
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 19 May 2022
Sec. Pediatric Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.885188
This article is part of the Research Topic Case Reports in Pediatric Surgery 2022 View all 21 articles
 Valeria Calcaterra1,2†
Valeria Calcaterra1,2† Gloria Pelizzo3,4*†
Gloria Pelizzo3,4*† Andreana Pipolo1
Andreana Pipolo1 Giulio Montecamozzo5
Giulio Montecamozzo5 Valentina Fabiano1,3
Valentina Fabiano1,3 Roberta Grazi1
Roberta Grazi1 Patrizia Carlucci1
Patrizia Carlucci1 Gianvincenzo Zuccotti1,3
Gianvincenzo Zuccotti1,3
Neuropsychiatric symptoms are rarely described as a manifestation of hyperparathyroidism, especially in children. We describe the case of an adolescent with hypercalcemia related to and hyperfunctioning parathyroid adenoma presenting with acute neuropsychiatric symptoms. A 14-year-old-girl presented into the Emergency Service Department because of an acute onset of marked asthenia, muscle weakness with difficulty in walking, and altered mental status, which included nonsensical speech. No other neurological signs were present. Abdominal, cardiac, and thoracic examination were unremarkable. There was no recent history of trauma or infection. Family history was negative for neurologic disorders. Her past medical history was unremarkable. A head CT scan showed negative results. The laboratory work-up showed elevated levels of calcium level (14.35 mg/dl; nv 9–11 mg/dl), parathyroid hormone (PTH; 184 pg/ml; nv 3.5–36.8 pg/ml), and creatinine (1.23 mg/dl; nv 0.45–0.75 mg/dl). Sodium, potassium, chloride, thyroid function, glycemia, and insulin values were normal. Neck ultrasonography showed a solid, oval, capsulated, hypoechoic neoformation, with discrete vascularization localized to the inferior pole of the right thyroid lobe, referring to parathyroid tissue. Scintigraphy revealed a hyperfunctioning parathyroid tissue at the inferior pole of the right thyroid lobe. Massive intravenous hydration and diuretic therapy were started. The signs and symptoms of hypercalcemia improved after the initiation of therapy. The patient was submitted to right cervicotomy and muscle sparing for the removal of the adenoma of the right superior parathyroid gland. After surgery, a decrease in PTH levels (<4 pg/ml) and calcium levels (9.1 mg/dl) was recorded. During follow-up, calcium values remained stable; a progressive normalization of PTH was obtained. The oral calcium therapy was suspended after 3 months from surgery. No neuropsychiatric symptoms recurred. An evaluation of the serum calcium level is mandatory in children and adolescents with unexplained neurological signs or symptoms, and a check for hyperparathyroidism should be considered.
Hypercalcemia is an infrequent finding in children (1). Through the interplay of parathyroid, renal, and skeletal factors, the serum levels of calcium are maintained in the normal range. Parathyroid hormone (PTH), synthesized and secreted from the parathyroid glands, represents a crucial calciotropic hormone.
The pathogenic mechanisms of hypercalcemia are different and may be age specific, and many have an underlying genetic basis (2). In the differential diagnosis of hypercalcemia, two categories must be considered: PTH-dependent (parathyroid adenoma or carcinoma, familial primary hyperparathyroidism, multiple endocrine neoplasia (MEN) types I, IIa, IV, tertiary hyperparathyroidism) and PTH-independent (drugs, Addison’s disease, pheochromocytoma, malignancies, inborn errors of metabolism, tubular acidosis).
Parathyroid adenoma is responsible for 80%–85% of hyperparathyroidism (3). Primary hyperparathyroidism (PH) is less common in pediatric age than in adult age, with an incidence estimated at only 2–5 in 100,000 and without an apparent sex predilection (4). In the child or adolescent, PH is usually sporadic (65%), and it is due to a single parathyroid adenoma (80%–92%); rarely, parathyroid adenoma occurs as a part of MEN syndromes.
Parathyroid adenoma outgrowth causes the release of more PTHs and leads to dysregulation in calcium and phosphorus levels in the blood. The clinical features of hypercalcemia may be nonspecific and depend upon both the degree of hypercalcemia and the rate of onset of the elevation in the serum calcium concentration: hypercalcemia symptoms may range from an incidental asymptomatic biochemical finding to hypotonia, vomiting, constipation, abdominal pain, lethargy, anorexia, polyuria, polydipsia, poor feeding, and dehydration (1, 2, 5–7). As the calcium concentration increases, symptoms can become more severe: muscle weakness, renal failure, pancreatitis, anxiety, depression, confusion, and stupor can occur. These symptoms are rarely reported in pediatric age (5).
