
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Surg. , 01 June 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.889536
This article is part of the Research Topic Technology Advancements, Social Media and Innovations in Uro-Oncology and Endourology View all 15 articles
 Weipu Mao1,2,3†
Weipu Mao1,2,3† Shuqiu Chen1†
Shuqiu Chen1† Lijie Zhang1†
Lijie Zhang1† Tao Li1†
Tao Li1† Si Sun1
Si Sun1 Bin Xu1
Bin Xu1 Weidong Zhu1
Weidong Zhu1 Guangyuan Zhang1
Guangyuan Zhang1 Lei Zhang1*
Lei Zhang1* Jianping Wu1*
Jianping Wu1* Ming Chen1,2,3*
Ming Chen1,2,3*
Background: Orthotopic neobladder reconstruction has become the preferred method of urinary diversion after radical cystectomy in major medical centers. We performed modified Y-shaped ileal orthotopic neobladder reconstruction and presented the functional results and postoperative complications of the modified surgery.
Methods: We included 21 patients with bladder cancer who underwent radical cystectomy at our center between February 2019 and December 2019. All patients underwent robotic-assisted laparoscopic radical cystectomy and lymph node dissection plus modified Y-shaped ileal orthotopic neobladder reconstruction. We collected the demographic and pathological history of the patients, and perioperative and postoperative functional outcomes and postoperative complications were recorded.
Results: All surgeries were successful and no serious postoperative complications occurred. The mean operative time was 321.43 ± 54.75 min, including 101.67 ± 10.88 min required for neobladder reconstruction. Liquid intake was encouraged about 5 days after surgery, stent and catheter were removed after 13.52 ± 3.28 days, and the patients were discharged 1–2 days after removing the catheter. No ureteral anastomotic and neobladder urethral anastomotic strictures occurred. The volume of the neobladder at 1-year post-surgery was 195.24 ± 16.07 mL and the maximum urinary flow rate was 20.64 ± 2.22 mL/s.
Conclusion: We describe the robotic-assisted modified Y-shaped ileal orthotopic neobladder reconstruction performed at our center, which requires a simple suture and short neobladder construction time, minimizes the occurrence of anastomotic stenosis, facilitates smooth patient emptying, and is clinically scalable and applicable.
According to the 2020 global cancer statistics, bladder cancer (BCa) is the 12th most common cancer with 573,278 new cases, accounting for 3.0% of all new cancers, and 212,536 deaths, approximately 2.1% of all deaths (1). As the second most common malignancy of the genitourinary system, BCa can be categorized into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). BCa also shows a high recurrence rate and aggressiveness (2, 3). Surgery is the treatment of choice for patients with BCa. Transurethral resection of bladder tumors (TURBT) combined with postoperative intravesical infusion chemotherapy is the main treatment for NMIBC patients (4, 5). For patients with MIBC or high-grade recurrent T1 and/or carcinoma in situ, radical cystectomy (RC) is the best surgical intervention (6, 7). RC can significantly improve tumor control rates and patient survival time.
Urinary diversion after RC is a core treatment for improving patient quality of life and the methods of urinary diversion include an ileal conduit (IC), orthotopic neobladder reconstruction (ONB), and cutaneous ureterostomy (CU) (8, 9). Because ONB can significantly improve patients’ long-term quality of life compared to IC and CU, ONB is now gradually becoming the preferred urinary diversion method after RC in major medical centers (10, 11). Based on the study of Massimo et al (12), our center explored robot-assisted laparoscopic RC and modified Y-shaped ileal ONB in 2019. Here, we report our initial clinical experience, surgical procedures, complications thereof, and oncologic and functional outcomes.
A total of 21 patients with bladder cancer who underwent RC at our center between February 2019 and December 2019 were included in the current study. All patients underwent preoperative pelvic magnetic resonance imaging (MRI) or enhanced computed tomography (CT), and preoperative cystoscopic biopsy and the postoperative pathological test confirmed whether it was MIBC or refractory NMIBC. Patients with locally advanced or metastatic disease, stress urinary incontinence, impaired external urethral sphincter, impaired renal function (serum creatinine > 200 μmol/L), severe hepatic impairment, severe small bowel disease, positive prostatic urethral biopsy, and psychiatric disease were excluded. The basic preoperative information of the patients is summarized in Table 1. All patients were followed up until December 2021. The study was approved by the Ethics Committee of the Southeast University Zhongda Hospital (Nanjing, China), and all patients signed written informed consent.
