- 1Department of Pediatric Surgery, Binzhou Medical Unversity Hospital, Binzhou, China
- 2Department of Ultrasonic Medicine, Binzhou Medical Unversity Hospital, Binzhou, China
Introduction: Iliopsoas abscess with septicemia in the pediatric population is rare. Early diagnosis and effective management of this emergent disorder remain challenging for clinicians.
Case Presentation: A 14-year-old girl presented with right lateral and posterior hip pain and fever for 7 days before admission. Blood culture was positive for Staphylococcus aureus. Enhanced magnetic resonance imaging revealed abscesses located in the right iliopsoas muscle and on the surface deep to the fascia of the right sacroiliac joint that were 6.8 cm × 6.2 cm × 5.7 cm and 3.7 cm × 3.5 cm × 2.1 cm, respectively. A diagnosis of right iliopsoas abscesses with septicemia was made. The patient received intravenous antibiotics, underwent ultrasound-guided percutaneous catheter drainage, and recovered uneventfully. Medical literature regarding this issue published in the English language during the last two decades was reviewed.
Discussion: Primary synchronous psoas and iliacus muscle abscesses are rare and emergent disorders in the pediatric age group. The diagnosis is generally delayed owing to the deep anatomic location and nonspecific signs and symptoms. A comprehensive medical history, meticulous physical examination, and judicious use of imaging studies could establish a timely and accurate diagnosis. Surgeons should be aware of the occurrence of multiple abscesses. Prompt and adequate antibiotic therapy accompanied by a mini-invasive approach, such as ultrasound-guided, laparoscopic, or video-retroperitoneoscopic drainage of the infectious focus, if indicated and feasible, is important to achieve a good outcome in the management of iliopsoas abscess.
Introduction
Synchronous iliacus and psoas abscesses with septicemia are extremely rare, particularly in children (1). The clinical presentation of suppurative iliopsoas abscess is often subacute, and its differential diagnosis includes lumbar vertebral osteomyelitis, septic sacroiliitis, and regional suppurative lymphadenitis (2). Moreover, limited hip joint motility may occur even in the absence of actual involvement of the hip. Nonspecific signs and symptoms of iliopsoas abscess may also mimic septic arthritis of the hip (3, 4). Owing to the deep location of the infection and its adjacent structures, including the intestine, iliac vessels, and femoral nerve, it is difficult to identify a safe approach for draining the abscesses percutaneously. Ultrasound-guided aspiration and catheter drainage are most commonly used for single abscess, while open surgery is recommended for complex abscess (3, 5–7). Herein, we present a case of an adolescent with iliopsoas abscess accompanying septicemia. The patient recovered uneventfully with ultrasound-guided percutaneous catheter drainage and intravenous antibiotic therapy. The literature on its pathophysiology, diagnosis, and management is also reviewed.
Case Description
A 14-year-old female patient was brought to an outpatient clinic because of right hip pain and limp with mild fever for 7 days, for which she received acupuncture therapy and intravenous antibiotic therapy. Her pain worsened with persistent high fever, reaching 40°C. The patient had no cough, shortness of breath, vomiting, or abdominal pain or distension. There was no history of food or drug allergy or trauma, except for a history of long jump exercise 1 day before the onset of hip pain.
A physical examination demonstrated that the patient flexed her right thigh and rotated it outward along with the right sacroiliac joint, and she had paralumbar (L4, 5) tenderness.
The laboratory investigation showed a white blood cell (WBC) count of 14.5 × 109/L, neutrophil percentage of 77.4%, neutrophil count of 11.3 × 109/L, serum C-reactive protein (CRP) level of 179.10 mg/L (reference range for inflammation, <10), and erythrocyte sedimentation rate (ESR) of 120 mm/h. Plain radiographs of the pelvis showed that the articular surface was blurred with unwidened joint space. Soft tissue infection or inflammation adjacent to the right sacroiliac joint was suspected. An ultrasound examination revealed no abnormal alterations in the early stage.