Here, we describe the case of an adolescent with hypercalcemia related to and hyperfunctioning parathyroid adenoma presenting with acute neuropsychiatric symptoms.
A 14-year-old-girl presented into the Emergency Service Department because of an acute onset of marked asthenia, massive muscle weakness with difficulty in walking, and altered mental status, which included nonsensical speech.
During the last month, she experienced weight loss, abdominal pain, and a lack of appetite. There was no recent history of trauma or infection. Family history was negative for neurologic disorders. Her past medical history was unremarkable.
On admission, temperature, blood pressure, heart, and respiratory rate were normal. Weight: 42 kg (−1 SDS), Height: 135 cm (−3.9 SDS), and BMI: 24.01 kg/m2 (+1 SDS). Physical examination showed marked hyposthenia with difficulty in walking, bradykinesia, and slurred speech. No other neurological signs were present. Abdominal, cardiac, and thoracic examination were unremarkable.
A contrast-enhanced head CT scan showed negative results. The initial laboratory work-up showed elevated levels of calcium level (14.35 mg/dl; normal range: 9–11 mg/dl), PTH (184 pg/ml; normal range: 3.5–36.8 pg/ml), and creatinine (1.23 mg/dl, normal range: 0.45–0.75 mg/dl). Sodium, potassium, chloride, thyroid function, glycemia, and insulin values were normal. Toxicology report was negative.
Serial ECG recordings showed a slightly shortened QT interval and EEG was normal.
Neck ultrasonography showed a solid, oval, capsulated, hypoechoic neoformation (21 × 7 mm), with discrete vascularization localized to the inferior pole of the right thyroid lobe, referring to parathyroid tissue (Figure 1). Magnetic resonance imaging (MRI) showed a solid, oval formation at the lower pole of the right thyroid lobe with early vascularization compatible with the suspicion of parathyroid neoformation (Figure 2).
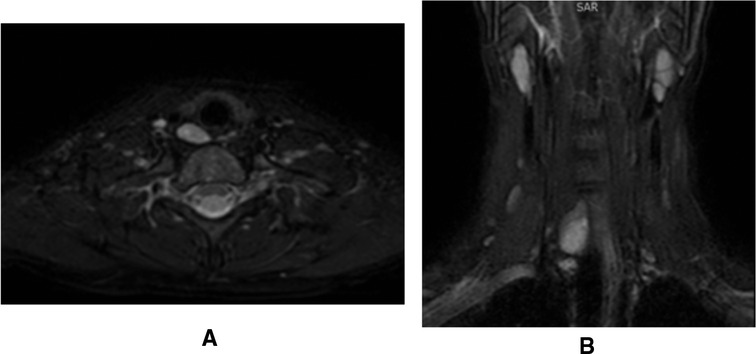
Figure 2. Parathyroid adenoma visualization on magnetic resonance imaging. (A) Transversal projection; (B) Coronal projection.
Planar scintigraphy revealed a hyperfunctioning parathyroid tissue at the inferior pole of the right thyroid lobe.
Massive hydration with intravenous fluids and diuretic therapy with furosemide were started upon admission to the Pediatric Department. The signs and symptoms of hypercalcemia rapidly improved after the initiation of therapy. During hospitalization, calcium levels improved but remained stably high.
Parathyroidectomy was finally performed. The patient was submitted to right cervicotomy and muscle sparing for the removal of a voluminous adenoma of the right superior parathyroid gland (Figure 3). Cautious blunt detachment of the pathological gland until identification of the vascular peduncle was performed. The lesion was found originating at the point of entry of the recurrent nerve into the larynx and to be adhering to the pretracheal region. Intraoperative pathologic evaluation confirmed the diagnosis of adenoma of the parathyroid with main oxyphil cell aspects.
No perioperative complications occurred, with a decrease in PTH levels (<4 pg/ml) and calcium levels (9.1 mg/dl).
The patient was discharged after 3 days from the intervention. As reported in Table 1, at discharge, calcium levels were normal and PTH levels were low; preventive calcium supplementation therapy was started upon discharge.
During follow-up, calcium values remained stable; a progressive normalization of PTH was obtained. The oral calcium therapy was suspended after 3 months from surgery.
During follow-up, no recurrence of neurological or psychiatric symptoms was noted.