The surgery was performed with the patient in the steep Trendelenburg position and a five-port transperitoneal approach was used: the first 12-mm trocar was placed 5 cm above the umbilicus along with the laparoscopic camera; an 8-mm trocar was placed along the lateral aspect of the rectus abdominis muscle where the No. 1 and No. 2 robotic arms were placed; an 8-mm trocar was placed medial to the left iliac spine where the No. 3 robotic arm was placed. In addition, a 12-mm trocar was placed medial to the right iliac spine and at the midpoint of the No. 1 robotic arm and the camera hole where the assistant instruments were placed (Figure 1).
A surgeon (MC) experienced in laparoscopic and robotic-assisted surgery for kidney, prostate and bladder cancers performed all the procedures.
Cystectomy was performed according to our center’s programmed optimization protocol, in the following general order: (1) separation of the right ureter; (2) separation of the bilateral seminal vesicle glands; (3) removal of the right lymph nodes; (4) separation of the right lateral bladder ligament; (5) separation of the left ureter; (6) removal of the left lymph nodes; (7) separation of the left lateral bladder ligament; (8) separation of the retropubic bladder space; (9) opening the pelvic floor and suture ligation of the dorsal deep vein complex (DVC); (10) separation of the lateral prostatic ligament and disconnecting the urethra. After disconnection of the urethra, the prostatic urethral stump is clamped shut using a surgical clip to prevent urine spillage from the bladder. The bladder was then placed in the endobag and finally removed from the incision.
The obturator, internal iliac, external iliac and presacral lymph nodes were cleared during extended pelvic lymph node dissection. Intraoperative frozen sections were used to assess whether the ureteral stump margin and urethral stump margin were positive.
The ileocecal portion was first located and the ONB was performed starting at 20 cm from the ileocecal flap, isolating approximately 45 cm of the ileum for ONB (Figure 2A). The midpoint of the ONB ileum was marked using absorbable sutures as the ileo-urethral anastomosis zone, the ends of the ONB ileum were marked, and a catheter was inserted through the urethra and connected to the ileo-urethral anastomosis zone and then pulled to keep the bowel in tension (Figure 2B). The bowel was dissected with scissors between the ileo-urethral anastomosis zone and the mesentery (Figure 2C), and the ureteral stent was used as a ruler to separate 15 cm of the bowel along each side of the marker (Figure 2D). Subsequently, the dorsal sides of the left and right intestinal canals were sutured throughout (Figure 2E). The intestinal canal was truncated using an ultrasonic knife at 5 cm from the end of the right intestinal canal suture and 8 cm from the end of the left intestinal canal suture. The lateral anastomosis was performed using an Endo-GIA stapler to restore the continuity of the intestine and then, the mesenteric foramen was closed (Figure 2F). The intestinal wall of the ileo-urethral anastomosis area was then anastomosed clockwise to the urethral stump (Figure 2G), and the anterior wall of the anastomotic site was suspended. A catheter and single J stents were inserted, and the ventral sides of the left and right intestinal tubes were sutured successively from the ileo-urethral anastomosis area, and the anterior wall of the reservoir was sutured (Figure 2H).
