- 1Department of General, Visceral and Transplant Surgery, Ludwig-Maximilians-University Munich, Munich, Germany
- 2Institute for Surgical Research Oberbayern, Hausham, Germany
- 3Department for General, Visceral, Endocrine and Vascular Surgery, Krankenhaus Agatharied GmbH, Hausham, Germany
Objective: Anastomotic leakage, surgical site infections, and other infectious complications are still common complications in gastrointestinal surgery. The concept of perioperative antibiotic bowel decontamination demonstrates beneficial effects in single randomized controlled trials (RCTs), but data from routine clinical use are still sparse. Our aim was to analyze the data from the routine clinical use of perioperative antibiotic bowel decontamination in gastrointestinal surgery.
Methods: Based on 20 years’ experience, we performed a retrospective analysis of all cases in oncologic gastrointestinal surgery with the use of antibiotic bowel decontamination in gastric, sigmoid, and rectal cancer. Clinical data and perioperative outcomes were analyzed, especially regarding anastomotic leakage, surgical site infections, and other infectious complications.
Results: A total of n = 477 cases of gastrointestinal surgery in gastric cancer (n = 80), sigmoid cancer (n = 168), and rectal cancer (n = 229) using a perioperative regimen of antibiotic bowel decontamination could be included in this analysis. Overall, anastomotic leakage occurred in 4.4% (2.5% gastric cancer, 3.0% sigmoid cancer, 6.1% rectal cancer) and surgical site infections in 9.6% (6.3% gastric cancer, 9.5% sigmoid cancer, 10.9% rectal cancer). The incidence of all infectious complications was 13.6% (12.5% gastric cancer, 11.3% sigmoid cancer, 15.7% rectal cancer). Mortality was low, with an overall rate of 1.1% (1.3% gastric cancer, 1.8% sigmoid cancer, 0.4% rectal cancer). Antibiotic decontamination was completed in 98.5%. No adverse effects of antibiotic bowel decontamination could be observed.
Conclusion: Overall, in this large cohort, we can report low rates of surgery-related serious morbidity and mortality when perioperative antibiotic bowel decontamination is performed. The rates are lower than other clinical reports. In our clinical experience, the use of perioperative antibiotic bowel decontamination appears to improve patient safety and surgical outcomes during gastrointestinal oncologic procedures in a routine clinical setting.
Introduction
Digestive tract surgery is associated with high rates of surgical site infections (SSIs) as well as other infectious complications (1–5) and major elevation of treatment costs (6). The rate is the highest in colorectal cancer surgery, where infectious complications affect up to 26% of patients (7–9). The most severe complication of digestive tract surgery however is anastomotic leakage (AL), with an incidence in colorectal resections ranging from 5% to 15% and an associated mortality rate of 6%–30% (10–13). The leakage rate of esophagojejunal anastomosis following total gastrectomy is reported to be between 4% and 15% in recent literature (14–16), and the mortality in case of AL reaches up to 60% (17). AL of upper and lower gastrointestinal tract surgery not only causes morbidity and postoperative mortality but also impairs long-term cancer survival (2, 18–21).
While the role of bacteria in the development of SSI is unquestioned, their role in the pathogenesis of AL is not well accepted (10, 22–24). Today however there is experimental and clinical evidence, indicating that microbiota is directly involved in the pathogenesis of intestinal AL (10, 23, 25, 26). In 1994, Schardey demonstrated that deliberate postoperative contamination of esophagointestinal anastomoses with virulent Pseudomonas aeruginosa in rats resulted in AL rates of 95% (24). A topical application of nonresorbable antibiotics administered perioperatively until the 10th postoperative day reduced bacterial counts by 95%, and no AL occurred (24). He modified the selective decontamination of the digestive tract regimen (SDD), originally reported by Stoutenbeek et al. for the prevention of pneumonia in ventilated patients, adding vancomycin for double antibiotic coverage of relevant germs (27).
In a clinical multicenter randomized controlled trial (RCT), for the first time, Schardey demonstrated a significant reduction of AL in patients using the modified SDD regimen in patients with total gastrectomy for topical decontamination in gastric cancer surgery (28). It is also noteworthy that the number of postoperative pneumonia decreased significantly, and treatment costs were reduced by about 20% (28, 29). In a further clinical RCT, this modified SDD regimen was used in patients undergoing (low) anterior resection for rectal cancer (30). There was a significant reduction of AL in treatment compared to the control group, with a cost reduction in the treatment group of up to 37% (30).
Nevertheless, the use of bowel decontamination in gastrointestinal surgery is not widespread in Europe or United States (31–33), despite reliable data are available from prospective studies, meta-analyses, and large clinical registry cohorts (34, 35). Currently, several randomized trials have recently been published on the role of perioperative antibiotic bowel decontamination in colorectal surgery to prevent SSI, AL, and other infectious complications (9, 36, 37). However, only sparse data from the routine clinical use of decontamination in gastrointestinal surgery are available at present. Furthermore, there are other concepts in which antibiotic bowel decontamination is performed only preoperatively with or without combination with mechanical bowel preparation (38).
Based on the work by Schardey et al., there is 20 years’ experience in the routine use of antibiotic decontamination in nearly all patients undergoing gastric or colorectal surgery with primary anastomosis (28, 30, 39, 40). Especially patients with intestinal anastomoses to the esophagus, rectum, and anus have a higher risk for AL compared to other localizations in the gastrointestinal tract (10, 13, 28). The aim of this work is to analyze the routine clinical use of decontamination in surgery for gastric and colorectal cancer (CRC) concerning AL, SSI, and possible side effects over the available 20 years’ period in a single center.
Materials and Methods
Study Design
We designed a single-center retrospective cohort study including patients who received the preoperative and postoperative (modified) SDD regimen in upper and lower GI cancer surgery between 1999 and 2020 (on treatment) in an academic teaching hospital. The study was approved by the local review board (19-621 and 22-0013).
All elective procedures of gastric cancer surgery and of lower GI surgery for sigmoid and rectal cancer were analyzed. The hospital’s electronic database was used to identify all patients undergoing gastrectomy for gastric cancer as well as a sigmoid or rectal resection for CRC with primary anastomosis. In colorectal cancer surgery, cases without primary anastomosis (Hartmann procedure) or abdomino-perineal rectal amputations were excluded from the analysis. Overall, n = 477 cases met the selection criteria and received perioperative antibiotic bowel decontamination (SDD), with n = 80 cases of gastric, n = 168 cases of sigmoid, and n = 229 cases of rectal cancer.
Antibiotic Decontamination (SDD) Regimen
An SDD regimen consisting of polymyxin B (100 mg), gentamicin (80 mg), and amphotericin B (500 mg) in sigmoid resections and a modified SDD regimen with additional use of vancomycin (125 mg) in gastric and rectal cancer surgery (PTVA) were used as previously described (28, 30). Patients without any perioperative SDD treatment were excluded from analysis (on treatment). The medication was administered four times daily. Amphotericin B was administered 30 min after the antibiotics. SDD application was usually started in the evening before surgery and continued every 6 h until the 7th postoperative day. For patients undergoing gastrectomy, the antimicrobial agents were dissolved in distilled water and administered as a solution per os (28). Patients with surgery for sigmoid or rectal cancer took these antibiotics as capsules per os (40). If a diverting stoma was created, an unblocked Foley catheter was placed transanally after the creation of the anastomosis, and antibiotics were then applied topically via the catheter dissolved in distilled water (30). The compliance of application as well as the completeness of decontamination regimen was controlled by evaluation of all patient files. All patients undergoing rectal cancer surgery received additional mechanical bowel preparation; the patient with sigmoid cancer had mild laxative therapy only. Gastric cancer patients received no additional bowel preparation.