After admission, the patient received intravenous antibiotics (cefazolin sodium) at a dose of 1 g bid. After treatment for 2 days, there was a slight reduction in the fever; however, no significant improvement was noted in the regional pain. On hospital day 3, the blood cultures were positive for Staphylococcus aureus, which was sensitive to oxacillin. Enhanced magnetic resonance imaging (MRI) revealed an abscess located posterolateral to the right psoas and was 6.8 cm × 6.2 cm × 5.7 cm, while another one was located within the swollen right iliacus muscle and was 3.7 cm × 3.5 cm × 2.1 cm (Figure 1). The diagnosis of synchronous primary right iliacus and psoas abscesses with septicemia was confirmed. Intravenous antibiotics (cefoperazone sulbactam) were administered based on the results of blood cultures and antibiotic sensitivity tests.
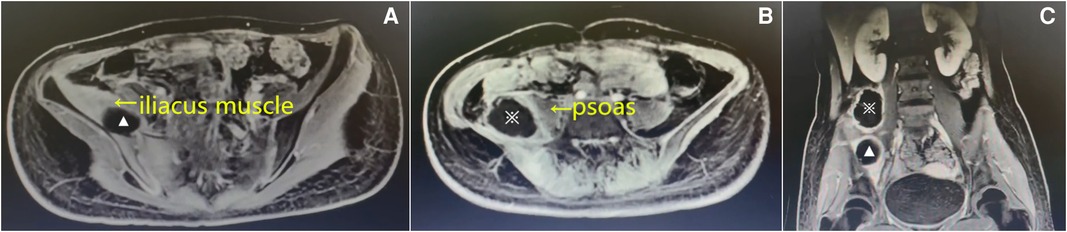
Figure 1. Enhanced magnetic resonance imaging (MRI) on the 3rd admission day. Axial (A) and (B) and coronal T1-weighted images (C) showing that the abscesses were located in the right iliacus muscle (▴) and posterolateral to the right psoas (※).
The fever and regional pain were relieved; however, the patient still could not move her right leg. Ultrasound images revealed mixed echo cystic lesions located posteriorly to the right psoas and within the swollen right iliacus muscle that were 7.1 cm × 4.3 cm and 4.9 cm × 1.9 cm, respectively. Ultrasound-guided percutaneous catheter drainage of the abscess was planned and performed on hospitalization day 14. The abscesses were drained via a different anterior route above the anterior superior iliac spine (Figure 2). Using the Seldinger technique, two 8-F pigtail drainage catheters were placed separately in the abscess cavities, and 50 mL of thick yellowish pus from the psoas abscess and 30 mL of thin yellowish pus from the iliacus muscle abscess were drained out. The catheters were then properly fixed and connected to two aseptic suction drainage systems. The growth of S. aureus was observed in the pus from both abscesses, and it was sensitive to oxacillin.
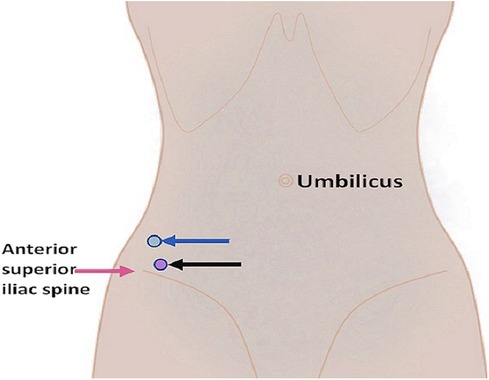
Figure 2. Diagram illustrating the puncture sites for the psoas abscess (blue arrow) and the iliacus muscle abscess (black arrow).
The intravenous administration of cefoperazone sulbactam was continued for 2 weeks. Normal saline was intermittently applied to flush the abscess cavities separately to ensure sufficient drainage. The regional pain was significantly relieved on the first day after drainage. On the third day postdrainage, the patient was afebrile and could leave the bed and move around independently. On the fifth postoperative day, the drainage of the collection was significantly reduced. Ultrasonography revealed no obvious residual abscesses on the eighth day after drainage, and the WBC count and serum CRP level were within the normal range (10 mg/L). The bacterial culture of the drainage fluid was negative. The drainage catheters were removed on the 12th postdrainage day. The patient was discharged from the hospital on the 14th postdrainage day.
