
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Surg. , 24 June 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.882625
This article is part of the Research Topic Anastomotic and Intestinal Wound Healing: Recent advances and future directions View all 11 articles
 F.H.M. Chaim
F.H.M. Chaim L.M.V. Negreiros
L.M.V. Negreiros K.M. Steigleder
K.M. Steigleder N.S.N. Siqueira
N.S.N. Siqueira L.M. Genaro
L.M. Genaro P.S.P. Oliveira
P.S.P. Oliveira C.A.R. Martinez
C.A.R. Martinez M.L.S. Ayrizono
M.L.S. Ayrizono J.J. Fagundes
J.J. Fagundes R.F. Leal*
R.F. Leal*
Anastomotic leakage is a major complication in gastrointestinal and colorectal surgery and its occurrence increases morbidity and mortality. Its incidence is even higher in Crohn’s disease surgeries. Several authors have identified factors involved in the pathophysiology of anastomotic leak in the literature, aiming to reduce its occurrence and, therefore, improve its surgical treatment. Surgical technique is the most discussed topic in studies on guiding the performance of side-to-side stapled anastomosis. Preoperative nutritional therapy also has been shown to reduce the risk of anastomotic leakage. Other factors remain controversial – immunomodulator use and biologic therapy, antibiotics, and gut microbiota – with studies showing a reduction in the risk of complication while other studies show no correlation. Although mesenteric adipose tissue has been related to disease recurrence, there is no evidence in the literature that it is related to a higher risk of anastomotic leakage. Further exploration on this topic is necessary, including prospective research, to support the development of techniques to prevent anastomotic leakage, in this way benefiting the inflammatory bowel disease patients who have to undergo a surgical procedure.
The inflammatory bowel disease (IBD) therapeutic arsenal has broadened in the last decades due to pharmacological advances and the development of new drugs (1). Nevertheless a considerable percentage of patients need to undergo surgical treatment one or more times during their lifetime. From 50 to 90% of Crohn’s disease (CD) patients, will require some type of surgical procedure along with medical follow-up and treatment period (2–4). The need to perform a surgical procedure is about 4 times lower (approximately 18%) among patients diagnosed with ulcerative colitis (UC) (2). And yet, the performance of surgical propaedeutic requires the implementation of procedures with greater invasiveness and risk to the patients.
Implementation of surgical treatment incurs the risk of occurrence of postoperative inherent complications, including hemorrhage, intraperitoneal collection, wound infection and dehiscence, fistulas, pulmonary complications, and thromboembolic events. Among those complications, anastomotic leakage (AL) is a major complication in gastrointestinal and colorectal surgery and its occurrence contributes significantly to the increase in morbidity and mortality (5).
The pathophysiology of CD involves the occurrence of acute complications (e.g., bleeding, bowel obstruction, perforations; severe acute colitis) and chronic complications (e.g., strictures and stenosis, internal or external fistulas, adhesions, abdominal masses) as well as disease forms refractory to pharmacological therapy. Intestinal or colon resection is the required basis of surgery in CD and the consequent need to perform an anastomosis is a common fact during surgery. Anastomosis and reoperation are intrinsically related. The realization of an anastomosis increases the risk for urgent reintervention due to AL and in long term, postoperative recurrence typically occurs at the anastomotic site (3).
The surgically related incidence of anastomotic dehiscence in the literature varies widely. In a recent observational study involving more than 36.000 subjects who submitted to surgery due to colorectal carcinoma, AL incidence was 4.1% (6). Its incidence is even higher in CD surgeries. Recent studies enrolling CD patients showed AL occurrence in 6.4% up to 14% of patients submitted to surgical treatment (7–9). Therefore, surgeons are in constant pursuit of practices to prevent AL, in this way benefiting IBD patients who have to undergo a surgical procedure.
Mesenteric adipose tissue (MAT) and its mediators are increasingly more implicated in CD pathogenesis. Recent accumulating evidence also highlights the role of creeping fat in contributing to disease recurrence, to the point that it has become a well-known feature of CD (10, 11).
There is ample work in the literature concerning the healing process of anastomosis and AL after colorectal surgery in the context of neoplastic disease. However, the information on IBD and specifically in CD surgical treatment is sparse. This article aims to discuss and summarize the main topics present in the literature and identify potential areas for future research on the subject.
Despite recent advances in gastrointestinal/colorectal surgical technique and perioperative care, anastomotic healing is still a matter of concern in CD patients submitted to surgical treatment and AL remains a major complication. Its etiology is not yet completely understood. However, it is multifactorial and not only influenced by surgery-related factors but also by factors related to the disease and its behavior.
The decision concerning anastomotic configuration depends on the surgical team’s preference, the surgeon’s experience, the availability of surgical materials (for example staplers and surgical threads), and the financial reality of each hospital.
Historically, hand-sewn anastomosis was the most common, and these produced variations related to the surgical thread and the type of stitches used. With the development and wide use of various types of staplers, even more, possible variations were added to this debate.
Side-to-side stapled anastomosis significantly reduces the incidence of short-term complications in surgical patients with CD when compared to end-to-end anastomosis (OR 0.54; 95% CI, 0.34 to 0.83). Specifically for the AL rate, side-to-side stapled anastomosis determines a decrease from 14.1 to 2% (95 percent confidence interval 1.7–22.2; p = 0.02). A reduction in mean postoperative hospital stays from 12.3 to 9.7 days (p = 0.03) was also observed (9, 12) (Figure 1A).
