to improve the inflammation state, particularly to reduce the level of NLR among female patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QZ contributed to the acquisition, analysis of data for the work and drafting the work; PY contributed to design of the work and interpretation of data for the work and revising it critically; HS contributed to the conception of the study and revising it critically; PY contributed to the analysis and interpretation of data for the work and revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.890116/full#supplementary-material.
1. World Health Organization. Obesity and overweight (2021). Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html (Cited 10 October 2021).
2. Du X, Dai R, Zhou HX, Su ML, Lu C, Zhou ZG, et al. Bariatric surgery in China: How is this new concept going? Obes Surg. (2016) 26(12):2906–12. doi: 10.1007/s11695-016-2204-2
3. Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: Inflammation bridges the two. Curr Opin Pharmacol. (2016) 29:77–89. doi: 10.1016/j.coph.2016.07.005
4. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. (2011) 29:415–45. doi: 10.1146/annurev-immunol-031210-101322
5. Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: Baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg. (2019) 29(3):782–95. doi: 10.1007/s11695-018-3593-1
6. Randell EW, Twells LK, Gregory DM, Lester KK, Daneshtalab N, Dillon C, et al. Pre-operative and post-operative changes in CRP and other biomarkers sensitive to inflammatory status in patients with severe obesity undergoing laparoscopic sleeve gastrectomy. Clin Biochem. (2018) 52:13–9. doi: 10.1016/j.clinbiochem.2017.10.010
7. McFadyen JD, Zeller J, Potempa LA, Pietersz GA, Eisenhardt SU, Peter K. C-reactive protein and its structural isoforms: An evolutionary conserved marker and central player in inflammatory diseases and beyond. Subcell Biochem. (2020) 94:499–520. doi: 10.1007/978-3-030-41769-7_20
8. Adamina M, Steffen T, Tarantino I, Beutner U, Schmied BM, Warschkow R. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br J Surg. (2015) 102:590–8. doi: 10.1002/bjs.9756
9. Tian R, Tian M, Wang L, Qian H, Zhang S, Pang H, et al. C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: A meta-analysis. Cytokine. (2019) 117:59–64. doi: 10.1016/j.cyto.2019.02.005
10. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev Cardiovasc Ther. (2016) 14(5):573–7. doi: 10.1586/14779072.2016.1154788
11. Liu CC, Ko HJ, Liu WS, Hung CL, Hu KC, Yu LY, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine. (2019) 98(43):e17537. doi: 10.1097/MD.0000000000017537
12. Yilmaz H, Ucan B, Sayki M, Unsal I, Sahin M, Ozbek M, et al. Usefulness of the neutrophil-to-lymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr. (2015) 9(4):299–304. doi: 10.1016/j.dsx.2014.04.009
13. Da Silva M, Cleghorn MC, Elnahas A, Jackson TD, Okrainec A, Quereshy FA. Postoperative day one neutrophil-to-lymphocyte ratio as a predictor of 30-day outcomes in bariatric surgery patients. Surg Endosc. (2017) 31(6):2645–50. doi: 10.1007/s00464-016-5278-y
14. Huguet E, Maccallini G, Pardini P, Hidalgo M, Obregon S, Botto F, et al. Reference values for neutrophil to lymphocyte ratio (NLR), a biomarker of cardiovascular risk, according to age and sex in a Latin American population. Curr Probl Cardiol. Mar. (2021) 46(3):100422. doi: 10.1016/j.cpcardiol.2019.04.002
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
16. Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc Imaging. (2009) 2(1):80–4. doi: 10.1016/j.jcmg.2007.12.007
17. Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function a study in normals and dilated cardiomyopathy. J Car Diol. (1995) 26:357–66. doi: 10.1016/S0894-7317(05)80111-7
18. Bulur O, Öztürk D, Ertuğrul DT, Sayın S, Asiltürk Z, Dal K, et al. Effects of sleeve gastrectomy on neutrophil–lymphocyte ratio. J Basic Clin Physiol Pharmacol. (2021). Online ahead of print. doi: 10.1515/jbcpp-2020-0179
19. Sams VG, Blackledge C, Wijayatunga N, Barlow P, Mancini M, Mancini G, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc. (2016) 30(8):3499–504. doi: 10.1007/s00464-015-4638-3
20. Shen Y, Liu Y, Zheng SQ, Han J, Pei EL, Li ZH, et al. Effects of left gastric artery ligation versus sleeve gastrectomy on obesity-induced adipose tissue macrophage infiltration and inflammation in diet-induced obese rats. Med Sci Monit. (2019) 25:6719–26. doi: 10.12659/MSM.915532