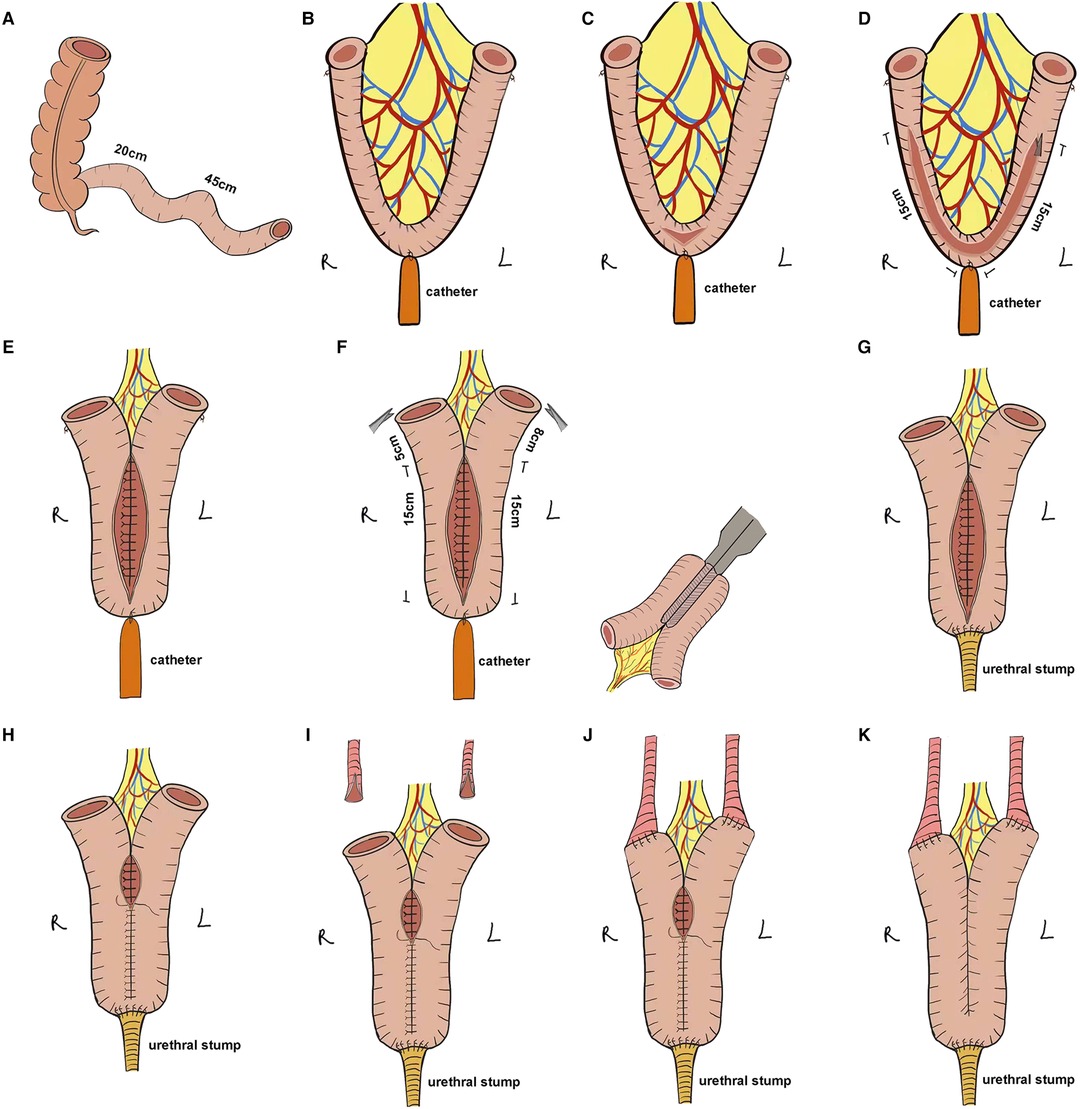
Figure 2. Stepwise configuration of the complete Y-shaped ileal neobladder. (A) Approximately 45 cm of ileum was separated for ONB. (B) The ileo-urethral anastomosis zone and the ends of the ONB ileum were marked, and the catheter was connected to the ileo-urethral anastomosis area to keep the bowel in tension. (C) The intestine between the ileo-urethral anastomosis area and the mesentery was dissected. (D) Using the ureteric stent as a ruler, 15 cm of the bowel along each side of the marker was separated. (E) The dorsal sides of the left and right intestinal canals were sutured throughout. (F) The intestinal canal was truncated at 8 cm from the end of the right intestinal canal suture and 5 cm from the end of the left intestinal canal suture, and the stapler laterally anastomosed the intestine to preserve the intestinal continuity. (G) The intestinal wall of the ileo-urethral anastomosis area was anastomosed clockwise to the urethral stump. (H) The anterior wall was sutured to the reservoir. (I) The ureter was split 1 cm at each end. (J) Wallance anastomosis was performed to suture the ends of the ureter to the Y-shaped arms on either side of the reservoir in a continuous manner. (K) The remaining anterior wall of the reservoir was anastomosed.
