- West China Hospital, Sichuan University, Chengdu, China
Pancreatic neurofibroma is a very rare benign neurogenic tumor unrelated to neurofibromatosis type 1 (NF-1). As the volume increases, it has the risk of malignant transformation. The surgical prognosis of pancreatic neurofibroma is good, but its preoperative imaging features are very similar to those of malignant tumors, which may affect the formulation of treatment plans. This article reports a case of giant neurofibroma of the pancreas with contrast-enhanced ultrasound (CEUS) as one of the diagnostic methods and discusses the tumor’s preoperative clinical features, laboratory examinations, and imaging features.
Introduction
Pancreatic neurofibroma is a benign tumor derived from nerve cells. In the absence of neurofibromatosis type 1 (NF-I), its malignant potential is very low. Isolated neurofibroma unrelated to NF-I is very rare and is not found until they grow too large to cause serious complications (1). This study focuses on analyzing the imaging data of pancreatic neurofibroma and discussing its imaging features to improve the understanding of the disease. Previously reported cases of neurofibromas were all diagnosed using CT or MRI before surgery (2–5). Here, we report a case of pancreatic neurofibroma with contrast-enhanced ultrasound as one of the diagnostic methods.
Case Presentation
A 35-year-old male patient had dull pain in his right abdomen for 2 months without obvious cause or medical history. At the local hospital, computed tomography (CT) of the upper abdomen revealed a large mass on the neck of the pancreas. Five days later, he came to the biliary surgery department of our hospital for further treatment. On physical examination after admission, the abdomen was soft, and a slight mass was palpable in the upper abdominal area, with poor mobility and mild tenderness. Laboratory examination results showed that CA19-9 was slightly increased, and the values of other tumor markers were normal. White blood cell count, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly increased, while other laboratory data, such as amylase, lipase and total bilirubin, were not abnormal.
Contrast-enhanced magnetic resonance (MR) revealed an 8.2 × 5.7 cm circular confounding signal mass on the pancreatic neck (Figure 1). It had a slightly higher T1 signal and equal T2 signal with a small patch of high T2 signal inside. Contrast enhancement was heterogeneous high enhancement. The adjacent common hepatic artery and splenic artery were pushed, causing their lumens to narrow slightly, and their local boundaries with the tumor were not clear. The fat space between the splenic vein and the tumor is not clear. The main pancreatic duct and bile duct were not dilated. MR finally considered pancreatic malignant cystadenoma or solid pseudopapillary tumor (SPT). Two-dimensional ultrasonography revealed a slightly weaker echogenic mass of approximately 8.6 × 6.4 cm in the pancreatic neck body area, with clear boundaries, clear capsules, heterogeneous internal echoes, and small hypoechoic areas (Figure 2A). CEUS showed rapid and high enhancement at the margin and equal enhancement at the interior of the mass in the arterial phase. In the parenchymal phase, the overall enhancement was low. CEUS considered mostly SPT (Figures 2B,C).
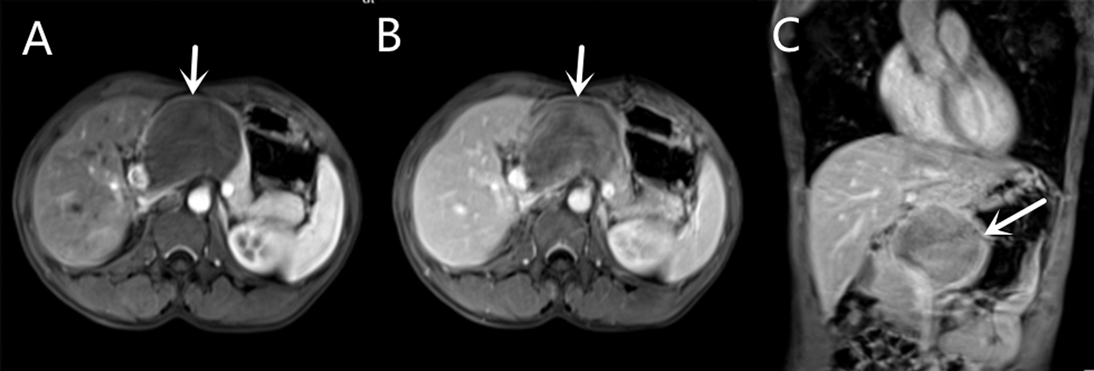
Figure 1. Contrast-enhanced magnetic resonance (MR) of a mass on the pancreatic neck-body. (A) T1W Cross-sections MR in arterial phase. (B) T1W Cross-sections MR in portal phase. (C) T2W Axial MR in arterial phase.