In a 6-month follow-up period by a telephone interview or the outpatient clinic, the patient recovered completely.
The medical literature was scanned for pediatric iliopsoas abscess, iliopsoas abscess in children, psoas abscess in children, or psoas abscess in neonates during the last two decades (from January 2002 to December 2021). There are 44 papers published in the English language, and the data associated with these issues are usually in the form of case report(s) (8–50). Clinical data included age (0–18 years), gender, medical history, symptoms and signs, inflammatory parameters, bacterial culture, imaging, treatment options, and outcomes. Sixty-nine cases met the inclusion criteria. The clinical characteristics are summarized in Table 1. Onset time has ranged from newborn to 18 years of age, about 75% of the cases have been reported in the age group of 4–18 years, and the ratio of male to female is 1.5:1. Only 1.3% of the cases have predisposing factors, such as diabetes, immune deficiency, and suspected trauma. Fever is the most common symptom; however, 1.3% of the cases are afebrile, especially in newborn patients. More than half of the cases present hip mobility limitations and a painful hip. Our case displayed high fever, painful hip, and poor mobility with a flexion deformity. The diagnosis delay ranges from 4 to 30 days. Nearly all cases with primary iliopsoas abscess have significant elevations in inflammatory parameters, such as WBC and CRP levels. Ultrasonography and/or MRI are the main imaging tools used for making the diagnosis. In the present case, MRI indicated the abscess location. In most cases, the cause is not detected; however, 11 cases have secondary abscess from inflammation of perforated appendicitis, sacroiliitis, or Crohn’s disease with fistula. S. aureus is the most frequently isolated organism from blood and/or pus in pediatric iliopsoas abscesses. Five cases have methicillin-resistant S. aureus infection. The second causative microorganism isolated is Streptococci. Regarding the management of pediatric iliopsoas abscess, antibiotic therapy alone is sufficient in 11 cases; however, abscess aspiration or drainage is required in nearly 85% of the cases, including more than half of them undergoing open surgical drainage. In most cases, the duration of antibiotic therapy lasts over 3 to 6 weeks. The outcome of iliopsoas abscess usually is good.
Discussion
Iliopsoas abscess was usually classified as being primary or secondary. Hematogenous spread of causative pathogens from a distal focus can cause primary iliopsoas abscess. The spread from an adjacent suppurative lesion, including appendicitis, Crohn’s disease, pancreatitis, pyelonephritis, ipsilateral septic arthritis, spondylodiscitis, lumbar vertebral osteomyelitis, septic sacroiliitis, and thoracolumbar spinal tuberculosis, can cause secondary iliopsoas abscess. Iiopsoas abscess in the pediatric population is rare and often primary. Although the precise cause of iliopsoas abscess is unclear, bacterial lymphadenitis, previous traumatism (as in the present case), and hematogenous seeding of the bacteria have been proposed as initial factors (51–56). Transient bacteremia can affect previously healthy children after strenuous or vigorous exercise or following localized and possibly unnoticed trauma (1, 2), as in the present case. Patients with decreased immune response, especially with primary immune deficiencies, will be more susceptible to pyogenic infections, including iliopsoas abscess. In more than three-fourths of cases, S. aureus has been causing bacteria in primary iliopsoas abscess, but for the secondary type, Gram-negative or anaerobic bacteria usually grow on pus culture (51–56).
In general, iliopsoas abscess has a subacute clinical course, and patients seek medical assistance in an average of 5–6 days after the onset of signs and symptoms. Children may present with a more acute presentation (3, 57). Pyomyositis, including iliopsoas abscess(es), has three consecutive clinical stages (58, 59). The onset is generally insidious with edema and pain in the involved muscle group. Only a few patients (approximately 2%) present at this early stage (diffuse muscle infection). Most patients present in the second phase (abscess formation). The third phase (sepsis) is characterized by the presence of systemic toxic symptoms such as high fever or septic shock (58, 59). The present patient had a history of extenuating exercise a day before the onset of the disorder and progressed rapidly, and the organism of blood and pus cultures was S. aureus, which is consistent with most cases reported in the literature.