The genetic make-up with the next-generation sequency-based gene panel test (NGS—Custom Panel Enrichment and Nextera Flex Enrichment) was performed to exclude the genetic forms of PH. No significant alterations in the coding sequences of the examined genes (AIP, APC, BRAF, CASR, CDC73, CDKN1B, DICER1, EPAS1, FXN, GCM2, GDNF, GNAS, GPR101, HAPB2, HRAS, KIF1B, KIT, KRAS, MAP2K5, MAX, MEN1, NF1, NRAS, PARP4, PRKAR1A, PTEN, RET, SDHA, SDHAF2, SDNB, SDNC, SDHD, TMEM127, VHL) were detected.
We reported a rare presentation of PH in an adolescent in which neurological symptoms occurred as a manifestation of hypercalcemia.
In pediatrics, PH is most commonly the presenting manifestation of a single parathyroid adenoma. PH can also be an autosomal dominant genetic disorder that is typically associated with multigland hyperplasia. Our patient had no family history of parathyroid disease or other endocrinopathies, and, consequently, the suspicion for MEN disease was low. However, sporadic MEN syndrome can still be a possibility, where de novo mutations in MEN1 are found in 10% of MEN1 patients; therefore, especially in younger age groups, genetic testing is important to exclude MEN and/or other genetic forms of hypercalcemia.
Neuropsychiatric symptoms are rarely described as a manifestation of hyperparathyroidism, especially in children. The most common symptoms are anxiety, depression, and cognitive dysfunction. More severe symptoms, including lethargy, confusion, stupor, and coma, may occur in patients with severe hypercalcemia (8); these symptoms are more likely to occur in older adults and in those with rapidly rising calcium concentrations (9). Less information is available in the literature on pediatric age. Minelli et al. (10) described the case of an adolescent with neuropsychiatric symptoms caused by hypercalcemia due to a mediastinal parathyroid adenoma. Babar et al. (11) reported the case of a 17-year-old adolescent male, who presented with an acute psychosis coinciding with severe hypercalcemia caused by a benign parathyroid adenoma. Teodoriu et al. (12) described the case of a 16-year-old adolescent girl, who presented with a disturbance of affectivity and mild memory impairment caused by hypercalcemia due to a parathyroid adenoma. As in our case, symptoms vanished after treatment, supporting the fact that neuropsychiatric symptoms are believed to represent a direct effect of hypercalcemia on the central nervous system.
The pathogenesis of neuropsychiatric symptoms is not completely understood: high calcium levels can be a catalyst for neuronal demise, possibly due to glutaminergic excitotoxicity and dopaminergic and serotonergic dysfunction (13, 14). Calcium appears to play an important role in causing changes in monoamine metabolism, determining the modification of dopaminergic and cholinergic metabolism and release at several neuroregulatory stages, and it may affect behavior and mood in some patients (15, 16). An alternate hypothesis is that the psychiatric symptoms may be related to a number of factors like premorbid adjustment and sociocultural influences (17). Also, severe hypercalcemia inhibits neuromuscular and myocardial depolarization, leading to muscle weakness and arrhythmias.
Patients with severe (calcium > 14 mg/dl) or symptomatic hypercalcemia usually require saline hydration as initial therapy, adjusted to maintain the urine output at 100 to 150 ml/h (18). Concurrent treatment with bisphosphonates with or without calcitonin may be required to treat moderate-to-severe hypercalcemia. Administration of a loop diuretic is not routinely recommended (19). Medical treatment may improve the neurological symptoms related to hypercalcemia; however, surgery remains the cornerstone of the management of PHPT.
This case shows that an evaluation of the serum calcium level is mandatory in children and adolescents with unexplained neurological signs or symptoms, and a check for hyperparathyroidism should be considered. Medical therapy and surgery has been associated with a resolution of the hypercalcemia and neuropsychological symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not provided for this study on human participants because for publication of the case reports, according to institutional requirement, the approvation of the ethics committee is not required. We obtained a written, informed consent from legal guardian of the patient for the publication of this case report. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
VC, GP, and AP conceptualized the study design and project management. VC, GP, AP, GM, VF, RG, PC, and GZ were responsible for the conceptualization and design of forms, data management and quality control, writing, and editing. VC, GP, and GZ participated in the study supervision. All authors contributed to this article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McNeilly JD, Boal R, Shaikh MG, Ahmed SF. Frequency and aetiology of hypercalcaemia. Arch Dis Child. (2016) 101(4):344–7. doi: 10.1136/archdischild-2015-309029.
2. Davies JH, Shaw NJ. Investigation and management of hypercalcaemia in children. Arch Dis Child. (2012) 97(6):533–8. doi: 10.1136/archdischild-2011-301284.