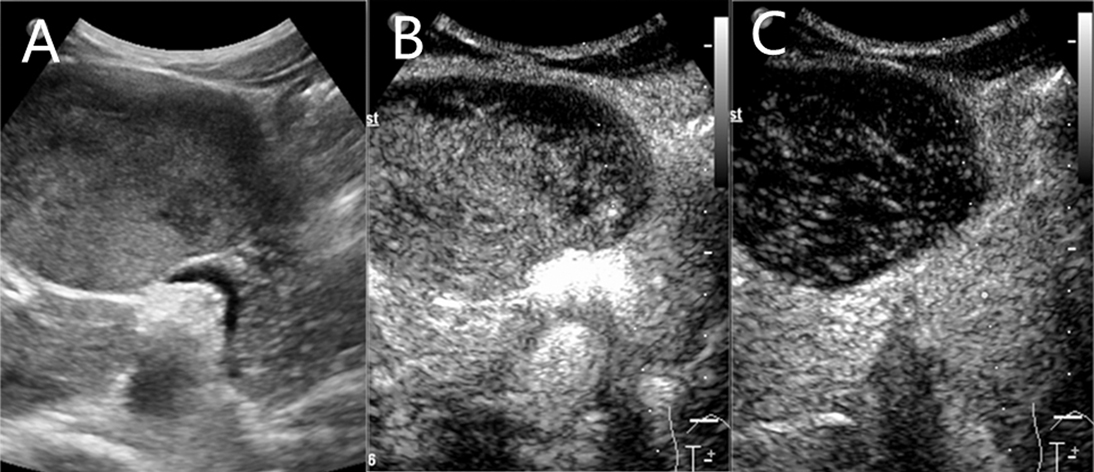
Figure 2. A slightly weaker echogenic mass on ultrasound. (A) Two-dimensional ultrasonography. (B) contrast-enhanced ultrasound (CEUS) in arterial phase. (C) CEUS in parenchymal phase.
Due to the large size of the mass, the clinician decided to perform an exploratory laparotomy. During the operation, a hard mass of approximately 10 × 8 × 9 cm with a capsule was found above the neck of the pancreas and the portal vein. The tumor surrounds surrounding tissues and organs, including the proper hepatic artery, splenic artery, portal vein and left adrenal gland. Based on the intraoperative findings, the surgeon decided to perform total tumor resection.
Histopathology found that the tumor was mainly composed of spindle cells with cytologically bland wavy nuclei in a collagenous matrix (Figure 3). Immunohistochemical staining was S100 (+), CD34 (+), and Ki67 (+2%) (Figures 4A,B). Combined with histological morphology and immunohistochemical results, it was considered a peripheral nerve tumor, and its subtype was consistent with solitary neurofibroma. After two years of follow-up, CT, MRI and CEUS were used alternately for examination, and there was no sign of tumor recurrence Supplementary Materials.
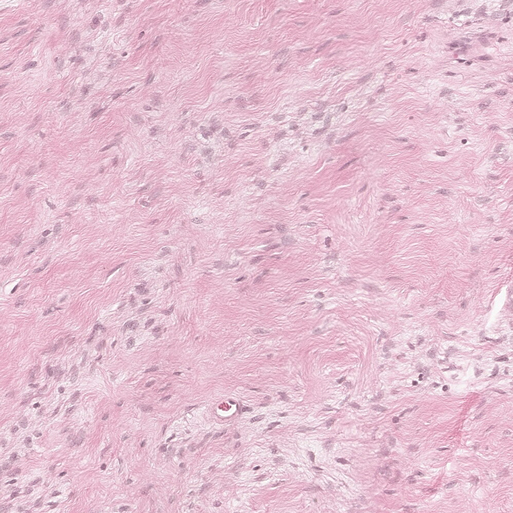
Figure 3. Photomicrograph of pancreatic neurofibroma. Collagen fibers contain a large number of spindle cells (H&E stain, ×100).