Suppurative nontuberculous abscesses of the psoas and iliacus muscles in children are uncommon (1). The variety of symptoms and signs resembling those of primary infection in the hip or intra-abdominal organs and the deep location of the muscles make early and accurate diagnosis challenging (60). Delays in diagnosis are the major cause of morbidity and can even be life-threatening (5–7). Regional pain corresponding to the affected area, a common symptom of iliacus and psoas abscess, occurs in more than 90% of cases in the pediatric population (7). In the present case, the patient experienced hip pain after strenuous exercise 1 day before the onset of the hip pain. The pain was aggravated, and a high fever occurred. However, some cases of iliacus and psoas abscesses have no typical clinical manifestations. Acute pain in the lumbosacral region, buttocks, groin region, hip joint, and thigh should be considered for this condition (8, 54). Although blood tests are often nonspecific, the abnormal elevation of WBC counts, ESR, and CRP indicates the existence of an acute systemic inflammatory response (8). In the present case, the blood and pus cultures were positive for S. aureus, suggesting primary synchronous iliacus and psoas abscesses. Early blood and pus bacterial cultures are vital to assist clinicians with selecting appropriate antibiotics for the management of this acute condition (6, 61). Despite imaging investigations, a high index of suspicion of this disease and the use of imaging modalities, such as repeated ultrasonography or MRI with contrast, may help confirm the diagnosis earlier (61, 62). Ultrasonography is a common and important method for diagnosing iliopsoas abscess (55). Ultrasonography is free from radiation effects and easy to use; however, it is a practitioner-dependent diagnostic tool. Moreover, it is difficult to display the retroperitoneal area owing to the presence of intestinal gases (8). In our case, an ultrasound examination was performed, and no significant alterations were found due to the absence of abscess formation in its early stage. A negative ultrasound is insufficient to exclude iliopsoas abscess. Repeated point-of-care ultrasonography and further investigations are recommended to ensure an early diagnosis (63). MRI is the preferred diagnostic imaging modality which can play an important role in the early recognition of bacterial pyomyositis (58, 59). MRI can provide useful information to clinicians to make an early diagnosis and reveal the precise site and size of the abscess. Furthermore, MRI is preferred because it is superior to computed tomography (CT) for displaying soft tissues and does not require intravenous contrast material to display the abscess wall and peripheral structures (61, 62). In our case, an enhanced MRI was conducted, and two separate abscesses were revealed and confirmed later by ultrasound-guided percutaneous catheter drainage. We recommend that patients suspected of having iliopsoas abscess undergo MRI for early diagnosis and treatment planning.
The management of this emergency status should consist of the early use of appropriate antibiotics along with abscess drainage. Due to the deep location and adjacent structures, such as the intestine, iliac vessels, and femoral nerve, it is difficult to identify a safe approach for placing a catheter to drain the abscesses percutaneously. The common organism causing iliacus muscle or psoas abscess is S. aureus, and antistaphylococcal antibiotics should be administered as early as possible after admission (64, 65). The patient in the present case received intravenous cefazolin sodium after admission; thereafter, the antibiotic was adjusted to cefoperazone sulbactam according to the positive blood culture of S. aureus. Accordig to the patients’ clinical status and lesion size, patients generally receive antibiotics for 1 week to 10 days intravenously, for a total of 5–6 weeks orally (58). Occasionally, intravenous therapy is administered for 6 weeks because the organism is multiresistant to oral antibiotics (66). In the present case, the duration of antibiotics depended on the clinical response, and antibiotics were continued for at least 2 weeks after drainage and fever resolution.