Rectal cancer surgery was performed according to current technical standards, especially the total mesorectal excision (TME) technique was used for all low anterior rectal resections. Circular double-row staplers (Ethicon Circular Stapler, Ethicon Endo-Surgery, Johnson and Johnson, USA) were used for anastomoses in different sizes in gastric, sigmoid, and rectal cancer surgery. Intraoperative routine leak testing with a methylene blue solution was performed in every case. The extent of resection in gastric cancer patients depended on preoperative pathhistologic report and localization of the tumor according to medical evidence and national guidelines (41, 42). In all cases, a D2 lymphadenectomy was performed (43).
Outcome Measures
Perioperative data (extent and type of surgery: subtotal/total/transhiatal extended gastrectomy, sigmoid resection, (low) anterior rectal resection [(L)AR] and multivisceral resection, use of minimally invasive surgery (MIC), TNM stage and UICC classification, all perioperative 30 day complications like infectious complications (AL, SSIs, urinary tract or pulmonary infections), and general complications (myocardial infarction, stroke, mortality)) were documented as well as other demographic data. The Charlson comorbidity index was calculated for all patients (44). Perioperative complications were classified according to the Clavien–Dindo classification (45), and additionally, the Clavien–Dindo comprehensive complication index (CCI) was calculated (46). Laboratory values such as white blood cell count and C-reactive protein (CRP) were assessed perioperatively. Potential adverse events associated with the SDD/PTVA regimen were also examined. Multivisceral resection was defined as additional resection of the small bowel, liver, or urogenital tract.
As previously described (39), AL was defined and classified according to the recommendation of the International Study Group for Rectal Cancer (47). AL was usually diagnosed by endoscopy, CT scan, or relaparotomy. Due to the retrospective design, only cases with clinically apparent AL could be included.
The primary endpoint is the rate of AL. Secondary endpoints are rates of surgical site infections (SSIs), infectious complications, overall morbidity and mortality, and adverse events related to the SDD/PTVA regimen.
Statistical Analysis
For statistical analysis, SPSS 28 (IBM) and Graph Pad Prism V7 (V7 (GraphPad Software, Inc.)) were used. We performed a descriptive evaluation of perioperative outcome since no comparison of groups was possible as all patients received SDD treatment. Comparative analysis of patients with or without above-mentioned complications was carried out. A correlation of the Charlson comorbidity index and other risk factors with perioperative outcome was performed and an ROC analysis of laboratory parameters with regard to infectious complications. Patient characteristics and perioperative data were summarized using descriptive statistics and calculation of mean values. For comparison between different groups, we used the Mann–Whitney U-test (MW) for non-normally distributed values and Student’s t-test for normally distributed values. The normal distribution of mean differences was tested with the Kolmogorov–Smirnov test. Fisher’s exact and χ2 tests were used to compare data between subgroups involving nominal or categorical data. p values <0.05 were considered statistically significant.
Results
Patient Characteristics
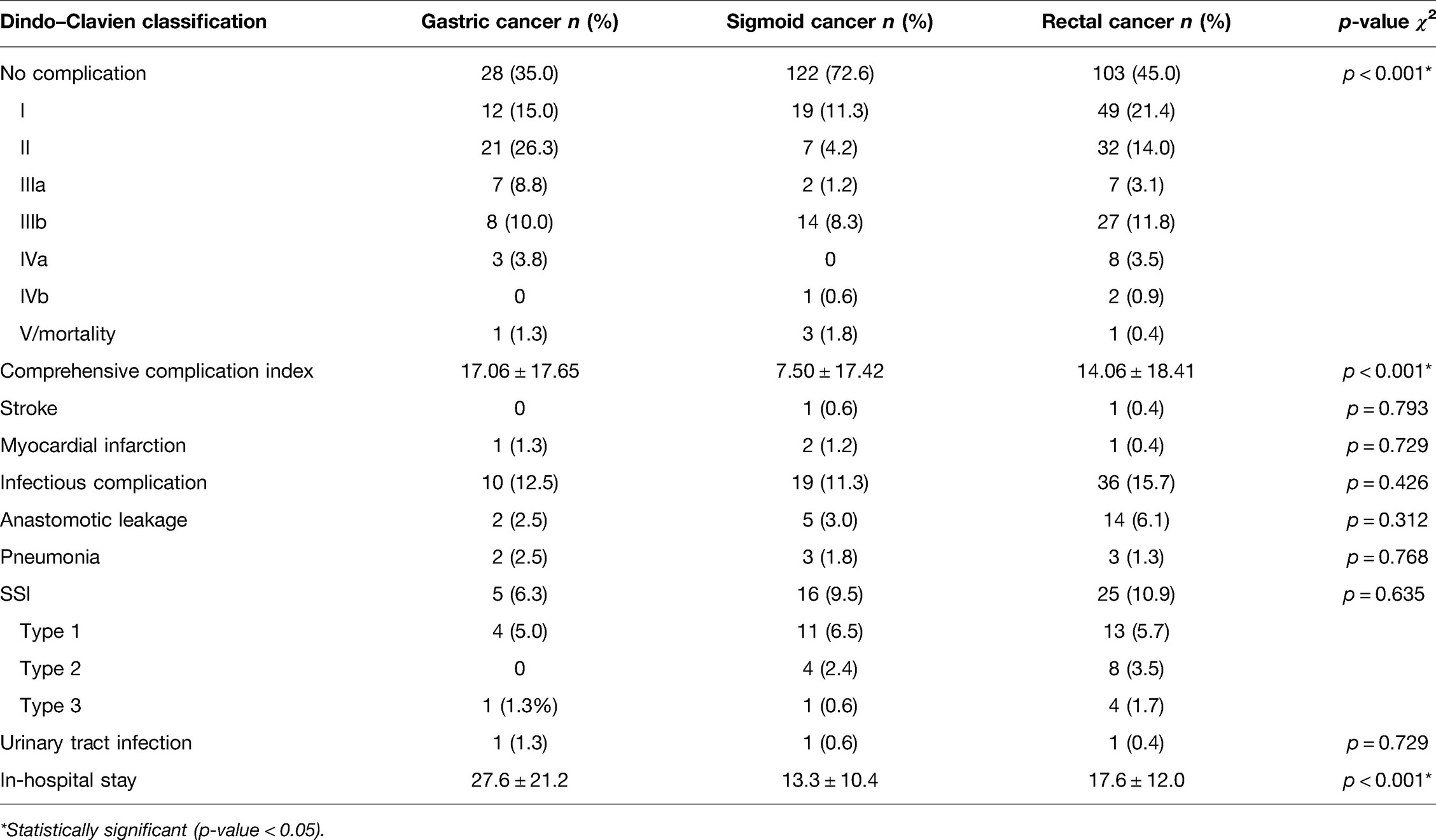
In total, 477 surgical procedures with primary anastomosis and perioperative SDD treatment were included. Patients’ characteristics are summarized in Table 1.