After splitting the ends of the ureters by 1 cm and anastomosing it with the Y-shaped arms with one stitch (Figure 2I), the single J stent was placed into the ureter up to the renal pelvis, followed by continuous suturing of the ends of the ureters to the Y-shaped arms on both sides of the reservoir using Wallance anastomosis (Figure 2J). Finally, the remaining anterior wall of the reservoir was anastomosed (Figure 2K), and the bilateral single J stent and catheter were exported from the ureter.
Perioperative and postoperative functional outcomes, postoperative complications, and postoperative voiding were evaluated in the 21 patients, and relevant imaging was performed to determine the volume of the neobladder.
Data were analyzed using SPSS software (version 24.0). Continuous variables were expressed as mean ± standard deviation.
The procedure was completed in all the patients without serious complications and conversion to open surgery. The mean operative time was (321.43 ± 54.75) min including the mean time of (101.67 ± 10.88) min required to reconstruct the neobladder. The mean intraoperative blood loss was (129.09 ± 73.55) mL, and only one patient required emergency blood transfusion. The number of intraoperative lymph node dissection was 16.10 ± 6.28, one patient had positive dissected lymph nodes (2/20), and all patients had negative soft tissue surgical margins. The postoperative histopathological staging was slightly altered from that in the preoperative period, with 8 patients being diagnosed with NMIBC (1 with cTis and 7 with cT1) and 13 patients with MIBC (10 with cT2 and 3 with cT3). During the perioperative period, patients resumed fluid intake at a mean of 5.14 ± 0.79 days, and stents and catheters were removed at 13.52 ± 3.28 days. The mean total length of hospital stay for the patients was 14.71 ± 3.20 days (Table 2).
All patients were followed up for more than 24 months, and the mean follow-up duration was (29.76 ± 3.65) months, during which no patient died. A total of eight patients developed Clavien-Dindo grade II complications after surgery, including one patient who required blood transfusion therapy, four patients with lung infections, one patient with anemia and two patients with hypoproteinemia (Table 3). Patients voided freely starting two months after surgery, and at the 1-year postoperative follow-up, the mean volume of the patients’ neobladder was 195.24 ± 16.07 mL and the maximum urinary flow rate was 20.64 ± 2.22 mL/s (Figure 3 and Supplementary Figure S1). During the follow-up period, no patient had difficulty in urination and showed recurrence of urethral tumor, neobladder urolithiasis, ureteral anastomotic stricture, and stricture of the neobladder urethral anastomosis. However, one patient developed postoperative incomplete bowel obstruction but the symptoms improved with appropriate treatment (Table 4).
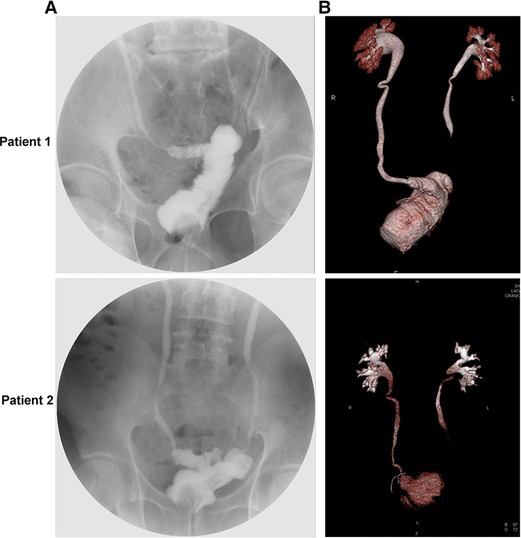
Figure 3. Cystogram and three-dimensional reconstruction images of a representative postoperative Y-shaped neobladder. (A) Postoperative cystogram image. (B) Postoperative three-dimensional reconstruction image.