There are a few reports regarding image-guided percutaneous drainage for the management of iliacus and psoas abscesses. Most patients could be managed using radiologically or ultrasound-guided percutaneous drainage with optimal results, even in a neonate with an iliopsoas abscess (9, 10). Ultrasound-guided drainage of the iliacus and/or psoas abscess(es) conducted by experienced physicians has several benefits, including precision, minimal invasiveness, fast recovery, avoidance of radiation exposure, and invisible scars (67). Ultrasound-guided percutaneous needle aspiration or catheter drainage might be the first choice according to the available facilities and experienced physicians in ultrasonic medicine (8, 11, 56, 68). In our case, using the ultrasound-guided method, two 8-Fr pigtail catheters were successfully placed in two separate abscesses. An intermittent flush with normal saline was recommended to ensure sufficient drainage. However, if a high risk of adjacent important structure injury related to aspiration or multiloculated abscess with very thick pus was assessed by imaging, laparoscopic-assisted, retroperitoneoscopic-assisted, and even traditional open surgical drainage (6, 10, 12, 13, 62, 69–72) remains an alternative option.
The indication for catheter removal may include the following parameters: complete regional pain relief, normal body temperature for more than 3 days, freely able to leave the bed, WBC count and CRP levels within the normal range, negative drainage fluid bacterial culture, and disappearance of the abscess cavity on ultrasonography (73).
In conclusion, primary iliopsoas abscess is extremely rare. The diagnosis is generally delayed owing to the deep location and unspecific signs and symptoms. A comprehensive medical history, meticulous physical examination, and judicious use of imaging studies could establish an accurate diagnosis. MRI is invaluable for establishing the diagnosis and anatomical site. Surgeons should be aware of the occurrence of multiple abscesses. In the era of minimally invasive surgery, prompt and adequate antibiotic therapy accompanied by mini-invasive approaches, such as ultrasound-guided, laparoscopic or video-retroperitoneoscopic drainage of the infectious focus, if indicated and feasible, is important to achieve a good outcome and significantly decrease the overall morbidity and mortality in the management of iliopsoas abscess (56, 74–76).
Based on the literature about pediatric iliopsoas abscess (51, 53, 56, 12, 77–93), a flow chart of its diagnosisa and treatment was recommended as shown in Figure 3.
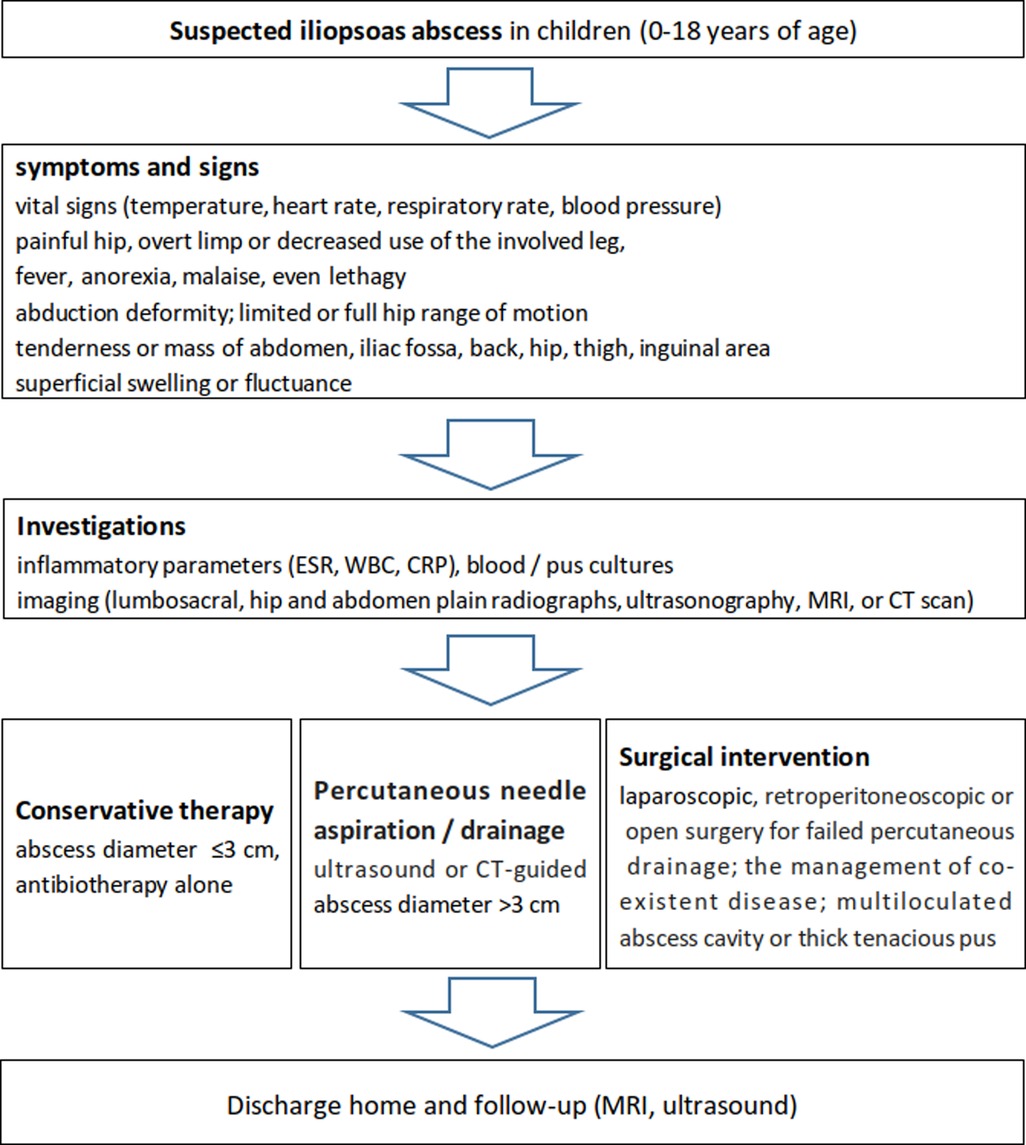