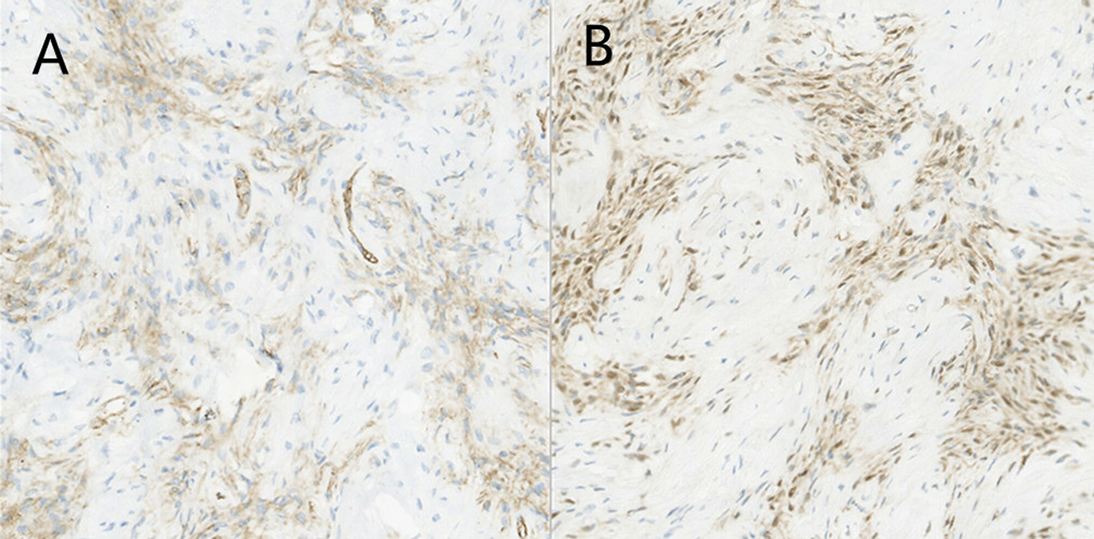
Figure 4. Photomicrograph of pancreatic neurofibroma. (A) Immunohistochemical staining [CD34(+)]. (B) Immunohistochemical staining [S100(+)].
Discussion
Neurofibroma is a benign neurogenic tumor that can be divided into solitary neurofibroma and neurofibromatosis. Neurofibroma generally originates from peripheral or central nerve cells and is composed of Schwann cells and fibroblasts. Most of them are isolated masses in the dermis or subcutaneous tissue. Neurofibroma occurring locally in the body is the most common, accounting for approximately 90%. It is rare that it appears in the pancreas, and solitary neurofibroma of the pancreas not related to NF-I is even rarer (6).
The solitary neurofibromas that have been reported now mostly appear under the skin, in the bones and in the gastrointestinal tract (3, 5, 7–13). The number of reports of solitary neurofibromas that appeared in the pancreas is very limited (Table 1). Moletta et al. reported that pancreatic neurofibroma combined the cerebral neurofibroma (14). NF-1 was suspected but the patient refused genetic tests. The other three cases were not related to NF-1 (2, 15, 16).
Unlike superficial neurofibromas, which can be easily palpated, abdominal neurofibromas are most often found because of abdominal pain (2, 17). By this time, the large tumor has already produced a space-occupying effect. Tsai et al. reported that patients with pancreatic neurofibroma had a history of acute pancreatitis, so they believed that the occurrence of solitary neurofibroma might be related to chronic inflammation, ischemia, trauma and other long-term chronic stimulation (2). In this case, the tumor capsule was intact, and the boundary with surrounding tissues was clear. In contrast, the pancreatic neurofibroma we reported was infiltrative growth invading surrounding organs and tissues. Both imaging features and intraoperative findings were very similar to malignant tumors (17).