Most of the patients underwent surgery for CRC (sigmoid cancer n = 168, rectal cancer n = 229), and most often LAR (39.1%; n = 187), sigmoid resection (32.8%; n = 156) and AR (11.3%; n = 54) were performed. In patients with gastric cancer (n = 80), total gastrectomy (55%; n = 44) and partial gastrectomy (45%; n = 36) were performed. In surgery for gastric cancer, all procedures were carried out using the conventional open technique, whereas in 44% (n = 74) of patients undergoing surgery for sigmoid cancer and in 18.3% (n = 42) of patients with rectal cancer, the procedures were performed using the minimal invasive surgical technique (MIC). Multivisceral resection was necessary in approximately 15%–20% of procedures independent of underlaying disease (Table 1).
CRC
Patients with CRC had a mean age of 67.9 ± 11.2 years and 67.8 ± 10.7 years for sigmoid and rectal cancer, respectively. The mean Charlson comorbidity index for patients with sigmoid cancer was 6.0 ± 2.4 and that for rectal cancer was 5.8 ± 2.4. Patients with sigmoid cancer were mostly classified as UICC III-IV with 51.8% of cases (n = 87) and 47.0% of cases UICC I-II (n = 79). In rectal cancer patients, 53.9% were classified as UICC (y0)I-II (n = 130) and 41.1% were classified as UICC III-IV (n = 99) (Table 1). Decontamination was completed in n = 165 (98.2%) cases in sigmoid and n = 227 (99.1%) cases in rectal cancer patients.
Gastric Cancer
For gastric cancer, patients were slightly older, with a mean age of 71.6 ± 10.4 years. The mean Charlson comorbidity index for patients with gastric cancer was 6.2 ± 2.3. The majority of patients with gastric cancer were classified UIC I-II in 67.5% (n = 54) and UICC III-IV in 32.5% (n = 26) (Table 1). The decontamination regimen was complete in n = 78 (97.5%) of gastric cancer patients and not completed in n = 2 cases (2.5%).
Perioperative Outcome
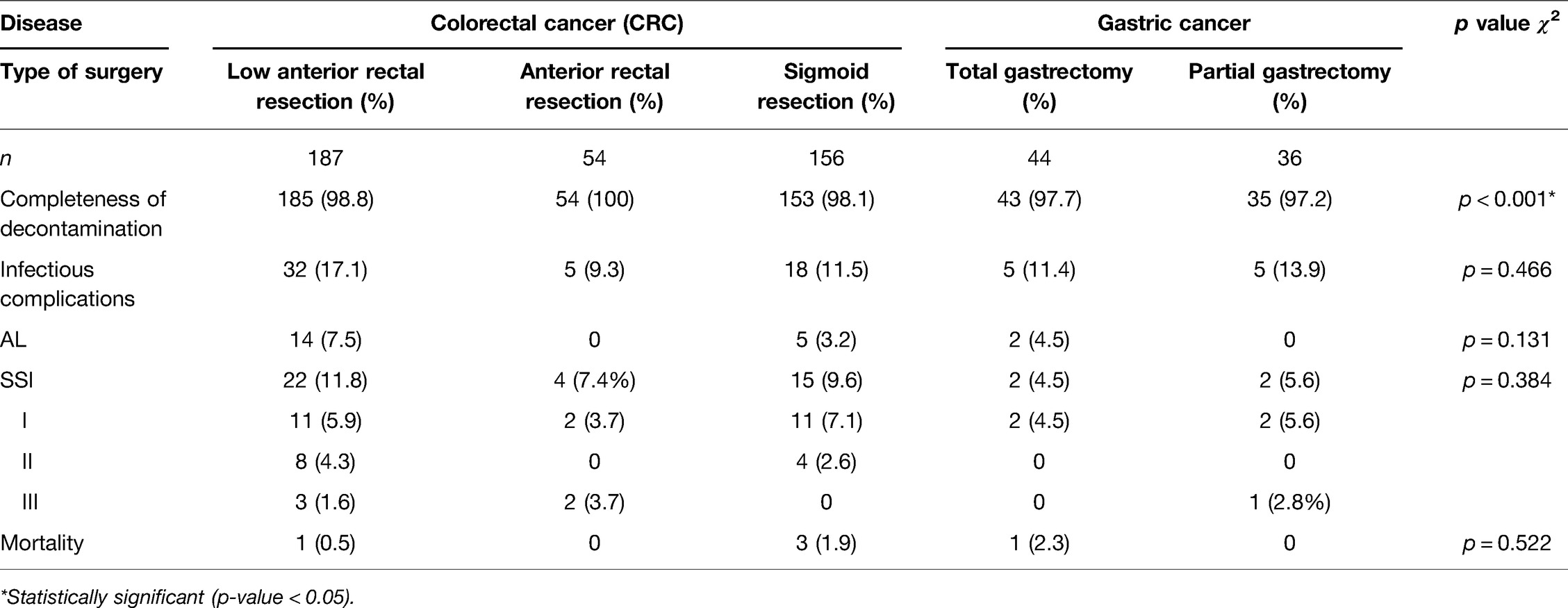
Outcome parameters are summarized in Table 2 (separated for diagnosis) and Table 3 (separated for surgical procedures). The CCI was the highest with a mean of 17.06 ± 17.65 for gastric cancer, with 23.75% major morbidity Clavien–Dindo IIIa–V (n = 19). In 35% (n = 28), no complications were reported, and in 41.3% (n = 33), only minor complications (Clavien–Dindo I–II) occurred.
For sigmoid cancer in 72.6% (n = 122), no complications occurred, whereas in 15.5% (n = 26), minor complications (Clavien–Dindo I–II) were reported. In 11.9% of cases (n = 20), major complications occurred (Clavien–Dindo IIIa–V). The mean CCI was 7.50 ± 17.42.
In rectal cancer, in 45.0% of cases (n = 103), no complication and in 35.4% minor complications (n = 81) were documented. In 19.7% (n = 45), major morbidity (Clavien–Dindo IIIa–V) occurred. The mean CCI was 14.06 ± 18.41. The distribution of complications according to the Clavien–Dindo classification was different between gastric, sigmoid, and rectal cancer (χ2: p < 0.001) as well as between different surgical procedures (χ2: p < 0.001).
CCI was different between different diagnoses (KW: p < 0.001) and between the surgical procedures (KW: p < 0.001). Both stroke and myocardial infarction occurred only in three cases of CRC patients and in one patient suffering from gastric cancer.
Anastomotic Leakage
AL occurred in a total of n = 21 cases and was most frequent in rectal cancer surgery (n = 14; 6.1%). Regarding the procedure, AL occurred only in LAR (n = 14) and not in AR (n = 0) procedures. Another n = 5 cases occurred in sigmoid resections (3.0%) and n = 2 in surgery for gastric cancer (2.5%) (Table 2).