In addition, postoperative daytime and night-time urinary incontinence was recorded for all patients. As shown in Table 5, it was observed that the rates of postoperative daytime and night-time incontinences were 42.86% (9/21) and 61.90% (13/21) at 3 months, 23.81% (5/21) and 38.10% (8/21) at 6 months, and 9.52% (2/21) and 19.05% (4/21) at 1 year, respectively.
In the present study, we evaluated the clinical outcomes in 21 patients who underwent modified Y-shaped ileal ONB and found that robot-assisted modified Y-shaped ileal ONB at our center showed satisfactory perioperative and postoperative functional outcomes (Table 6).

Table 6. Evaluation of the effectiveness of several robotic-assisted Y-shaped ileal orthotopic neobladder.
There are several approaches to urinary diversion after RC, depending on the clinical characteristics and surgeon’s considerations. Nevertheless, ONB has become a common choice for patients due to its similarity with the voiding mechanisms (16). Open RC was a surgical approach with a high success rate but the associated uretero-intestinal anastomosis was difficult to visualize due to high surgical difficulty and postoperative complications (17). After the report published by Gill et al. (18) laparoscopy-based RC and ONB have been further developed and matured. Robot-assisted laparoscopic radical cystectomy and neobladder reconstruction have also recently gained popularity due to the introduction of the da Vinci® SI system, and ONB reconstruction is performed at an increasing number of centers (18–20). In some medical centers, the proportion of ONB after RC has increased to 50%–90%. In 2007, Hautmann et al. (11) retrospectively summarized a total of 7,129 reports on postoperative ureteral diversion methods after RC from multiple medical centers worldwide, of which ONB accounted for 46.9%, IC accounted for 32.7%, and controlled ureteral diversion skin stoma accounted for 7.6%. However, in 2015, Hugen (21) reported that 75% of patients at the largest medical center in the United States receive ONB, with only a minority of patients (less than 15%) opting for IC.
ONB can be classified as gastric ONB, ileal ONB, ileocolonic ONB, and sigmoid ONB depending on the segment of the GI tract used (22). Due to the convenience of ileal retrieval and low pressure of the neobladder after detubularization, which is conducive to the protection of the patient’s renal function, the ileum is currently most widely used to construct the neobladder (22, 23). The Camey II ileal neobladder was a modification of Camey I ileal neobladder reconstruction (24). The Camey II ileal neobladder reconstruction method separates a 65-cm long section of the ileum, arranges the bowel in a U-shape, and performs a ureter-ileal anastomosis using the Le Due method. The Camey II ileal neobladder reconstruction achieves a low-pressure reservoir that better protects the upper urinary tract function and improves daytime urinary control. Mrini et al. (25) reported that after the Camey II procedure, patients showed a postoperative ureteral reflux rate of 6.8% and ureteral anastomotic stricture rate of 8.6%, with 64.2% of patients achieving daytime urinary control immediately post-surgery and 80% daytime urinary control at 3 months post-surgery.
In 2012, Massimo and colleagues modified the Camey II procedure by decannulating a 45-cm section of the ileum and arranging it in a vertical Y-shape (12). The advantage, therein, was that the intercepted bowel was shorter and the volume of the constructed storage bladder was relatively smaller, which, together with the Y-shaped bladder, was more conducive to postoperative bladder emptying and avoidance of residual urine, thereby reducing metabolic acidosis. Massimo found that all 237 patients who received the Camey II modification were able to empty their bladders postoperatively, with daytime and night-time urinary control rates of 93.5% and 83.9%, respectively (12).
However, the incidences of ureteral neobladder stricture, neobladder urethral stenosis, and neobladder urolithiasis in Massimo’s modified approach were 3.3%, 6.3%, and 16.0%, respectively. After exploring the causes of complications in Massimo’s Y-shaped neobladder approach, we further improved it. Firstly, we used the da Vinci robotic operating system, which utilizes the advantages of minimally invasive robotic surgery. Secondly, we used absorbable thread in suturing the neobladder instead of staples, which, too, reduced the incidence of neobladder urolithiasis. Thirdly, we split the end of the ureter and sutured it to the intestinal break of the reservoir using the Wallance anastomosis to reduce the incidence of ureteral neobladder stenosis. Finally, we reserved a longer urethral stump and the anterior wall of the anastomotic site was draped during the neobladder urethral anastomosis, which reduced the incidence of neobladder urethral stricture and improved urinary control in patients postoperatively.