3. Colognesi A, de Tullio D, Messina F, Ferrocci G, Stano R, Azzena G. Primary hyperparathyroidism related to a parathyroid adenoma: the dramatic clinical evolution of a misdiagnosed patient and its surgical solution. Minerva Chir. (2006) 61(1):51–6. PMID: 16568023
4. Roizen J, Levine MA. Primary hyperparathyroidism in children and adolescents. J Chin Med Assoc. (2012) 75(9):425–34. doi: 10.1016/j.jcma.2012.06.012.
5. Lietman SA, Germain-Lee EL, Levine MA. Hypercalcemia in children and adolescents. Curr Opin Pediatr. (2010) 22(4):508–15. doi: 10.1097/MOP.0b013e32833b7c23.
6. Nishiyama S. Hypercalcemia in children: an overview. Acta Paediatr Jpn. (1997) 39(4):479–84. doi: 10.1111/j.1442-200x.1997.tb03624.x.
7. Stokes VJ, Nielsen MF, Hannan FM, Thakker RV. Hypercalcemic disorders in children. J Bone Miner Res. (2017) 32(11):2157–70. doi: 10.1002/jbmr.3296.
8. Shane E, Hypercalcemia ID. Pathogenesis, clinical manifestations, differential diagnosis, and management. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism, Vol. 6. Washington, DC: American Society for Bone and Mineral Research (2006).
9. Ohrvall U, Akerström G, Ljunghall S, Lundgren E, Juhlin C, Rastad J. Surgery for sporadic primary hyperparathyroidism in the elderly. World J Surg. (1994) 18(4):612–18. doi: 10.1007/BF00353779.
10. Minelli R, Meoli A, Tiri A, Fanelli U, Iannarella R, Gismondi P, et al. An atypical presentation of primary hyperparathyroidism in an adolescent: a case report of hypercalcaemia and neuropsychiatric symptoms due to a mediastinal parathyroid adenoma. Front Endocrinol. (2020) 11:581765. doi: 10.3389/fendo.2020.581765.
11. Babar G, Alemzadeh R. A case of acute psychosis in an adolescent male. Case Rep Endocrinol. (2014) 2014:937631. doi: 10.1155/2014/937631.
12. Teodoriu L, Botezatu C, Grigorovici A, Ciobanu D, Stefanescu C, Preda C. Psychiatric disorders in juvenile primary hyperparathyroidism. Case report. Bull Integr Psychiatry. (2018) 24:95.
13. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. (2010) 47(2):122–9. doi: 10.1016/j.ceca.2010.01.003.
14. Nagy L, Mangini P, Schroen C, Aziz R, Tobia A. Prolonged hypercalcemia-induced psychosis. Case Rep Psychiatry. (2020) 2020:6954036. doi: 10.1155/2020/6954036.
15. Athanassenas G, Papadopoulos E, Kourkoubas A, Tsitourides S, Gabriel J, Hoïdas S, et al. Serum calcium and magnesium levels in chronic schizophrenics. J Clin Psychopharmaco. (1983) 3(4):212–16. PMID: 6886033
16. Snyder SH. Molecular mechanism of psychotropic drug action. Proceedings of the 136th American Psychiatric Association Annual Meeting; New York, NY, USA (1983).
17. Alarcón RD, Franceschini JA. Hyperparathyroidism and paranoid psychosis. Case report and review of the literature. Br J Psychiatry. (1984) 145:477–86. doi: 10.1192/bjp.145.5.477.
18. Hosking DJ, Cowley A, Bucknall CA. Rehydration in the treatment of severe hypercalcaemia. Q J Med Autumn. (1981) 50(200):473–81. PMID: 7342172
Keywords: neuropsychiatric symptoms, hypercalemia, hyperparathyroidism, parathyroid, adenoma children, adolescents
Citation: Calcaterra V, Pelizzo G, Pipolo A, Montecamozzo G, Fabiano V, Grazi R, Carlucci P and Zuccotti G (2022) Hypercalcemia and Neurological Symptoms: A Rare Presentation of Hyperfunctioning Parathyroid Adenoma in an Adolescent. Front. Surg. 9:885188. doi: 10.3389/fsurg.2022.885188
Received: 27 February 2022; Accepted: 28 April 2022;
Published: 19 May 2022.
Edited by:
Haoyong Yu, Shanghai Sixth People's Hospital, Shanghai Jiao Tong University, ChinaReviewed by:
Heba Taher, Cairo University, EgyptCopyright © 2022 Calcaterra, Pelizzo, Pipolo, Montecamozzo, Fabiano, Grazi, Carlucci and Zuccotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gloria Pelizzo Z2xvcmlhLnBlbGl6em9AdW5pbWkuaXQ=
†These authors have contributed equally to this work and share first authorship
Specialty section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.