In patients with gastric carcinoma, there was one AL classified as grade B and C. In patients with sigmoid carcinoma, all cases of AL required surgical therapy (grade C). In patients with rectal cancer, AL was classified as grade A (n = 2; 1.9%), grade B (n = 4; 1.7%), and grade C (n = 8; 3.5%), requiring surgical treatment. The mean time (range) to the diagnosis of AL was 17 days (13–20) in gastric cancer, 7.6 days (5–10) in sigmoid cancer, and 8.6 days (1–15) in patients with rectal cancer surgery.
There was no significant difference in rates of AL between groups regarding the type of surgical procedure (LAR, AR, sigmoid resection, total gastrectomy, subtotal gastrectomy; χ2: p = 0.064). Also, multivisceral resection was not associated with increased rates of AL (Fisher: p = 0.252). There was no difference in rates of AL in open vs. MIC surgery (Fisher: p = 0.404), and rates of AL were not higher if conversion to open surgery was necessary (Fisher: p = 0.835). AL significantly prolonged the in-hospital stay (MW: p < 0.001).
Patients with AL had a significantly higher Charlson comorbidity index (MW: p = 0.048) across all diagnoses. Age did not significantly differ between patients with and without AL (MW: p = 0.258).
Infectious Complications
Overall, none of the diagnoses (rectal, sigmoid, or gastric carcinoma) showed an increased rate of infectious complications in general compared to the others (χ2: p = 0.426). However, there was a nonsignificant trend toward fewer infectious complications with minimally invasive surgery (χ2: p = 0.071). In the case of conversion to open surgery, infectious complications did not occur more frequently (Fisher: p = 0.425).
Patients with infectious complications showed a significantly higher Charlson comorbidity index than patients without infectious complications (MW: p = 0.010). These patients were significantly older than patients without infectious complications (MW: p = 0.049). As expected, hospital stay was significantly prolonged in patients with infectious complications (MW: p < 0.001).
Surgical Site Infection
SSIs occurred in 6.3% of cases in gastrectomies. SSI grade I–III was reported in 9.5% of cases for sigmoid cancer surgery (n = 16) and in 10.9% of cases (n = 25) for rectal cancer surgery (Table 2).
SSIs were distributed equally between groups of gastric, sigmoid, and rectal cancer surgery (χ2: p = 0.635). Even for the different types of surgical procedures, the rates of SSI were not different (χ2: p = 0.384). The in-hospital stay of patients suffering from SSI was significantly longer (30.5 ± 15.3 days vs. 16.1 ± 13.4 days; MW: p < 0.001). The Charlson comorbidity index was significantly higher in patients with SSI (6.7 ± 2.8 vs. 5.8 ± 2.3; MW: p = 0.042).
There was no significant difference in rates of SSI for the use of minimally invasive surgery (χ2: p = 0.187), conversion to open surgery (χ2: p = 0.478), or multi-visceral resection (χ2: p = 0.234). There was no difference in the distribution of SSI in different UICC stages (χ2: p = 0.335). Completed decontamination had no significant impact on the rate of SSI (χ2: p = 0.767).
Mortality
In gastric cancer cohort, there was a mortality rate of 1.3% (n = 1), 1.8% (n = 3) in sigmoid cancer and 0.4% (n = 1) rectal cancer surgery. Overall, the distribution of mortality was equal between gastric, sigmoid, and rectal cancer (χ2: p = 0.419). Patients who eventually died had a significantly higher age (79.6 ± 8.7 vs. 68.34 ± 10.8 years; MW: p = 0.028) and Charlson comorbidity index (9.2 ± 1.3 vs. 5.87 ± 2.4; MW: p = 0.003) than patients without in-hospital mortality. Patients who died had a significantly longer in-hospital stay than those who survived (24.2 ± 4.0 vs. 17.7 ± 14.4 days; MW: p = 0.022). Mortality rates were not different between MIC and open surgery (Fisher: p = 0.647) or if conversion to open surgery was necessary (Fisher: p = 0.959). In cases of multivisceral resections, mortality was not increased (Fisher: p = 0.214). The distribution of mortality was not different for UICC stages (χ2: p = 0.836). Complete decontamination did not have a significant impact on mortality rates (χ2: p = 0.926).
In the gastric cancer cohort, there was one patient who died due to AL-related septic complications. In patients with sigmoid cancer, one patient with AL and wound healing disorder developed a status epilepticus and died from septic complications and another patient died due to septic complications following grade II SSI with progressive multiorgan failure and pneumonia after aspiration, respectively. One patient developed a rapid cancer progression and associated pulmonary complications and died from respiratory insufficiency. In rectal cancer surgery, only one patient died from AL-related septic complications. This patient refused the necessary surgical therapy for AL.
Analysis for Risk Factors in Univariate Analysis
In univariate analysis, the Charlson comorbidity index and multivisceral resection had a significant impact on the incidence of infectious complications, SSI, and AL. Additionally, the UICC stage had a significant impact on infectious complications in general only. However, diagnosis, use of MIC surgery, and completeness of decontamination had no effect on the occurrence of infectious complications, SSI, and AL. The univariate analysis revealed no significant risk factors for mortality (Table 4).