To date, there were several different ileal neobladder procedures, including Studer ileal neobladder, Camey II ileal neobladder, M/W-shaped ileal neobladder, Kock ileal neobladder, Mainz ileal neobladder, and Le Bag ileal neobladder (10, 11, 26). The Y-shaped neobladder was first described by Fontana in 2004 (27), who demonstrated that the ileal Y-shaped neobladder showed good functional outcomes comparable to the mainly diffused ileal neobladder, and several subsequent studies have confirmed the feasibility of ileal Y-shaped neobladder surgery (13–15, 28, 29). Compared to other ileal neobladder procedures, our modified Y-shaped ileal neobladder requires only one bowel fold and simple suturing. Combined with the advantages of minimally invasive robotic surgery, the construction time of the neobladder is relatively short, which greatly reduces the surgery time and facilitates the patient’s postoperative recovery. The modified Y-shaped ileal neobladder requires a shorter bowel, which is more conducive to postoperative bladder emptying and avoiding residual urine and impaired renal function, and none of the patients experienced acute kidney injury. Unlike the Y-shaped neobladder described by Fontana, we used absorbable thread instead of staples in suturing the neobladder, and none of the patients developed neobladder urolithiasis postoperatively. In addition, unlike other Y-shaped neobladder approaches, we drew bilateral single J stents from the urethra, which facilitated fixation and reduced the number of perforations in the abdominal wall, which facilitated the patient’s postoperative recovery.
Although the volume of the modified Y-shaped ileal neobladder immediately after surgery was lower, after 1 year, patients achieved a volume of 200 mL and daytime and nocturnal urinary control rates of 90.5% and 81.0%, respectively. In addition, we used the Wallance anastomosis for the ureteral neobladder anastomosis, which increased the area of the ureteral ileum and greatly reduced the incidence of ureteral neobladder stenosis. Also, the sides of the Y-shaped bowel of the neobladder were connected to the bilateral ureters on both sides, the ureters did not need to be displaced again, and both sides of the intestine could play an anti-urinary reflux effect.
There were a few limitations of this study. Since this study was conducted only at our center, the number of included cases was small, and the patients were followed up for just over two years. A multicenter study with a longer follow-up period needs to be conducted. In addition, one patient in this study presented with an emergency blood transfusion. A retrospective review of the patient’s clinicopathological data revealed that the patient was older and had a large tumor size and abundant blood supply, which may have caused the transfusion and required us to develop a reasonable indication. Moreover, several medical centers do not have a robotic operating system due to gaps across the regional medical centers, which may cause differential effects that need to be evaluated with further research on this technology.
We report the clinical efficacy of a robot-assisted modified Y-shaped ileal ONB at our center. Although the follow-up period was less, preliminary results were encouraging, with a low rate of postoperative complications and better postoperative functional outcomes. This also suggested that robot-assisted laparoscopic modified Y-shaped orthotopic ileal ONB is a scalable option for ONB.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study was approved by the Ethics Committee of the Southeast University Zhongda Hospital (Nanjing, China), and all patients signed written informed consent. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MC, JW and LZ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MC, JW, LZ and WM. Acquisition of data: WM and SC. Analysis and interpretation of data: WM and SS. Drafting of the manuscript: WM, SC and LZ. Critical revision of the manuscript for important intellectual content: MC, JW, LZ, SC, BX and WZ. Statistical analysis: WM and SC. Obtaining funding: None. Administrative, technical, or material support: SS, BX and WZ. Supervision: MC and JW. Other (figure drawing and production): LZ and TL. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81672551), Jiangsu Provincial Key Research and Development Program (BE2019751), Innovative Team of Jiangsu Provincial (2017ZXKJQW07), and The National Key Research and Development Program of China (SQ2017YFSF090096).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.889536/full#supplementary-material.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) 63(2):234–41. doi: 10.1016/j.eururo.2012.07.033
3. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. (2000) 164(3 Pt 1):680–4. doi: 10.1016/S0022-5347(05)67280-1
4. Witjes JA, Babjuk M, Bellmunt J, Bruins HM, De Reijke TM, De Santis M, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-an international collaborative multistakeholder effort(dagger): under the auspices of the EAU-ESMO guidelines committees. Eur Urol. (2020) 77(2):223–50. doi: 10.1016/j.eururo.2019.09.035
5. Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. (2017) 198(3):552–9. doi: 10.1016/j.juro.2017.04.086
6. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 update. Eur Urol. (2019) 76(5):639–57. doi: 10.1016/j.eururo.2019.08.016
7. Mao W, Huang X, Wang L, Zhang Z, Liu M, Li Y, et al. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J Exp Clin Cancer Res. (2019) 38(1):169. doi: 10.1186/s13046-019-1136-9
8. Hautmann RE, Abol-Enein H, Davidsson T, Gudjonsson S, Hautmann SH, Holm HV, et al. ICUD-EAU international consultation on bladder cancer 2012: urinary diversion. Eur Urol. (2013) 63(1):67–80. doi: 10.1016/j.eururo.2012.08.050
9. Lee RK, Abol-Enein H, Artibani W, Bochner B, Dalbagni G, Daneshmand S, et al. Urinary diversion after radical cystectomy for bladder cancer: options, patient selection, and outcomes. Bju Int. (2014) 113(1):11–23. doi: 10.1111/bju.12121
10. Hautmann RE, Volkmer BG, Schumacher MC, Gschwend JE, Studer UE. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol. (2006) 24(3):305–14. doi: 10.1007/s00345-006-0105-z
11. World Health Organization Consensus Conference on Bladder C, Hautmann RE, Abol-Enein H, Hafez K, Haro I, Mansson W, et al. Urinary diversion. Urology. (2007) 69(1 Suppl):17–49. doi: 10.1016/j.urology.2006.05.058.17280907
12. Porena M, Mearini L, Zucchi A, Zingaro MD, Mearini E, Giannantoni A. Perugia ileal neobladder: functional results and complications. World J Urol. (2012) 30(6):747–52. doi: 10.1007/s00345-012-0985-z
13. Hassan AA, Elgamal SA, Sabaa MA, Salem KA, Elmateet MS. Evaluation of direct versus non-refluxing technique and functional results in orthotopic Y-ileal neobladder after 12 years of follow up. Int J Urol. (2007) 14(4):300–4. doi: 10.1111/j.1442-2042.2006.01716.x
14. Sim A, Todenhofer T, Mischinger J, Halalsheh O, Fahmy O, Boettge J, et al. Y pouch neobladder-a simplified method of intracorporeal neobladder after robotic cystectomy. J Endourol. (2015) 29(4):387–9. doi: 10.1089/end.2014.0507
15. Hassan AA, Elbendary M, Radwan M, Abo-Farha MO, Gamil T, Azab SS, et al. Long-term evaluation of modified orthotopic Y-shaped ileal neobladder (Tanta pouch) with left retro-colic chimney. Int Urol Nephrol. (2020) 52(4):681–6. doi: 10.1007/s11255-019-02345-5
16. Helmy Aly A, Ezzat A, Hamed A. Orthotopic neobladder reconstruction after radical cystectomy in patients with a solitary functioning kidney: clinical outcome and evaluation. J Egypt Natl Canc Inst. (2011) 23(4):133–40. doi: 10.1016/j.jnci.2011.10.005