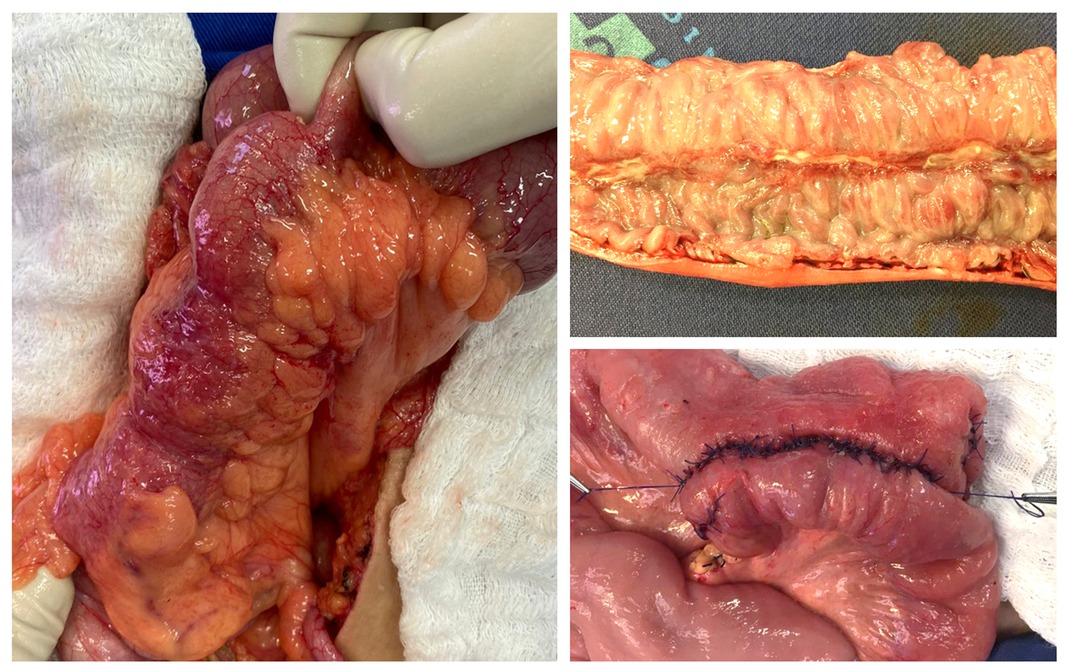
Figure 1. Aspects of an anastomosis followed by an enterectomy for Crohn’s disease (CD). (A) Surgical aspect of the ileum affected by CD. (B) Surgical specimen showing a longitudinal deep ulcer in the inflamed intestinal mucosa by the disease. (C) Side-to-side anastomosis. Source of the photographs: Colorectal Surgery Unit, Unicamp.
The technique chosen must consider the location of anastomotic performance (accessibility), the caliber of the intestinal segments (presence of edema may influence), surgical contamination, and the progress of the disease. According to the ECCO Consensus, the anastomotic diameter is an important discrimination factor that must be considered (13). Some authors suggest that for resections performed in the ileum, side-to-side anastomoses are indicated, in order to have a wider anastomotic lumen, while end-to-end anastomoses are performed in colonic segments, which have a larger caliber (14).
Due to the high recurrence rate characteristics of CD pathophysiology, repeat surgery is often required. In this sense, performing surgical procedures in CD patients with previous intestinal resection (reoperation) is an independent risk factor for AL and the number of previous resections is correlated with increased risk (8) (Figure 1B).
Recently, the laparoscopic approach has become more frequent and has become the standard of care in most situations. Robotic-assisted surgery also has been gaining acceptance in colorectal benignant and malignant surgery with intracorporeal anastomosis. Evidence on digital and robotic platform surgery applied to CD is scarce and recent, so it is still liable to selection bias. However, it seems to point to a lower occurrence of AL in patients undergoing robotic surgery (15). It has also already been verified that CD patients seem to be the most technically difficult group to apply the robotics procedures (16, 17). Despite the numerous benefits of these less invasive approaches, complications associated with stapled tissue continue to be a concern.
Considering that most CD patients will require surgical treatment over time (2–4), it becomes a persistent and recurrent dilemma in the daily practice of the surgeon: the decision between indicating an early surgery (incurring all the risks related to the surgical intervention) or continuing to try clinical treatments (at the risk of having to approach the patient later with an even more deteriorated clinical condition).
The literature is controversial regarding the optimal timing of surgery. Even the period that is taken into account to define a surgical intervention as early is inconstant, varying from 6 to 18 months after CD diagnosis (18, 19). Earlier surgical approaches would be related to the performance of technically easier procedures, smaller resections and consequently lower postoperative complications.
In a study performed by An et al., 31.3% of patients who were initially treated with drugs for ileocolonic CD required surgery within 5 years. In addition, patients in this cohort who underwent early surgery demonstrated a more benign course of the disease with fewer future surgical interventions and fewer hospitalizations (18). A prospective randomized controlled trial enrolling 134 localized ileocecal CD patients, demonstrated that both early and delayed resections are comparable in terms of their influence on the quality of life and that early resection is more cost-effective and associated with lower clinical recurrence (20).
Reliable predictors for the need for surgical interventions are yet to be established, to assist the surgical decision-making and individualize the treatment for each patient. Despite all the data that elucidate the advantages of performing early surgical interventions, the well-being of the patient should also be considered, especially in terms of the psychological aspects, such as anxiety, and also the consequences of surgeries such as the performance of stomas, the peri and postoperative risks, in addition to the possibility of CD recurrence even after surgical resection (21). Post-surgical complications such as infections, bleeding, anastomotic leakage, and mortality are questions that must be considered before choosing the intervention, in addition to factors such as the technical skills of the surgeons who will perform it. The final decision on the ideal moment of surgical therapy must be individualized for each patient, considering the characteristics of the disease, such as its phenotype, the risk factors involved in the process, and the patient’s opinion regarding the procedure (22).
The indication of surgical treatment for CD patients may occur in one of two different settings: emergent operations due to acute decompensating or life-threatening events in patients without a previous diagnosis, or elective operations which are indicated due to failure of clinical treatment. This distinction is relevant because in the latter situation, patients are in use of one or more drugs and it may influence the anastomotic healing process.