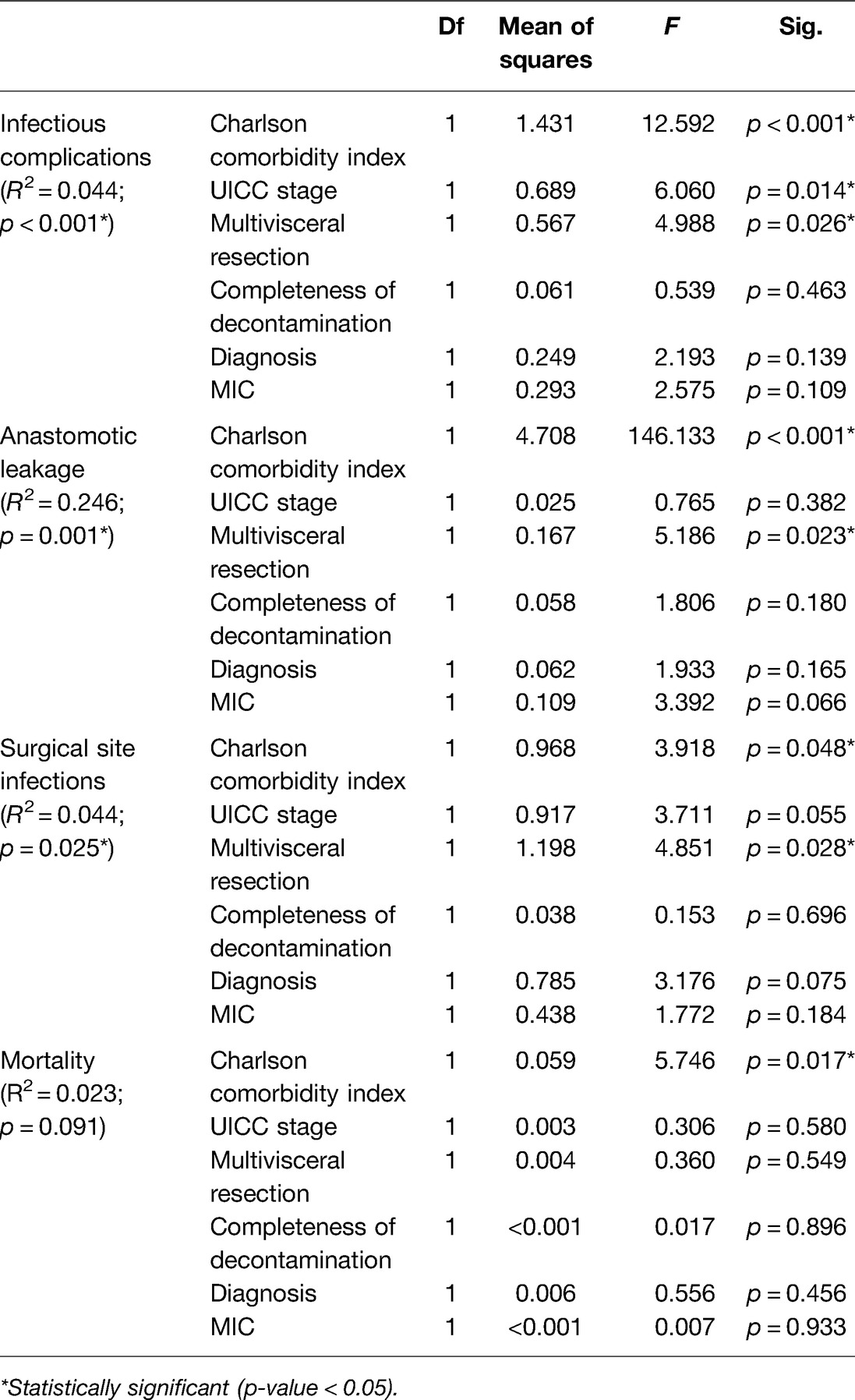
Table 4. Univariate analysis for infectious complications, anastomotic leakage, SSI, and mortality (p values < 0.05 are marked with an *).
Diagnosis of Infectious Complications and Anastomotic Leakage Based on CRP Values
Whereas the white blood cell count was not significantly different between patients with and without infectious complications or AL, the course of CRP values differed significantly (Figures 1A,B). ROC analysis showed that CRP values on days 4 and 5 discriminate not as good for diagnosis of infectious complications (AUC 0.739 and 0.737; Figure 2A) as for diagnosis of AL (AUC 0.826 and 0.830; Figure 2B) on days 4 and 5, respectively.

Figure 1. Laboratory values such as white blood cell count (WBC) and C-reactive protein (CRP) were assessed perioperatively from the day before surgery until the 7th postoperative day. (A) Comparison of the course of parameters between patients with (red) and without (blue) infectious complications. (B) Comparison of the course of the parameters between patients with (red) and without (blue) AL.
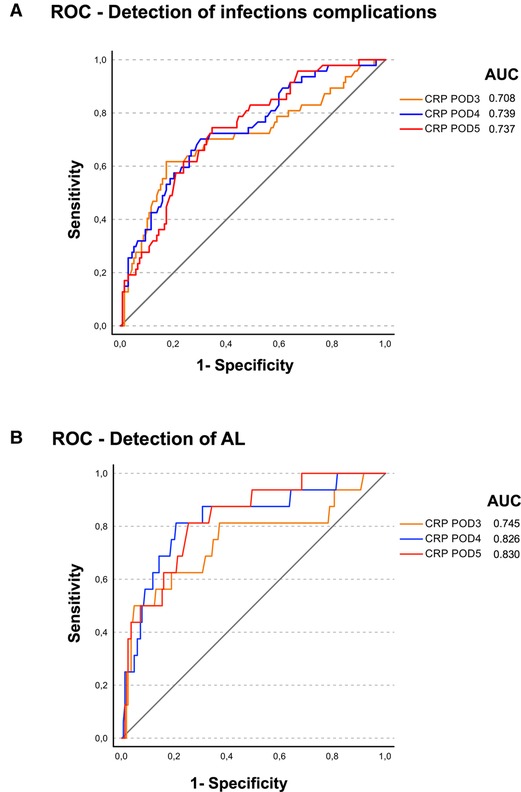
Figure 2. ROC analysis was perfomed for the postoperative CRP levels from postoperative days (POD) 3, 4, and 5 for (A) diagnosis of infectious complications and (B) diagnosis of AL with the area under curve (AUC) given in the figures.
Adverse Events Related to the SDD Regimen
Overall, in n = 2 patients with gastric cancer and n = 3 patients with sigmoid cancer, the SDD regimen was not completed due to nausea and possible intolerance, whereas in rectal cancer surgery in n = 2 cases, the catheter at the anastomotic site was dislocated or removed accidentally so that the SDD regimen could not be continued. Other side effects such as allergic reactions or intolerance did not occur.
Only in rectal cancer surgery there was one patient with clostridium difficile-associated diarrhea.
Discussion
The routine clinical use of antibiotic decontamination in 477 patients with gastric, sigmoid, and rectal cancer surgery seems to be not only feasible but also successful with regard to the overall low rates of SSI, AL, and mortality. Certainly, the rates for AL and SSIs were higher in colorectal compared to gastric cancer surgery.
Although this is a retrospective study lacking a control group, the complication rates compare well with results achieved in double-blind RCTs for gastric (28) and rectal cancer surgery with the use of this SDD/PTVA regimen (30). Mortality rates were low and major complications were more frequent in gastric and rectal compared to sigmoid cancer patients. Patients with SSI, AL, and infectious complications in general and mortality had a significantly higher Charlson comorbidity index compared to patients without infectious complications. Hospital stay was significantly prolonged in these patients. Nearly all patients completed the perioperative antibiotic decontamination regimen, and no adverse events could be detected.
Data on Gastric Cancer Surgery
In a recent review, AL of esophagointestinal anastomosis was reported with an incidence between 2.1% and 14.6% and associated mortality of up to 50% (48). Yoo et al. reported AL in 6.7% following curative resection of gastric cancer. Poor performance status and tumor localization were risk factors for leakage in the latter study (17). In our data, the Charlson comorbidity index was higher for patients with gastric cancer compared to CRC patients. Nonetheless, rates for infectious complications in our gastric cancer patients were low by any standard. In our patients, leaks occurred late in the postoperative course, which may be an effect of decontamination. In our experience, late leaks are less dangerous compared to leaks in the early postoperative course. Overall, only scarce data are available about the use of perioperative antibiotic decontamination in gastric cancer surgery. Scheufele et al. recently conducted a systematic review and meta-analysis of the current evidence for the role of SDD in RCTs of upper gastrointestinal tract surgery reporting a significant reduction in AL and postoperative pneumonia after total gastrectomy and esophagectomy using SDD regimens. These data support the routine use of the SDD regimen in gastrointestinal surgery (16).