Figure 3. Recommended flow chart for the diagnosis and management of iliopsoas abscess in the pediatric population according to the literature and our experience.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Binzhou Medical University Hospital. Written informed consent to participate in this study was provided by the participant's legal guardian/next of kin.
Author Contributions
KJ, TF, and LG: conceptualization. KJ, WZ, GF, GC, XL, SR, and LG: investigation. KJ, WZ, and TF: writing—original draft preparation. KJ, GC, TF, and LG: writing—review and editing. TF and LG: supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the patient and the family and appreciate all staff for their cooperation. The authors also thank Professor Xu Wang from the Department of Nuclear Medicine, Radiology, Binzhou Medical University Hospital for his support in rechecking the radiograph and MRI. The authors also acknowledge English editing from Editage.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schut JM, Meradji M, Oranje AP, Bergmeijer JH, Schuller JL. Double-sided psoas abscess in a young infant: sonographic and radiographic findings. Pediatr Radiol. (1988) 18:176–7. doi: 10.1007/BF02387569
2. Perry J, Barrack RL, Burke SW, Haddad RJ Jr. Psoas abscess mimicking a septic hip. Diagnosis by computed tomography. J Bone Joint Surg Am. (1985) 67:1281–3. PMID: 4055855
3. Christin L, Sarosi GA. Pyomyositis in North America: case reports and review. Clin Infect Dis. (1992) 15:668–77. doi: 10.1093/clind/15.4.668
4. Chen W-S, Wan Y-L. Iliacus pyomyositis mimicking septic arthritis of the hip joint. Arch Orthop Trauma Surg. (1996) 115:233–5. doi: 10.1007/BF00434562
5. Livne M, Serour F, Aladjem M, Vinograd I. General peritonitis induced by rectal examination: an unusual complication of primary psoas abscess. Eur J Pediatr Surg. (1994) 4:186–187. doi: 10.1055/s-2008-1066100
6. Charalampopoulos A, Macheras A, Charalabopoulos A, Fotiadis C, Charalabopoulos K. Iliopsoas abscesses: diagnostic, aetiologic and therapeutic approach in five patients with a literature review. Scand J Gastroenterol. (2009) 44:594–9. doi: 10.1080/00365520902745054
7. Li Y, Funakoshi H, Shiga T, Fujitani S. Iliopsoas abscess. Cleve Clin J Med. (2017) 84:833–4. doi: 10.3949/ccjm.84a.17002
8. Karlı A, Belet N, Danacı M, Avcu G, Paksu Ş, Köken Ö, et al. Iliopsoas abscess in children: report on five patients with a literature review. Turk J Pediatr. (2014) 56:69–74. PMID: 24827950
9. Alvi AR, Ur Rehman Z, Nabi ZU. Pyogenic psoas abscess: case series and literature review. Trop Doct. (2010) 40:56–8. doi: 10.1258/td.2009.090212