Conventional treatment has evolved to induce and maintain remission, thus avoiding complications, such as the need for surgical interventions. If this objective is not achieved, and the patient has to undergo surgical procedures, an evaluation of preoperative, perioperative, and postoperative medication uses is needed, and their implications for an increased risk of postoperative complications must be considered (23, 24).
Corticosteroids are anti-inflammatory drugs and have been widely used in the treatment of CD since 1950. Their use is only indicated for induction and not for maintenance of remission (25–28). However, they can have a negative influence, generating surgical complications and ineffective healing (29, 30). For elective surgeries, there is still no consensus regarding the recommendation to reduce the doses of corticosteroids before surgery, and in the studies that recommend doses reduction the preoperative interval varies from 3 to 6 weeks (13, 23, 31, 32).
Immunomodulators have been widely used for maintenance of remission or in conjunction with biological therapy to decrease surgical needs in CD patients. To date, studies have shown that its use does not adversely affect postoperative results (33, 34). Therefore, it is recommended to discontinue thiopurines on the day of surgery and reintroduce them along with all oral medications, if renal function remains normal. Methotrexate can be maintained pre-and post-operatively when the patient does not have an infection or renal failure (23, 35).
Biological medications, used in the treatment of various immune-mediated disorders, have revolutionized the treatment of CD. These medications are effective in containing inflammation and mucosal healing and reducing hospitalization and surgery rates (36–38). Despite the benefits established in the literature, this therapy has already been shown to be associated with an increased risk of postoperative septic complications in traditional abdominal surgeries for CD. Therefore, it is, recommended for elective surgeries to respect the longest possible interval between doses (e.g., 4 weeks for infliximab and a minimum of 2 weeks for adalimumab) (23, 39). Reintroduction is recommended approximately 3–4 weeks after definitive healing of the anastomosis (13).
Figure 2 summarizes the recommended management of immunosuppressors and biological therapy in CD patients who undergo abdominal surgery.
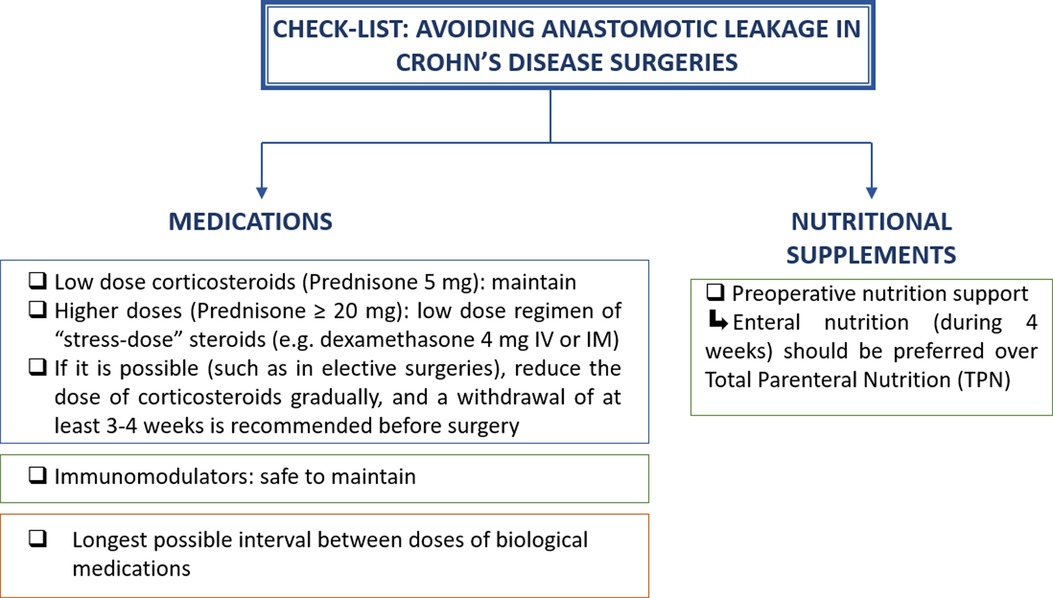
Figure 2. Check-list to guide surgeons to avoid complications in Crohn’s disease abdominal surgeries that require intestinal resection and anastomosis.
Aiming to reduce postoperative complications, surgeons have been making efforts to act beyond the surgical technique itself and control multidisciplinary perioperative issues to obtain better surgical results. In this sense, there is evidence that perioperative nutritional aspects are an important predictor of risks and complications (40).
Preoperative nutritional status is directly related to anastomotic healing since malnutrition directly interferes with collagen synthesis and fibroblast proliferation (40). Impaired preoperative nutritional status defined as anemia (hemoglobin ≤10.0 g/dL), or hypoproteinemia (albumin ≤3.2 g/dL) is significantly associated with complications (41). Preoperative hypoalbuminemia is an independent risk factor for intraabdominal septic complications after ileocolic resection (42, 43). These changes in perioperative albumin levels may reflect the severity of systemic inflammation, protein-losing enterocolopathy, malnutrition, or concurrent liver dysfunction (42).
In the postoperative period, malnutrition characterized by hypoalbuminemia can be a tool to identify patients at high risk of AL. Mean serum albumin levels on postoperative days 1 and 3 are significantly lower in patients that will present AL (44).
Even so, hypoalbuminemia is not a direct marker of preoperative nutritional status, nor is it the only biomarker that can be used for this purpose (42, 45). Several blood biomarkers, in addition to albumin, can be useful biochemical indicators to characterize malnutrition, even in the presence of chronic inflammation (e.g., pre-albumin, hemoglobin, total cholesterol, and total protein) (45). When malnutrition is detected in preoperative patients, nutritional optimization by enteral nutrition (EN) or total parenteral nutrition (TPN) is necessary to improve the surgical results reducing the overall rate of postoperative complications including AL (46). Nonetheless, further studies are needed to evaluate the best malnutrition biomarkers directly related to AL occurrence in CD surgeries.