Data on Colorectal Cancer Surgery
The complication rates for sigmoid and rectal cancer surgery were much higher than our previously reported data on surgery for diverticulitis using the same SDD regimen (40). For rectal cancer surgery, reliable data about outcome measures without the use of antibiotic bowel decontamination are available from a large German cohort with rates for AL of 11.9% and overall in-hospital mortality of 2.1% (13).
Roos et al., based on their data of a systematic review, stated that a combination of perioperative SDD and perioperative intravenous antibiotic prophylaxis in elective gastrointestinal surgery reduces the rate of postoperative infections, including AL, compared with the use of intravenous antibiotics alone (5). These results have been confirmed by Abis et al., who analyzed the use of SDD in esophageal, gastric, and colorectal surgeries (49).
Results from recently published RCTs and meta-analyses report contrary outcomes of combined bowel preparation. The SELECT trial using a perioperative SDD regimen demonstrated a significant reduction of SSI but not AL (9). The MOBILE trial adding neomycin and metronidazole to mechanical bowel preparation preoperatively only failed to show a relevant difference in SSI or AL between the treatment and control groups (36). However, both trials included only a limited number of left-sided colonic and rectal resections. A meta-analysis recently published by Rollins et al. demonstrated a reduction in SSIs and AL mostly based on the included registry data. The meta-analysis of the RCTs alone did not show a relevant reduction of AL (38).
The available data lack consistency as different types of antibiotic regimens and durations of application are used as well as different types of surgical procedures are included (4, 9, 30, 36, 38, 49). Compared to the available RCTs and other data on the use of a perioperative SDD regimen in combination with mechanical bowel preparation, our analysis shows similar results regarding rates of infectious complication, SSI, and AL, despite the fact that most of these studies excluded UICC stage IV patients, whereas about 18% of UICC stage IV cases are included in our analysis (4, 5, 9, 30, 49). In summary, the relevant data on the use of the perioperative SDD regimen in colorectal surgery support the strategy of topical antibiotics in a reasoned combination (4, 9, 30, 39, 40).
Effect of SDD on Multidrug Resistant Germs and Possible Side Effects
We are aware that there are increasing numbers of vancomycin-resistant Enterococci species (50), but published data on routine use of topical antibiotics like SDD in intensive care units show even a decrease in colonization of Enterococci species (51). Furthermore, there are reliable data on oral vancomycin, as it is widely used in Clostridioides difficile infections. Few antibiotic resistances to vancomycin occurred over time, with a treatment duration of 10 days or even longer (52–54). However, recent experimental data demonstrated a significant role of Enterococci species in the pathogenesis of AL (25, 55). Schardey et al. modified the SDD regimen for antibiotic decontamination by adding vancomycin to the usual SDD regimen. This modified SDD regimen seems to be much more efficacious as it covers a much larger spectrum of potentially pathogenic germs, most of them even twice, including Enterococci species, while these are not sufficiently covered by a conventional SDD regimen (24, 28, 30). On the other hand, the widespread use of antibiotics is a major concern regarding the development of antimicrobial resistance. Presently, the beneficial effect of topical antibiotics in the prevention of AL, in our opinion, outweighs the possible adverse side effects. In over 20 years of the use of these modified SDD regimens in gastrectomy and colorectal surgery, no adverse events regarding multidrug-resistant germs or other relevant side effects have been observed (30, 39, 40).
Risk Factors for Anastomotic Leakage and Other Infectious Complications
In our data, we could detect some risk factors in univariate analysis like the Charlson comorbidity index and multivisceral resections for AL, SSI, and infectious complications in general. Other data already demonstrated male sex, obesity, neoadjuvant (radio)chemotherapy, an impaired preoperative physical and nutritional state or ASA ≥ 3 patients, smoking, UICC stage, and operative factors like level of anastomosis, surgeon volume, and not creating a diverting stoma in low anterior rectal resections as risk factors for AL (12, 13, 56). In our data, due to a limited number of events, no reliable analysis of risk factors for anastomotic leakage and other infectious complications despite the results of the univariate analysis has been possible.
Furthermore, our analysis shows that CRP levels on postoperative days 4 and 5, to some extent, seem to be predictive for AL and less for infectious complications in general in ROC analysis (Figures 1, 2). One can only speculate that due to less nonspecific infectious complications, CRP course on postoperative day 4/5 seems to be a more sensitive marker for the occurrence of AL. In a meta-analysis, Paradis et al. also investigated the diagnostic characteristics of CRP levels between postoperative days 3–5 (8). Overall, elevated CRP levels do not prove AL, but especially further increasing CRP levels are reliable markers for potential alterations of routine postoperative course and may result in further diagnostics (8).
Limitations
Due to the retrospective character, this study has several limitations. All patients were operated on over a period of 20 years in the same academic teaching hospital, which nonetheless is a low-volume community hospital, not expected to reach excellence. Also, due to technical improvements over time, more and more minimal invasive and robotic procedures have been performed (9, 57, 58) and neoadjuvant treatment concepts have been introduced into clinical practice (59–61). Over this time period, there have been major improvements in the perioperative management using “enhanced recovery after surgery” concepts (62, 63). Thus, we can only report on the surgical outcomes. In contrast to these expectations, the complication rates especially regarding SSI, AL, and mortality in this retrospective analysis of routine use of antibiotic decontamination in gastrointestinal surgery compare very well with the results of cancer surgery in currently published studies, reviews, and meta-analyses (4, 5, 9, 28, 30, 49).
Furthermore, one must assume that minor complications (Dindo–Clavien grade I–II) may be rather underrepresented. However, major complications with the need for interventional or surgical reintervention (Clavien–Dindo IIIa–V) are very well documented. Our data are heterogeneous as we report all cases using a perioperative antibiotic decontamination regimen representing high-risk anastomosis in gastric, sigmoid, and rectal cancer surgery. Our data lack a control group because in our center nearly all patients are on treatment using the SDD or modified SDD regimen. However, otherwise, a lot of outcome data and some comparable outcome data using similar SDD regimens are available in the literature for comparison (4, 5, 9, 13, 16, 17, 48, 49).
Conclusion
The concept of perioperative antibiotic bowel decontamination in gastrointestinal surgery based on the use of a (modified) SDD regimen may be able to improve patient safety and surgical outcome in gastrointestinal oncologic surgery in a routine clinical setting. Based on new experimental data, agents other than antibiotics, such as polyphosphates or protease inhibitors, may be an alternative in the future but have not yet been introduced into clinical practice (64, 65).
Contributions to the Field Statement
Antibiotic bowel decontamination and SDD are still not widely used concepts in gastrointestinal surgery, despite the existing evidence not only from registry data but also from the different available RCTs. The impact of bacterial factors on surgical site infections and especially anastomotic leakage is proven, but rates of surgical site infections and anastomotic leakage remain stable over the past years.
In our center, we have 20 years’ experience in the use of antibiotic bowel decontamination. Overall, in the here-presented large cohort, we have low rates for surgery-related major morbidity and mortality. Compared to available international data, we have low rates of AL and surgery-related mortality. No relevant side effects of SDD regimens occurred.