After the literature review, this study should be the first to report the use of CEUS to participate in the diagnosis and observation of pancreatic neurofibroma. CEUS showed that neurofibroma was a solid masse with signs of fast in and out, which led to the final diagnosis of low-grade SPT. MRI findings of tumor invasion of surrounding tissue made the diagnosis result more inclined to malignant pancreatic cystadenoma. Because the results of the two imaging results were inconsistent and the patient requested surgery, the clinician finally decided to open the abdominal exploration. Because pancreatic neurofibroma is very rare and there is a lack of large-scale research to statistically analyze its imaging characteristics and because the growth pattern of this tumor is very similar to that of malignant tumors, there is indeed a great possibility of misdiagnosis in clinical practice. Histopathology showed that the tumor was mainly composed of spindle cells, and the immunohistochemical results showed that the tumor expressed S-100 protein and CD34 protein, which was consistent with other reports (6, 18).
In the diagnosis process, pancreatic neurofibroma needs to be differentiated from pancreatic mucinous cystic neoplasms and SPT. Unlike pancreatic cystic tumors, pancreatic neurofibroma has little or no cystic components and is not connected to the main pancreatic duct, so there is no dilation of the main pancreatic duct (19). Moreover, although giant pancreatic neurofibroma infiltrates other organs, rarely causes to cause lymph node metastasis or other organ metastasis, such as pancreatic mucinous cystadenocarcinoma (20).
At present, the preoperative differential diagnosis of pancreatic neurofibroma and other pancreatic tumors is still difficult. In clinical practice, various imaging methods should be combined to fully understand the relationship between the tumor and surrounding tissues, and pathological biopsy should be taken to confirm the diagnosis if necessary. Radical resection is often used to treat pancreatic neurofibroma. Most patients have no recurrence or metastasis after surgery, which confirms the effectiveness of surgical resection. It should be noted that the clinician must confirm that it is not related to NF-1 after the diagnosis of neurofibroma (21). The patient had no family history of NF-1 and no symptoms such as café-au-lait spots and subcutaneous nodules, so he was ruled out.
We described the appearance of pancreatic neurofibroma under contrast-enhanced ultrasound, which accumulated experience for the diagnosis of this rare case in the future.
Conclusion
Pancreatic neurofibroma is a very rare benign tumor of the pancreas. It is very important to fully analyze the imaging data and adopt an individualized surgical approach for patients to preserve the function of the pancreas to the greatest extent and minimize postoperative complications while ensuring complete tumor resection.
Data Avaliability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Satatement
The studies involving human participants were reviewed and approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee, and West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LS and YL designed this study and drafted the report. JC provided the histological results. ZJ and BG reviewed the report critically and gived revisions. All authors issued final approval for the version to be submitted. All authors contributed to the article and approved the submitted version.
Acknowledgments
We express our thanks to Yan Luo and Jiawu Li for their help in finding this case and revising our article several times.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.874006/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology. (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
2. Tsai PJ, Liu KY, Wang SE, Shyr YM, Su CH, Chen TH. Solitary neurofibroma of the pancreas body not associated with type 1 neurofibromatosis. J Chin Med Assoc. (2012) 75(3):132–5. doi: 10.1016/j.jcma.2011.12.016
3. Chen N, Hsu Y-H, Lee Y-C. Solitary neurofibroma of eyelid masquerading as chalazion. Int Med Case Rep J. (2017) 10:177–9. doi: 10.2147/imcrj.S136255