The use of nutritional therapy (NT) has shown promise in modulating the inflammation in CD patients who required surgical resection, whether in exclusive or partial use (47, 48). Guo et al. evaluated the use of NT using exclusive EN with a polymeric formula that was infused continuously through a nasogastric tube (40). Two weeks of preoperative EN significantly increases albumin level, decreases C reactive protein, and also decreases AL incidence (48). Nutritional therapy in CD can reduce inflammation of intestinal and mesenteric fat, by reducing the expression of pro-inflammatory cytokines such as IL-1beta, IL-6, TNF-alpha, and leptin and increasing anti-inflammatory cytokines such as adiponectin (46, 49). This anti-inflammatory effect also may be able to improve wound healing ability (48).
The European Society for Parenteral and Enteral Nutrition has published guidelines addressing preoperative nutrition on IBD. Patients with severe nutritional risk (weight loss >10%–15% within six months; BMI <18.5 kg/m2; serum albumin <3.0 g/dL with no evidence of liver or kidney dysfunction) should have surgery delayed for 7–14 days whenever possible (50). This period needs to be used for EN supplementation, and if there are any contraindications such as intestinal obstructions or ileum or high output fistulas, TPN should be indicated (51, 52). Although it does not specifically address only AL, a recent and comprehensive meta-analysis enrolling 1111 CD patients showed that preoperative nutritional supplementation through the use of EN is a positive prognostic factor and significantly reduced the overall rate of postoperative complications from 73.2% to 21.9% (OR = 0.09, 95% CI, 0.06–0.13, p < 0.001), when compared to the group that received standard nutritional care. However, a consensus about differences in specific nutrition formulations and the duration of enteral nutrition is yet to be achieved (52). Although the use of TPN did not reach statistical significance, it pointed to a trend in reducing postoperative complications.
Figure 2 also summarizes the recommended management of nutrition in CD patients who undergo abdominal surgery (Figure 3).
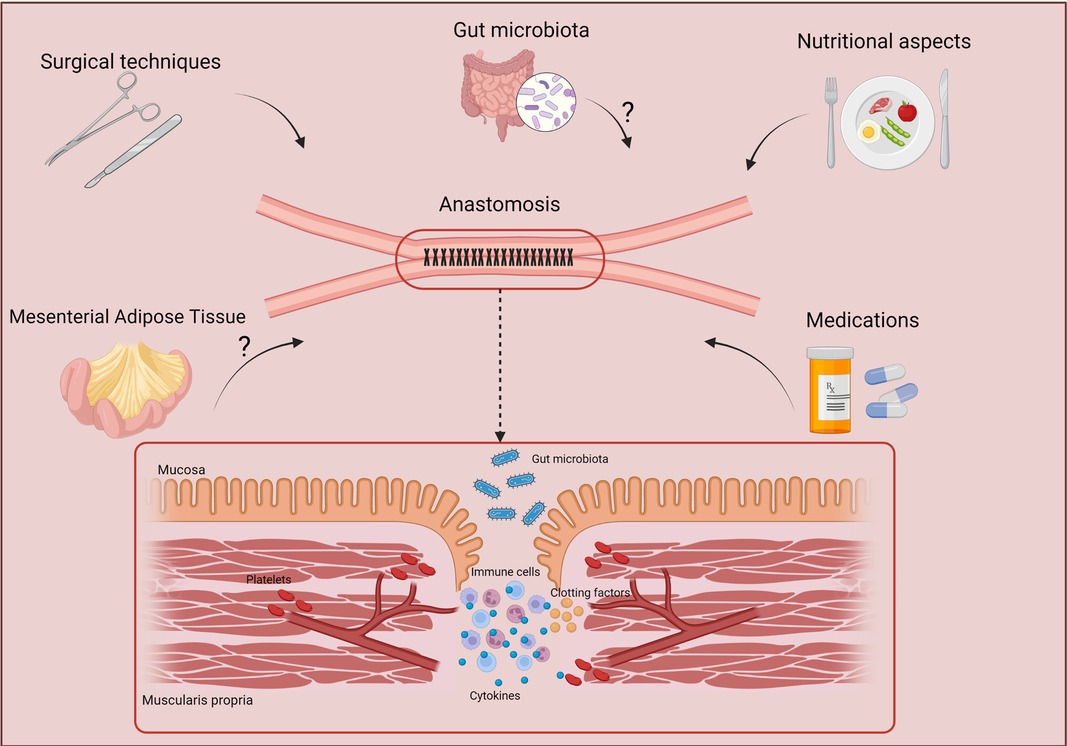
Figure 3. Factors that may influence the effective healing of anastomosis. Several factors may act directly on the correct healing of anastomoses, such as nutritional factors, intestinal microbiota, medications, and the tissues nearby the intestinal affected area such as the mesenteric adipose tissue. Created with BioRender.com.
Gut microbiota has gained extensive importance in IBD pathophysiology and, thus, intraluminal microbes may also interact and have a significant influence on the anastomotic healing and consequently the risk of AL occurrence. An impaired permeable intestinal barrier can lead to exposure of the microbiota and its metabolites to various components, therefore becoming triggers for changes in physiology and the immune responses (53, 54).
Dysbiosis, a loss of balance of the intestinal microbiota, is associated with several diseases, including CD, presenting an increase in the number of pathogenic bacteria and reducing the proportion of the beneficial ones (55, 56). Thereby, CD patients have a reduction in bacteria of the genus Firmicutes (57), and an increase in specimens of the Enterobacteriaceae family (58) in their stool, when compared with healthy individuals.