Therefore, the use of the SDD regimen seems to improve patient safety and surgical outcome in gastrointestinal oncologic surgery in a routine clinical setting, but further evidence from RCTs is still necessary.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethikkommission Medizinische Fakultät LMU München. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JS: conceptualization, methodology, data extraction and analysis, and writing the original draft. TA: methodology, data extraction and curation, and critical revision. ES: data extraction and curation and critical revision. AK: methodology, data extraction and curation, and critical revision. PZ: methodology, data curation, and critical revision. FK: methodology, project administration, and critical revision. JA: methodology, data curation, and critical revision. JW: conceptualization, methodology, supervision, and critical revision. HA: conceptualization, methodology, project administration, and critical revision. UW: conceptualization, methodology, data extraction and analysis, writing original draft, and project administration. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The author TvA is employed by Krankenhaus Agatharied GmbH, Hausham, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vazquez-Aragon P. Nosocomial infection and related risk factors in a general surgery service: a prospective study. J Infect. (2003) 46:17–22. doi: 10.1053/jinf.2002.1073
2. Li J, Zhang Y, Hu D-M, Gong T-P, Xu R, Gao J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: A systematic review and meta-analysis of 64 follow-up studies. Asian J Surg. (2020) 43:719–29. doi: 10.1016/j.asjsur.2019.10.007
3. Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. (2014) 21:3008–14. doi: 10.1245/s10434-014-3664-z
4. Roos D, Dijksman LM, Oudemans-van Straaten HM, de Wit LT, Gouma DJ, Gerhards MF. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg. (2011) 98:1365–72. doi: 10.1002/bjs.7631
5. Roos D, Dijksman LM, Tijssen JG, Gouma DJ, Gerhards MF, Oudemans-van Straaten HM. Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery: perioperative selective decontamination of the digestive tract. Br J Surg. (2013) 100:1579–88. doi: 10.1002/bjs.9254
6. Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. (2014) 18:1176–85. doi: 10.1007/s11605-014-2506-4
7. Ghuman A, Kasteel N, Brown CJ, Karimuddin AA, Raval MJ, Wexner SD, et al. Surgical site infection in elective colonic and rectal resections: effect of oral antibiotics and mechanical bowel preparation compared with mechanical bowel preparation only. Colorectal Dis. (2020) 22:1686–93. doi: 10.1111/codi.15153
8. Paradis T, Zorigtbaatar A, Trepanier M, Fiore JF, Fried GM, Feldman LS, et al. Meta-analysis of the diagnostic accuracy of C-reactive protein for infectious complications in laparoscopic versus open colorectal surgery. J Gastrointest Surg. (2020) 24:1392–401. doi: 10.1007/s11605-020-04599-2
9. Abis GSA, Stockmann HBAC, Bonjer HJ, van Veenendaal N, van Doorn-Schepens MLM, Budding AE, et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial): selective decontamination of the digestive tract in colorectal cancer surgery. Br J Surg. (2019) 106:355–63. doi: 10.1002/bjs.11117
10. Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg. (2013) 17:1698–707. doi: 10.1007/s11605-013-2227-0
11. Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. (2010) 251:807–18. doi: 10.1097/SLA.0b013e3181dae4ed
12. Matthiessen P, Hallböök O, Rutegard J, Simert G, Sjödahl R. Defunctioning ctoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. (2007) 246:207–14. doi: 10.1097/SLA.0b013e3180603024
13. Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H, et al. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget. (2015) 6:36884–93. doi: 10.18632/oncotarget.5170
14. Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M. Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg. (2011) 35:716–22. doi: 10.1007/s00268-010-0922-5
15. Inokuchi M. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. WJG. (2015) 21:9656. doi: 10.3748/wjg.v21.i32.9656
16. Scheufele F, Schirren R, Friess H, Reim D. Selective decontamination of the digestive tract in upper gastrointestinal surgery: systematic review with meta-analysis of randomized clinical trials. BJS Open. (2020) 4:bjs5.50332. doi: 10.1002/bjs5.50332
17. Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer: leakage and survival after gastrectomy. J Surg Oncol. (2011) 104:734–40. doi: 10.1002/jso.22045
18. Andreou A, Biebl M, Dadras M, Struecker B, Sauer IM, Thuss-Patience PC, et al. Anastomotic leak predicts diminished long-term survival after resection for gastric and esophageal cancer. Surgery. (2016) 160:191–203. doi: 10.1016/j.surg.2016.02.020
19. Boccola MA, Buettner PG, Rozen WM, Siu SK, Stevenson ARL, Stitz R, et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg. (2011) 35:186–95. doi: 10.1007/s00268-010-0831-7
20. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. (2011) 253:890–9. doi: 10.1097/SLA.0b013e3182128929
21. Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. (2016) 59:236–44. doi: 10.1097/DCR.0000000000000554
22. Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M, et al. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer: influence of preoperative biliary drainage on the biliary microbiome. Br J Surg. (2017) 104:e182–8. doi: 10.1002/bjs.10450
23. Alverdy JC, Hyoju SK, Weigerinck M, Gilbert JA. The gut microbiome and the mechanism of surgical infection: the gut microbiome and the mechanism of surgical infection. Br J Surg. (2017) 104:e14–e23. doi: 10.1002/bjs.10405
24. Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G, Schildberg FW. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother. (1994) 38:2564–7. doi: 10.1128/AAC.38.11.2564
25. Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. (2015) 7:286ra68. doi: 10.1126/scitranslmed.3010658
26. Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch M, et al. Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS ONE. (2012) 7:e44326. doi: 10.1371/journal.pone.0044326
27. Stoutenbeek CP, van Saene HKF, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisat ion and infection rate in multiple trauma patients. Intensive Care Med. (1984) 10:185–92. doi: 10.1007/BF00259435
28. Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A, et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination: a prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg. (1997) 225:172–80. doi: 10.1097/00000658-199702000-00005
29. Schardey HM, Joosten U, Finke U, Schauer R, Staubach KH, Exner H, et al. Cost reduction by decontamination to prevent anastomotic leakage following total gastrectomy. Chirurg. (1997) 68:416–24. doi: 10.1007/s001040050207
30. Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D, Jauch KW. Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorect Dis. (2020) 35:847–57. doi: 10.1007/s00384-020-03544-8
31. Devane LA, Proud D, O’Connell PR, Panis Y. A European survey of bowel preparation in colorectal surgery. Colorectal Dis. (2017) 19:O402–6. doi: 10.1111/codi.13905
32. McChesney SL, Zelhart MD, Green RL, Nichols RL. Current U.S. pre-operative bowel preparation trends: a 2018 survey of the American Society of Colon and Rectal Surgeons Members. Surg Infect. (2020) 21:1–8. doi: 10.1089/sur.2019.125