21. Zhang C, Zhang J, Liu Z, Zhou Z. More than an anti-diabetic bariatric surgery, metabolic surgery alleviates systemic and local inflammation in obesity more than an anti-diabetic bariatric surgery, metabolic surgery alleviates systemic and local inflammation in obesity. Obes Surg. (2018) 28(11):3658–68. doi: 10.1007/s11695-018-3400-z
22. Russel SM, Valle V, Spagni G, Hamilton S, Patel T, Abdukadyrov N, et al. Physiologic mechanisms of type II diabetes mellitus remission following bariatric surgery: A meta-analysis and clinical implications. J Gastrointest Surg. (2020) 24(3):728–41. doi: 10.1007/s11605-019-04508-2
23. Schiavon CA, Bersch-Ferreira AC, Santucci EV, Oliveira JD, Torreglosa CR, Bueno PT, et al. Effects of bariatric surgery in obese patients with hypertension: The GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension). Circulation. (2018) 137(11):1132–42. doi: 10.1161/CIRCULATIONAHA.117.032130
24. Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg. (2015) 19(3):429–36. doi: 10.1007/s11605-014-2678-y
25. Beiglböck H, Mörth E, Reichardt B, Stamm T, Itariu B, Harreiter J, et al. Sex-specific differences in mortality of patients with a history of bariatric surgery: A nation-wide population-based study. Obes Surg. (2022) 32(1):8–17. doi: 10.1007/s11695-021-05763-6
26. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. (2015) 402:113–9. doi: 10.1016/j.mce.2014.11.029
27. Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: A critical review and meta-analysis. Int J Obes. (2017) 41(5):672–82. doi: 10.1038/ijo.2017.31
28. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320(3):C375–91. doi: 10.1152/ajpcell.00379.2020
29. Hatziagelaki E, Pergialiotis V, Kannenberg JM, Trakakis E, Tsiavou A, Markgraf DF, et al. Association between biomarkers of low-grade inflammation and sex hormones in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. (2020) 128(11):723–30. doi: 10.1055/a-0992-9114.31461765
30. Majnarić L T, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-Lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. Biomolecules. (2021) 11(4):528. doi: 10.3390/biom11040528
31. Emami MR, Safabakhsh M, Khorshidi M, Moradi Moghaddam O, Mohammed SH, Zarezadeh M, et al. Effect of bariatric surgery on endogenous sex hormones and sex hormone-binding globulin levels: A systematic review and meta-analysis. Surg Obes Relat Dis. (2021) 17(9):1621–36. doi: 10.1016/j.soard.2021.05.003
32. Benito E, Gómez-Martin JM, Vega-Piñero B, Priego P, Galindo J, Escobar-Morreale HF, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. (2020) 105(9):dgaa439. doi: 10.1210/clinem/dgaa439
33. Varghese M, Griffin C, Singer K. The role of sex and sex hormones in regulating obesity-induced inflammation. Adv Exp Med Biol. (2017) 1043:65–86. doi: 10.1007/978-3-319-70178-3_5
34. Shin SH, Lee YJ, Heo YS, Park SD, Kwon SW, Woo SI, et al. Beneficial effects of bariatric surgery on cardiac structure and function in obesity. Obes Surg. (2017) 27(3):620–25. doi: 10.1007/s11695-016-2330-x
35. Takahari K, Utsunomiya H, Itakura K, Yamamoto H, Nakano Y. Impact of the distribution of epicardial and visceral adipose tissue on left ventricular diastolic function. Heart Vessels. (2022) 37(2):250–61. doi: 10.1007/s00380-021-01904-0
36. Lin HH, Lee JK, Yang CY, Lien YC, Huang JW, Wu CK. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol. (2013) 30(12):127. doi: 10.1186/1475-2840-12-127
37. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. (2018) 71:2360–72. doi: 10.1016/j.jacc.2018.03.509
38. Anzai T. Inflammatory mechanisms of cardiovascular remodeling. Circ J. (2018) 82(3):629–35. doi: 10.1253/circj.CJ-18-0063
39. Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflamm Res. (2019) 68(10):815–23. doi: 10.1007/s00011-019-01269-1
40. Bock C, Vogt B, Mattecka S, Yapici G, Brunner P, Fimpel S, et al. C-reactive protein causes blood pressure drop in rabbits and induces intracellular calcium signaling. Front Immunol. (2020) 11:1978. doi: 10.3389/fimmu.2020.01978.eCollection2020
Keywords: bariatric surgery, neutrophil-to-lymphocyte ratio, C-reactive protein, myocardial performance index, global longitudinal strain
Citation: Zhou Q, Yan P, Shi H and Yan P (2022) Might female patients benefit more from bariatric surgery with respect to inflammation. Front. Surg. 9:890116. doi: 10.3389/fsurg.2022.890116
Received: 5 March 2022; Accepted: 25 July 2022;
Published: 8 August 2022.
Edited by:
Marcel André Schneider, University Hospital of Zurich, SwitzerlandReviewed by:
Paolo Bernante, University of Bologna, Italy© 2022 Yan, Zhou, Yan and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Yan eWFucGluZ19odWFzaGFuQDE2My5jb20=
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.