Several studies have shown a relationship between intestinal microbiota and the healing process of anastomoses. In addition, external environmental factors such as comorbidities and surgeries directly impact the microbiota, being able to select and boost colonization by species considered more aggressive (59, 60). One of the products derived from the metabolism of intestinal bacteria is butyrate, a short-chain fatty acid, which acts as a source of energy for epithelial cells while decreasing their permeability and increasing the stiffness of junctions. In experimental studies, oral and rectal administration of butyrate during the perioperative period of intestinal resection demonstrated that the performed anastomoses were firmer (61). Similarly, it was demonstrated that the oral use of pectin in the rat model led to an increase in the production of short-chain fatty acid, thus contributing to accelerating the healing of the performed anastomoses (62). Although dietary factors are implicated in CD pathophysiology, the effects of dietary interventions based on fiber substrates with pectin supplementation are uncertain (63, 64).
Studies were also performed in humans to analyze the direct influence of the microbiota on the anastomoses. Comparing the microbiota of patients who had AL with that of patients whose anastomoses healed with no sign of leakage, it has been shown that patients with AL had lower microbial diversity and abundance in the Lachnospiraceae and Bacteroidaceae families, which may be directly related to AL due to its association with mucin-degrading bacteria (65).
Despite the availability of several approaches to modulation of gut microbiota for therapeutic benefit in CD patients (e.g., removal with antibiotics, replacement and reset with specific or multiple-bacteria probiotics), results to date have not found consistent evidence for the effectiveness of probiotics in these patients for the prevention of postoperative complications. Although it has been demonstrated that probiotics improve the bacterial variety, decrease the growth of pathogenic bacteria, and enhance the intestinal barrier, a detailed study demonstrating its benefit in clinical practice still lacking (66).
In recent years, MAT has gained increasing importance in the research of CD pathophysiology. Starting from the initial observation that chronic CD patients with transmural inflammation have MAT increasing nearby the affected intestinal area, alterations were found among the numerous functions of this tissue. It at least partially justifies the variations, the severity and contributes to the understanding of the disease.
Given the numerous mesentery intraoperative features found by surgeons (e.g., signs of inflammation, mesenteric thickening, edema extending circumferentially, presence of granulomata, increased mesenteric lymphatic vessel and lymph nodes density), it was postulated that inflammatory activity would result of the convergence of inflammatory inputs coming from both the submucosa and the mesentery. As a consequence, new mesenteric excision-based surgical strategies were formulated aiming to improve postoperative outcomes (67, 68).
Results from an international, multicenter, randomized controlled trial protocol comparing mesenteric excision or conservative limited resection in small intestine CD surgery, suggest that the inclusion of the mesentery during bowel resection improves the natural history of postoperative clinical and surgical recurrence of CD (11). Similarly, in another study, the mesentery resection technique was an independent determinant of postoperative recurrence rate in ileocolic resection for CD and the adoption of mesentery resection reduced the reoperation rate from 40% to 2.9% (69). Although mesenteric excision in CD may reduce postoperative disease recurrence, there is no robust data about the occurrence of morbidities, such as AL in cases that would require a larger resection of this tissue.
The extension of mesenteric resection has been evaluated to determine the effects of a more extensive or limited excision on early postsurgical outcomes. Available data until now shows that extensive mesenteric resection is associated with a longer postoperative recurrence-free survival time (70). The involvement of MAT in CD is a fact. However, remains uncertain adequate excision extension and is still to be determined effects of MAT resection on the early postoperative period and AL rates.
A configuration of antimesenteric hand-sewn functional end-to-end anastomosis nominated “Kono-S” has been developed in 2003, based on cautious mesenteric excision, a stabilizing structure, and a wide anastomotic lumen. Since its introduction in Japan, its performance has spread around the world with cumulative evidence of favorable results. Kono-S technique is associated with a lower recurrence rate when compared to the standard hand-sewn end-to-end anastomosis (69, 71, 72). A recent meta-analysis enrolling 676 CD patients not only demonstrated a very low clinical and endoscopic recurrence rate (5% CI, 0.00–0.15) but also a small incidence of anastomotic leakage (1% CI, 0.00–0.03) (73). In addition, depending on the affected topography of the gastrointestinal tract, the surgical approach will involve the resection of specific intestinal segments and, consequently, the performance of the corresponding anastomosis. In this context, the literature data favors the Kono-S anastomosis even further when performing an ileocolic anastomosis (73, 74).
The pursuit for the ideal anastomosis (technically easy, without the need for expensive materials, with a low rate of AL and low recurrence rate) is still ongoing. Based on data available until now, it is recommended to perform side-to-side anastomosis to obtain lower rates of AL. The benefits of pharmacological therapies to CD patients are irrefutable but in the perioperative setting, they may worsen the anastomotic healing process. In this sense, it is of extreme importance that the surgical team evaluate the medications that the patient uses in the context of elective surgeries to decide on their suspension or maintenance. Moreover, preoperative nutritional therapy impacts surgical outcomes by reducing AL rates. It is yet to be established whether there is a specific biomarker endpoint to be accomplished before performing elective surgeries to get lower AL rates.
Therefore, this review aimed to contribute to a better understanding of the anastomotic healing in CD patients and to highlight the factors that directly may affect it. CD is a chronic inflammatory disease that still has no cure, and many patients need surgical interventions at least once during the course of the disease. All these factors potentially involved with anastomotic healing are important and need to be analyzed carefully to provide a better outcome and avoid complications (Figure 3).
Concerning future developments on this topic, differences in intestinal microbiota have already been found between patients who develop AL and patients who suffered no complications in the postoperative period. This may become a future therapeutic topic. Another target worth exploring in future studies is the role of MAT in this whole process. MAT’s resection demonstrably reduces disease recurrence and the need for reoperation in long-term follow-up. However, its influence on anastomotic healing and the relationship between the degree of mesenteric involvement and early postoperative complication rates in CD are two current research gaps that have yet to be addressed in the literature.
FHMC, LMVN, KMS, NSNS, LMG, and RFL were involved in drafting the manuscript. PSPO, CARM, MLSA, JJF, and RFL were involved in critical revision. All authors contributed to the article and approved the submitted version.