4. Iqbal A, Tamgadge S, Tamgadge A, Chande M. Intraosseous neurofibroma in a 13-year-old male patient: A case report with review of literature. J Cancer Res Ther. (2018) 14(3):712–5. doi: 10.4103/0973-1482.176173
5. Sleiman Z, Abboud L, Mehanna E, Mahmoud R, Yaacoub E, Ghanime G. Mandibular neurofibroma: Case report of a rare tumor. Clin Pract. (2019) 9(4):111–3. doi: 10.4081/cp.2019.1143
6. Wang XY, Cai JP, Song LL, Huang CS, Chen W, Huang XT, et al. Identification of genomic alterations in sporadic pancreatic neurogenic tumors. Pancreas. (2020) 49(10):1393–7. doi: 10.1097/mpa.0000000000001680
7. Shah AM, Dash SP, Das SP, Rangan VS, Bhartiya SC. An unusual presentation of neurofibroma masquerading as a vascular hamartoma, post-iatrogenic intervention. J Clin Diagn Res. (2015) 9(1):PD18–9. doi: 10.7860/jcdr/2015/9852.5442
8. Tall A, N’Diaye C, Diom ES, Thiam I. Solitary neurofibroma originating from the posterior nasal septum: Transnasal endoscopic resection. Eur Ann Otorhinolaryngol Head Neck Dis. (2015) 132(4):223–5. doi: 10.1016/j.anorl.2015.03.001
9. da Silva LP, Goes Gonzaga AK, Santana T, Cobe Sena DA, de Souza LB. Solitary intraosseous neurofibroma: Report of a rare entity. J Oral Maxillofac Surg Med Pathol. (2018) 30(6):566–8. doi: 10.1016/j.ajoms.2018.06.001
10. Rotili A, De Maria F, Di Venosa B, Ghioni M, Pizzamiglio M, Cassano E, et al. Solitary breast neurofibroma: imaging aspects. Ecancermedicalscience. (2018) 12:800. doi: 10.3332/ecancer.2018.800
11. Sekhar P, Nandhini G, Kumar KR, Kumar AR. Solitary neurofibroma of the palate mimicking mucocele: A rare case report. J Oral Maxillofac Pathol. (2019) 23(Suppl 1):23–6. doi: 10.4103/jomfp.JOMFP_196_18
12. Behrad S, Sohanian S, Ghanbarzadegan A. Solitary intraosseous neurofibroma of the mandible: Report of an extremely rare histopathologic feature. Indian J Pathol Microbiol. (2020) 63(2):276–8. doi: 10.4103/ijpm.Ijpm_28_19
13. Gupta A, Jajoo SN. Intrascrotal solitary neurofibroma. J Clin Diagn Res. (2020) 14(6):PD06–8. doi: 10.7860/jcdr/2020/43656.13790
14. Moletta L, Milanetto AC, Lico V, Farinati F, Alaggio R, Pedrazzoli S, et al. Neuroendocrine pancreatic tumor associated with a cerebral neurofibroma. A case report. Neuroendocrinology. (2015) 102(1–2):152. doi: 10.1016/j.pan.2014.05.651
15. Imai H, Kobayashi J, Manabe R, Namio H, Ichinona T. A case of neurofibroma located in the retroperitoneum involving the uncinate process of the pancreas. Gastroenterol Jpn. (1989) 24(4):421–4. doi: 10.1007/bf02774351
16. Kato O, Hattori K, Matsuyama M, Yoshizaki S. Neurofibroma of the pancreas - differentiation from carcinoma. Am J Gastroenterol. (1982) 77(9):630–2.
17. Gupta P, Aggarwal R, Sarangi R. Solitary neurofibroma of the adrenal gland not associated with type-1 neurofibromatosis. Urol Ann. (2015) 7(1):124–6. doi: 10.4103/0974-7796.148661
18. Abdullah , Xing J-p. A case report of solitary neurofibroma of the Vas deferens. Urol Case Rep. (2020) 28:101057. doi: 10.1016/j.eucr.2019.101057
19. Burk KS, Knipp D, Sahani DV. Cystic pancreatic tumors. Magn Reson Imaging Clin N Am. (2018) 26(3):405–20. doi: 10.1016/j.mric.2018.03.006
20. Doulamis IP, Mylonas KS, Kalfountzos CE, Mou D, Haj-Ibrahim H, Nasioudis D. T Pancreatic mucinous cystadenocarcinoma: Epidemiology and outcomes. Int J Surg. (2016) 35:76–82. doi: 10.1016/j.ijsu.2016.09.017
Keywords: pancreas, neurofibroma, ultrasound diagnosis, general surgery, case report
Citation: Song L, Jiang Z, Cui J, Gao B and Luo Y (2022) Benign Pancreatic Neurofibroma with Malignant Imaging Features: A Case Report and Literature Review. Front. Surg. 9:874006. doi: 10.3389/fsurg.2022.874006
Received: 18 February 2022; Accepted: 28 March 2022;
Published: 7 April 2022.
Edited by:
Mengling Feng, National University of Singapore, SingaporeReviewed by:
José Manuel Lopes, Faculdade de Medicina, Universidade do Porto, PortugalJaques Waisberg, Faculdade de Medicina do ABC, Brazil
Copyright Copyright © 2022 Song, Jiang, Cui, Gao and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Luo slguangyu@163.com
Speciality section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Ling Song
Ling Song BinYang Gao
BinYang Gao Yan Luo
Yan Luo