17. van Son MJ, Lock M, Peters M, van de Putte EEF, Meijer RP. Treating benign ureteroenteric strictures: 27-year experience comparing endourological techniques with open surgical approach. World J Urol. (2019) 37(6):1217–23. doi: 10.1007/s00345-018-2475-4
18. Gill IS, Kaouk JH, Meraney AM, Desai MM, Ulchaker JC, Klein EA, et al. Laparoscopic radical cystectomy and continent orthotopic ileal neobladder performed completely intracorporeally: the initial experience. J Urol. (2002) 168(1):13–8. doi: 10.1016/S0022-5347(05)64821-5
19. Desai MM, Gill IS, de Castro Abreu AL, Hosseini A, Nyberg T, Adding C, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol. (2014) 192(6):1734–40. doi: 10.1016/j.juro.2014.06.087
20. Tan WS, Sridhar A, Goldstraw M, Zacharakis E, Nathan S, Hines J, et al. Robot-assisted intracorporeal pyramid neobladder. Bju Int. (2015) 116(5):771–9. doi: 10.1111/bju.13189
21. Hugen CM, Daneshmand S. Orthotopic urinary diversion in the elderly. World J Urol. (2016) 34(1):13–8. doi: 10.1007/s00345-015-1696-z
22. Hautmann RE. Urinary diversion: ileal conduit to neobladder. J Urol. (2003) 169(3):834–42. doi: 10.1097/01.ju.0000029010.97686.eb
23. Sevin G, Soyupek S, Armagan A, Hoscan MB, Oksay T. Ileal orthotopic neobladder (modified Hautmann) via a shorter detubularized ileal segment: experience and results. Bju Int. (2004) 94(3):355–9. doi: 10.1111/j.1464-410X.2004.04933.x
24. Barre PH, Herve JM, Botto H, Camey M. Update on the camey II procedure. World J Urol. (1996) 14(1):27–8. doi: 10.1007/BF01836341
25. el Mrini M, Aboutaieb R, el Fatimi A, Sarf I, Joual A, Bennani S, et al. Detubularized U-shaped enterocystoplasty after cystoprostatectomy. Ann Urol (Paris). (1996) 30(2):85–8.8767813
26. Nagele U, Anastasiadis AG, Stenzl A, Kuczyk M. Radical cystectomy with orthotopic neobladder for invasive bladder cancer: a critical analysis of long-term oncological, functional, and quality of life results. World J Urol. (2012) 30(6):725–32. doi: 10.1007/s00345-011-0649-4
27. Fontana D, Bellina M, Fasolis G, Frea B, Scarpa RM, Mari M, et al. Y-neobladder: an easy, fast, and reliable procedure. Urology. (2004) 63(4):699–703. doi: 10.1016/j.urology.2003.11.015
28. Asimakopoulos AD, Campagna A, Gakis G, Corona Montes VE, Piechaud T, Hoepffner JL, et al. Nerve sparing, robot-assisted radical cystectomy with intracorporeal bladder substitution in the male. J Urol. (2016) 196(5):1549–57. doi: 10.1016/j.juro.2016.04.114
29. Checcucci E, Manfredi M, Sica M, Amparore D, De Cillis S, Volpi G, et al. Robot-assisted-radical-cystectomy with total intracorporeal Y neobladder: analysis of postoperative complications and functional outcomes with urodynamics findings. Eur J Surg Oncol. (2021) 48(3):694–702. doi: 10.1016/j.ejso.2021.12.014
Keywords: bladder cancer, robot-assisted laparoscopic, radical cystectomy, modified Y-shaped ileal orthotopic neobladder, urinary diversion
Citation: Mao W, Chen S, Zhang L, Li T, Sun S, Xu B, Zhu W, Zhang G, Zhang L, Wu J and Chen M (2022) Robot-Assisted Laparoscopic Radical Cystectomy and Modified Y-Shaped Ileal Orthotopic Neobladder Reconstruction. Front. Surg. 9:889536. doi: 10.3389/fsurg.2022.889536
Received: 4 March 2022; Accepted: 16 May 2022;
Published: 1 June 2022.
Edited by:
Bhaskar K. Somani, University of Southampton, United KingdomReviewed by:
Maria Chiara Sighinolfi, Santi Paolo e Carlo Hospital, ItalyCopyright © 2022 Mao, Chen, Zhang, Li, Sun, Xu, Zhu, Zhang, Zhang, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Chen bWluZ2NoZW5zZXVAMTI2LmNvbQ== Jianping Wu ZG9jdG9yd3VqaWFucGluZ0AxMjYuY29t Lei Zhang bGVpMTk4NzExQDE2My5jb20=
†These authors have contributed equally to this work
Specialty section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.