This work was supported by National Council for Scientific and Technological Development (CNPq) [Grant no. #302557/2021-0 for R.F.L.]. K.M.S (co-author) received a doctoral scholarship from the National Council for Scientific and Technological Development (CNPq) [Grant number #140520/2019-8], N.S.N.S. (co-author) received a master’s degree scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel, (CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil), Finance Code 001, L.M.G. (co-author) received a Doctoral scholarship from the São Paulo Research Foundation (FAPESP) [Grant number #2020/01924-5].
We thank Dr. Tristan Torriani for the English revision of our manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Na SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver. (2019) 13(6):604–16. doi: 10.5009/gnl19019
2. Fucilini LMP, Genaro LM, Sousa DCE, Coy CSR, Leal RF, Ayrizono MLS. Epidemiological profile and clinical characteristics of inflammatory bowel diseases in a brazilian referral center. Arq Gastroenterol. (2021) 58(4):483–90. doi: 10.1590/s0004-2803.202100000-87
3. Slavu I, Alecu L, Tulin A, Mihaila D, Braga V, Voiosu T, et al. Reintervention rate following emergency surgery for crohn disease. Chirurgia (Bucur). (2018) 113(2):227–33. doi: 10.21614/chirurgia.113.2.227
4. Fichera A, Schlottmann F, Krane M, Bernier G, Lange E. Role of surgery in the management of Crohn’s disease. Curr Probl Surg. (2018) 55(5):162–87. doi: 10.1067/j.cpsurg.2018.05.001
5. Group ESoCC. Predictors for anastomotic leak, postoperative complications, and mortality after right colectomy for cancer: results from an international snapshot audit. Dis Colon Rectum. (2020) 63(5):606–18. doi: 10.1097/DCR.0000000000001590
6. Sparreboom CL, van Groningen JT, Lingsma HF, Wouters MWJM, Menon AG, Kleinrensink GJ, et al. Different risk factors for early and late colorectal anastomotic leakage in a nationwide audit. Dis Colon Rectum. (2018) 61(11):1258–66. doi: 10.1097/DCR.0000000000001202
7. Baelum JK, Qvist N, Ellebaek MB. Ileorectal anastomosis in patients with Crohn’s disease. Postoperative complications and functional outcome-a systematic review. Colorectal Dis. (2021) 23(10):2501–14. doi: 10.1111/codi.15839
8. Johnston WF, Stafford C, Francone TD, Read TE, Marcello PW, Roberts PL, et al. What is the risk of anastomotic leak after repeat intestinal resection in patients with Crohn’s disease? Dis Colon Rectum. (2017) 60(12):1299–306. doi: 10.1097/DCR.0000000000000946
9. Resegotti A, Astegiano M, Farina EC, Ciccone G, Avagnina G, Giustetto A, et al. Side-to-side stapled anastomosis strongly reduces anastomotic leak rates in Crohn’s disease surgery. Dis Colon Rectum. (2005) 48(3):464–8. doi: 10.1007/s10350-004-0786-6
10. Eder P, Adler M, Dobrowolska A, Kamhieh-Milz J, Witowski J. The role of adipose tissue in the pathogenesis and therapeutic outcomes of inflammatory bowel disease. Cells. (2019) 8(6):628–46. doi: 10.3390/cells8060628
11. Li Y, Mohan H, Lan N, Wu X, Zhou W, Gong J, et al. Mesenteric excision surgery or conservative limited resection in Crohn’s disease: study protocol for an international, multicenter, randomized controlled trial. Trials. (2020) 21(1):210. doi: 10.1186/s13063-020-4105-x
12. Feng JS, Li JY, Yang Z, Chen XY, Mo JJ, Li SH. Stapled side-to-side anastomosis might be benefit in intestinal resection for Crohn’s disease: a systematic review and network meta-analysis. Medicine (Baltimore). (2018) 97(15):e0315. doi: 10.1097/MD.0000000000010315
13. Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J Crohns Colitis. (2018) 12(1):1–16. doi: 10.1093/ecco-jcc/jjx061
14. Diseases BSGoIB. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. (2010) 47(3):313–25. doi: 10.1590/S0004-28032010000300019
15. Hota S, Parascandola S, Smith S, Tampo MM, Amdur R, Obias V. Robotic and laparoscopic surgical techniques in patients with Crohn’s disease. Surg Endosc. (2021) 35(8):4602–8. doi: 10.1007/s00464-020-07885-x
16. Johnson CS, Kassir A, Marx DS, Soliman MK. Performance of da Vinci Stapler during robotic-assisted right colectomy with intracorporeal anastomosis. J Robot Surg. (2019) 13(1):115–9. doi: 10.1007/s11701-018-0828-z
17. Crippa J, Carvello M, Kotze PG, Spinelli A. Robotic surgery in inflammatory bowel disease. Curr Drug Targets. (2021) 22(1):112–6. doi: 10.2174/1389450121999200820125918
18. An V, Cohen L, Lawrence M, Thomas M, Andrews J, Moore J. Early surgery in Crohn’s disease a benefit in selected cases. World J Gastrointest Surg. (2016) 8(7):492–500. doi: 10.4240/wjgs.v8.i7.492
19. Zhu M, Feng Q, Xu X, Qiao Y, Cui Z, Yan Y, et al. Efficacy of early intervention on the bowel damage and intestinal surgery of Crohn’s disease, based on the Lémann index. BMC Gastroenterol. (2020) 20(1):421. doi: 10.1186/s12876-020-01575-7
20. Stevens TW, Haasnoot ML, D’Haens GR, Buskens CJ, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: Retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol Hepatol. (2020) 5(10):900–7. doi: 10.1016/S2468-1253(20)30117-5
21. Singh S, Al-Darmaki A, Frolkis AD, Seow CH, Leung Y, Novak KL, et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and eta-analysis of population-based studies. Gastroenterology. (2015) 149(4):928–37. doi: 10.1053/j.gastro.2015.06.001