33. Buia A, Post S, Buhr HJ, Hanisch E. Darmvorbereitung bei elektiven kolorektalen Resektionen in Deutschland 2017: Ergebnisse einer Umfrage unter den Mitgliedern der DGAV. Chirurg. (2019) 90:564–9. doi: 10.1007/s00104-018-0773-4
34. Cannon JA, Altom LK, Deierhoi RJ, Morris M, Richman JS, Vick CC, et al. Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum. (2012) 55:1160–6. doi: 10.1097/DCR.0b013e3182684fac
35. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg. (2015) 262:331–7. doi: 10.1097/SLA.0000000000001041
36. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet. (2019) 394:840–8. doi: 10.1016/S0140-6736(19)31269-3
37. Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset B, et al. Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. (2010) 252:863–8. doi: 10.1097/SLA.0b013e3181fd8ea9
38. Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg. (2019) 270:43–58. doi: 10.1097/SLA.0000000000003145
39. Wirth U, Rogers S, Haubensak K, Schopf S, von Ahnen T, Schardey HM. Local antibiotic decontamination to prevent anastomotic leakage short-term outcome in rectal cancer surgery. Int J Colorect Dis. (2018) 33:53–60. doi: 10.1007/s00384-017-2933-2
40. Wirth U, Schardey J, von Ahnen T, Zimmermann P, Kühn F, Werner J, et al. Oral antibiotic bowel decontamination in open and laparoscopic sigmoid resections for diverticular disease. Int J Colorectal Dis. (2021) 36:1667–76. doi: 10.1007/s00384-021-03890-1
41. Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Ann Surg. (1999) 230:170. doi: 10.1097/00000658-199908000-00006
42. Moehler M, Al-Batran S-E, Andus T, Anthuber M, Arends J, Arnold D, et al. S3-Leitlinie “Magenkarzinom”. Z Gastroenterol. (2011) 49:461–531. doi: 10.1055/s-0031-1273201
43. Ravichandran D, Carty N, Lamah M, Johnson C. Extended lymph node dissection (D2 resection) should now be performed routinely in the curative surgical treatment of gastric carcinoma. Ann R Coll Surg Eng. (1995) 77:431–6. PMID: 8540662; PMCID: PMC2502460
44. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
45. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
46. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien P-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. (2013) 258:1–7. doi: 10.1097/SLA.0b013e318296c732
47. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery. (2010) 147:339–51. doi: 10.1016/j.surg.2009.10.012
48. Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, Terashima M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. (2019) 49:187–96. doi: 10.1007/s00595-018-1726-8
49. Abis GSA, Stockmann HBAC, van Egmond M, Bonjer HJ, Vandenbroucke-Grauls CMJE, Oosterling SJ. Selective decontamination of the digestive tract in gastrointestinal surgery: useful in infection prevention? a systematic review. J Gastrointest Surg. (2013) 17:2172–8. doi: 10.1007/s11605-013-2379-y
50. Markwart R, Willrich N, Haller S, Noll I, Koppe U, Werner G, et al. The rise in vancomycin-resistant Enterococcus faecium in Germany: data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob Resist Infect Control. (2019) 8:147 (eCollection). doi: 10.1186/s13756-019-0594-3
51. van der Bij AK, Frentz D, Bonten MJM. Gram-positive cocci in Dutch ICUs with and without selective decontamination of the oropharyngeal and digestive tract: a retrospective database analysis. J Antimicrob Chemother. (2016) 71:816–20. doi: 10.1093/jac/dkv396
52. Gonzales M, Pepin J, Frost EH, Carrier JC, Sirard S, Fortier LC, et al. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis. (2010) 10:363. doi: 10.1186/1471-2334-10-363
53. van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B, et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. (2021) 27(Suppl 2):S1–S21. doi: 10.1016/j.cmi.2021.09.038
54. Eubank TA, Gonzales-Luna AJ, Hurdle JG, Garey KW. Genetic mechanisms of vancomycin resistance in Clostridioides difficile: a systematic review. Antibiot (Basel). (2022) 11(258):258. doi: 10.3390/antibiotics11020258
55. Jacobson RA, Wienholts K, Williamson AJ, Gaines S, Hyoju S, van Goor H, et al. Enterococcus faecalis exploits the human fibrinolytic system to drive excess collagenolysis: implications in gut healing and identification of druggable targets. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G1–G9. doi: 10.1152/ajpgi.00236.2019
56. Sciuto A, Merola G, Palma GDD, Sodo M, Pirozzi F, Bracale UM, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. WJG. (2018) 24:2247–60. doi: 10.3748/wjg.v24.i21.2247
57. Andolfi C, Umanskiy K. Appraisal and current considerations of robotics in colon and rectal surgery. J Laparoendosc Adv Surg Tech. (2019) 29:152–8. doi: 10.1089/lap.2018.0571
58. Blackmore AE. Evolution of laparoscopy in colorectal surgery: an evidence-based review. WJG. (2014) 20:4926. doi: 10.3748/wjg.v20.i17.4926
59. Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, Nakajima Y, et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today. (2020) 50:30–7. doi: 10.1007/s00595-019-01896-5
60. Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. (2020) 271:440–8. doi: 10.1097/SLA.0000000000003471
61. Lagarde SM, Navidi M, Gisbertz SS, van Laarhoven HWM, Sumpter K, Meijer SL, et al. Prognostic impact of extracapsular lymph node involvement after neoadjuvant therapy and oesophagectomy. Br J Surg. (2016) 103:1658–64. doi: 10.1002/bjs.10226
62. Cavallaro P, Bordeianou L. Implementation of an ERAS pathway in colorectal surgery. Clin Colon Rectal Surg. (2019) 32:102–8. doi: 10.1055/s-0038-1676474
63. Wind J, Polle SW, Fung Kon Jin PHP, Dejong CHC, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. (2006) 93:800–9. doi: 10.1002/bjs.5384
64. Jacobson RA, Williamson AJ, Wienholts K, Gaines S, Hyoju S, van Goor H, et al. Prevention of anastomotic leak via local application of tranexamic acid to target bacterial-mediated plasminogen activation: a practical solution to a complex problem. Ann Surg. (2019) 274:e1038–e1046. doi: 10.1097/SLA.0000000000003733
Keywords: antibiotic bowel decontamination, gastrointestinal surgery, anastomotic leakage, SDD, colorectal cancer, gastric cancer
Citation: Schardey J, von Ahnen T, Schardey E, Kappenberger A, Zimmermann P, Kühn F, Andrassy J, Werner J, Arbogast H and Wirth U (2022) Antibiotic Bowel Decontamination in Gastrointestinal Surgery—A Single-Center 20 Years’ Experience. Front. Surg. 9:874223. doi: 10.3389/fsurg.2022.874223
Received: 11 February 2022; Accepted: 25 April 2022;
Published: 16 May 2022.
Edited by:
Sven Flemming University Hospital of Wuerzburg, GermanyReviewed by:
John Chris Alverdy, University of Chicago, United StatesMatthias Kelm, University Hospital Würzburg, Germany
Copyright © 2022 Schardey, Von Ahnen, Schardey, Kappenberger, Zimmermann, Kühn, Andrassy, Werner, Arbogast and Wirth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrich Wirth VWxyaWNoLldpcnRoQG1lZC51bmktbXVlbmNoZW4uZGU=
†ORCID: Ulrich Wirthorcid.org/0000-0003-2366-8524
‡These authors have contributed equally to this work and share senior authorship
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Josefine Schardey1,2
Josefine Schardey1,2 Florian Kühn
Florian Kühn Joachim Andrassy
Joachim Andrassy Helmut Arbogast
Helmut Arbogast Ulrich Wirth
Ulrich Wirth