22. Maruyama BY, Ma C, Panaccione R, Kotze PG. Early laparoscopic ileal resection for localized ileocecal Crohn’s disease: hard sell or a revolutionary new norm? Inflamm Intest Dis. (2022) 7(1):13–20. doi: 10.1159/000515959
23. Lightner AL, Shen B. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new “biological era”. Gastroenterol Rep (Oxf). (2017) 5(3):165–77. doi: 10.1093/gastro/gow046
24. Fleshner P. Expert commentary on perioperative management of biologic and immunosuppressive medications in Crohn’s disease. Dis Colon Rectum. (2018) 61(4):431–2. doi: 10.1097/DCR.0000000000001073
25. Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. (2008) (2):CD006792. doi: 10.1002/14651858.CD006792.pub2
26. Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, et al. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. (2015) (6):CD000296. doi: 10.1002/14651858.CD000296.pub4
27. Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. (2011) 106(4):590–9, quiz 600. doi: 10.1038/ajg.2011.70
28. Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. (2003) (4):CD000301. doi: 10.1002/14651858.CD000301
29. Subramanian V, Saxena S, Kang JY, Pollok RC. Preoperative steroid use and risk of postoperative complications in patients with inflammatory bowel disease undergoing abdominal surgery. Am J Gastroenterol. (2008) 103(9):2373–81. doi: 10.1111/j.1572-0241.2008.01942.x
30. Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. (1998) 11(6):277–85.10326344
31. Lightner AL. Perioperative management of biologic and immunosuppressive medications in patients with Crohn’s disease. Dis Colon Rectum. (2018) 61(4):428–31. doi: 10.1097/DCR.0000000000001072
32. Preiß JC, Bokemeyer B, Buhr HJ, Dignaß A, Häuser W, Hartmann F, et al. [Updated German clinical practice guideline on “Diagnosis and treatment of Crohn’s disease” 2014]. Z Gastroenterol. (2014) 52(12):1431–84. doi: 10.1055/s-0034-1385199
33. Colombel JF, Loftus EV, Tremaine WJ, Pemberton JH, Wolff BG, Young-Fadok T, et al. Early postoperative complications are not increased in patients with Crohn’s disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol. (2004) 99(5):878–83. doi: 10.1111/j.1572-0241.2004.04148.x
34. Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. (2003) 125(2):320–7. doi: 10.1016/S0016-5085(03)00883-7
35. Afzali A, Park CJ, Zhu K, Hu JK, Sharma P, Sinanan MN, et al. Preoperative use of ethotrexate and the risk of early postoperative complications in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2016) 22(8):1887–95. doi: 10.1097/MIB.0000000000000780
36. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. (2017) 389(10080):1741–55. doi: 10.1016/S0140-6736(16)31711-1
37. Misselwitz B, Juillerat P, Sulz MC, Siegmund B, Brand S, Swiss IBDnet aowgotSSoG. Emerging treatment options in inflammatory bowel disease: Janus kinases, stem cells, and more. Digestion. (2020) 101(Suppl 1):69–82. doi: 10.1159/000507782
38. Scott FI. Infliximab versus biosimilars for IBD: is it better to fight than switch? Dig Dis Sci. (2020) 65(8):2158–60. doi: 10.1007/s10620-020-06283-6
39. Kienle P. Impact of modern drug therapy on surgery: Crohn’s disease. Visc Med. (2018) 34(6):422–5. doi: 10.1159/000495127
40. Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. (2018) 24(21):2247–60. doi: 10.3748/wjg.v24.i21.2247
41. Zhu QL, Feng B, Lu AG, Wang ML, Hu WG, Li JW, et al. Laparoscopic low anterior resection for rectal carcinoma: Complications and management in 132 consecutive patients. World J Gastroenterol. (2010) 16(36):4605–10. doi: 10.3748/wjg.v16.i36.4605
42. Shah RS, Bachour S, Jia X, Holubar SD, Hull TL, Achkar JP, et al. Hypoalbuminaemia, not biologic exposure, is associated with postoperative complications in Crohn’s disease patients undergoing ileocolic resection. J Crohns Colitis. (2021) 15(7):1142–51. doi: 10.1093/ecco-jcc/jjaa268
43. Cambi MPC, Yamamoto T, Kotze PG. Importance of nutrition and hypoalbuminaemia in postoperative morbidity in Crohn’s disease: thinking outside of the box on biologics as single risk factors. J Crohns Colitis. (2021) 15(8):1401–2. doi: 10.1093/ecco-jcc/jjab019
44. Shimura T, Toiyama Y, Hiro J, Imaoka H, Fujikawa H, Kobayashi M, et al. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J Surg. (2018) 41(1):30–8. doi: 10.1016/j.asjsur.2016.07.009
45. Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. (2017) 9(8):829–48. doi: 10.3390/nu9080829
46. Feng Y, Li Y, Mei S, Zhang L, Gong J, Gu L, et al. Exclusive enteral nutrition ameliorates mesenteric adipose tissue alterations in patients with active Crohn’s disease. Clin Nutr. (2014) 33(5):850–8. doi: 10.1016/j.clnu.2013.10.009
47. Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori M, et al. Crohn's disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. (2019) 157(2):440–50.e8. doi: 10.1053/j.gastro.2019.04.021
48. Guo Z, Guo D, Gong J, Zhu W, Zuo L, Sun J, et al. Preoperative Nutritional Therapy Reduces the Risk of Anastomotic Leakage in Patients with Crohn’s Disease Requiring Resections. Gastroenterol Res Pract. (2016) 2016:5017856. doi: 10.1155/2016/5017856
49. Yamamoto T, Nakahigashi M, Saniabadi AR, Iwata T, Maruyama Y, Umegae S, et al. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: a prospective study. Inflamm Bowel Dis. (2007) 13(12):1493–501. doi: 10.1002/ibd.20238
50. Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. ESPEN practical guideline: clinical Nutrition in inflammatory bowel disease. Clin Nutr. (2020) 39(3):632–53. doi: 10.1016/j.clnu.2019.11.002
51. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. (2020) 14(2):155–68. doi: 10.1093/ecco-jcc/jjz187
52. Brennan GT, Ha I, Hogan C, Nguyen E, Jamal MM, Bechtold ML, et al. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn’s disease patients: a meta-analysis. Eur J Gastroenterol Hepatol. (2018) 30(9):997–1002. doi: 10.1097/MEG.0000000000001162
53. Zulian A, Cancello R, Ruocco C, Gentilini D, Di Blasio AM, Danelli P, et al. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study. PLoS One. (2013) 8(10):e78495. doi: 10.1371/journal.pone.0078495
54. Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. (2011) 3(9):559–72. doi: 10.1002/emmm.201100159
55. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. (2017) 474(11):1823–36. doi: 10.1042/BCJ20160510
56. Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. (2015) 33(2):131–6. doi: 10.1159/000369534
57. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. (2006) 55(2):205–11. doi: 10.1136/gut.2005.073817
58. Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. (2003) 52(2):237–42. doi: 10.1136/gut.52.2.237
59. Yu YN, Fang JY. Gut Microbiota and Colorectal Cancer. Gastrointest Tumors. (2015) 2(1):26–32. doi: 10.1159/000380892
60. Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. (2006) 55(Pt 5):617–24. doi: 10.1099/jmm.0.46198-0
61. Bosmans JW, Jongen AC, Boonen BT, van Rijn S, Scognamiglio F, Stucchi L, et al. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis. (2017) 32(3):305–13. doi: 10.1007/s00384-016-2718-z
62. Yamada F, Endo N, Miyatake S, Ebisu G, Hino K. Enteral feeding with low-methoxyl pectin accelerates colonic anastomosis healing in rats. Nutrition. (2018) 45:94–8. doi: 10.1016/j.nut.2017.07.013
63. Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, Parian A, Matarese LE, Bracewell K, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev. (2019) 2:CD012839. doi: 10.1002/14651858.CD012839.pub2
64. Gerasimidis K, Nichols B, McGowan M, Svolos V, Papadopoulou R, Kokkorou M, et al. The effects of commonly consumed dietary fibres on the gut microbiome and its fibre fermentative capacity in adults with inflammatory bowel disease in remission. Nutrients. (2022) 14(5):1053–67. doi: 10.3390/nu14051053
65. van Praagh JB, de Goffau MC, Bakker IS, Harmsen HJ, Olinga P, Havenga K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: a pilot study. Surg Endosc. (2016) 30(6):2259–65. doi: 10.1007/s00464-015-4508-z
66. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. (2020) 145(1):16–27. doi: 10.1016/j.jaci.2019.11.003
67. Coffey JC, O’Leary DP, Kiernan MG, Faul P. The mesentery in Crohn’s disease: friend or foe? Curr Opin Gastroenterol. (2016) 32(4):267–73. doi: 10.1097/MOG.0000000000000280
68. Peltrini R, Bucci L. “Mesentery-based surgery” to prevent surgical recurrence in Crohn’s disease: from basics to surgical practice. Int J Colorectal Dis. (2019) 34(2):353–4. doi: 10.1007/s00384-018-3197-1
69. Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, et al. Inclusion of the mesentery in ileocolic resection for crohn’s disease is associated with reduced surgical recurrence. J Crohns Colitis. (2018) 12(10):1139–50. doi: 10.1093/ecco-jcc/jjx187
70. Zhu Y, Qian W, Huang L, Xu Y, Guo Z, Cao L, et al. Role of extended mesenteric excision in postoperative recurrence of Crohn’s colitis: a single-center study. Clin Transl Gastroenterol. (2021) 12(10):e00407. doi: 10.14309/ctg.0000000000000407
71. Alshantti A, Hind D, Hancock L, Brown SR. The role of Kono-S anastomosis and mesenteric resection in reducing recurrence after surgery for Crohn’s disease: a systematic review. Colorectal Dis. (2021) 23(1):7–17. doi: 10.1111/codi.15136
72. Kono T, Fichera A. Surgical treatment for Crohn’s disease: a role of Kono-S Anastomosis in the West. Clin Colon Rectal Surg. (2020) 33(6):335–43. doi: 10.1055/s-0040-1714236
73. Ng CH, Chin YH, Lin SY, Koh JWH, Lieske B, Koh FH, et al. Kono-S anastomosis for Crohn’s disease: a systemic review, meta-analysis, and meta-regression. Surg Today. (2021) 51(4):493–501. doi: 10.1007/s00595-020-02130-3
Keywords: anastomotic healing, inflammatory bowel disease, surgical complications, Crohn’s disease, anastomosis, mesenteric adipose tissue, postoperative complications/prevention & control, suture techniques
Citation: Chaim F, Negreiros L, Steigleder K, Siqueira N, Genaro L, Oliveira P, Martinez C, Ayrizono M, Fagundes J and Leal R (2022) Aspects Towards the Anastomotic Healing in Crohn’s Disease: Clinical Approach and Current Gaps in Research. Front. Surg. 9:882625. doi: 10.3389/fsurg.2022.882625
Received: 24 February 2022; Accepted: 6 June 2022;
Published: 24 June 2022.
Edited by:
Sven Flemming, University Hospital of Wuerzburg, GermanyReviewed by:
Matthias Kelm, University Hospital Würzburg, GermanyCopyright © 2022 Chaim, Negreiros, Steigleder, Siqueira, Genaro, Oliveira, Martinez, Ayrizono, Fagundes and Leal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:Raquel Franco Leal cmFmcmFuY28udW5pY2FtcEBnbWFpbC5jb20=
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.