10. Nisar MU, Sikander S, Noorain Z, Baig MS, Akhtar N. Primary Iliopsoas abscess in a neonate. J Coll Physicians Surg Pak. (2019) 29:S45–7. doi: 10.29271/jcpsp.2019.06.S45
11. Elliott C. Paediatric Iliopsoas abscess: a case report. Australas J Ultrasound Med. (2013) 16:198–201. doi: 10.1002/j.2205-0140.2013.tb00248.x
12. Katara AN, Shah RS, Bhandarkar DS, Unadkat RJ. Retroperitoneoscopic drainage of a psoas abscess. J Pediatr Surg. (2004) 39:e4–5. doi: 10.1016/j.jpedsurg.2004.05.033
13. Han YM, Kim AY, Lim RK, Park KH, Byun SY, Kim SH, et al. Neonatal iliopsoas abscess: the first Korean case. J Korean Med Sci. (2015) 30:1203–6. doi: 10.3346/jkms.2015.30.8.1203
14. Al-Zaiem MM, Bajuifer SJ, Fattani MO, Al-Zaiem FM. Bilateral iliopsoas abscess associated with right hip septic arthritis in a neonate. Saudi Med J. (2014) 35:743–6. PMID: 25028234
15. Belet N, Akyurt B, Karlı A, Gülman B, Selçuk MB, Şensoy G. Psoas abscess with septic arthritis of the hip: a case report. Turk J Pediatr. (2014) 56:320–3. PMID: 25341610
16. Okada Y, Yamataka A, Ogasawara Y, Matsubara K, Watanabe T, Lane GJ, et al. Ilio-psoas abscess caused by methicillin-resistant Staphylococcus aureus (MRSA): a rare but potentially dangerous condition in neonates. Pediatr Surg Int. (2004) 20:73–4. doi: 10.1007/s00383-003-1088-0
17. Kamiya Y, Hasegawa T, Takegami Y, Horiba K, Ando S, Torii Y, et al. Primary psoas abscess caused by group A streptococcus in a child: case report with microbiologic findings. J Infect Chemother. (2016) 22:811–4. doi: 10.1016/j.jiac.2016.06.011
18. Okan F, Ince Z, Coban A, Can G. Neonatal psoas abscess simulating septic arthritis of the hip: a case report and review of the literature. Turk J Pediatr. (2009) 51:389–91. PMID: 19950852
19. Bingöl-Koloğlu M, Ciftçi AO, Senocak ME, Tanyel FC, Karnak I, Büyükpamukçu N. Xanthogranulomatous pyelonephritis in children: diagnostic and therapeutic aspects. Eur J Pediatr Surg. (2002) 12:42–8. doi: 10.1055/s-2002-25085
20. Gentile Á, Bakir J, Ensinck G, Cancellara A, Casanueva EV, Firpo V, et al. Grupo de Trabajo de Staphylococcus aureus. Community-acquired methicillin-resistant Staphylococcus aureus infections: hospitalization and case fatality risk in 10 pediatric facilities in Argentina. Arch Argent Pediatr. (2018) 116:e47–e53. English, Spanish. doi: 10.5546/aap.2018.eng.e47
21. Tural-Kara T, Özdemir H, Fitöz S, Çiftçi E, Yalçınkaya F. Bone marrow aspiration complications: Iliopsoas abscess and sacroiliac osteomyelitis. Turk J Pediatr. (2016) 58:562–5. doi: 10.24953/turkjped.2016.05.019
22. Diacinti D, Gioia C, Vullo F, Cannavale G, Catalano C, Valesini G. Magnetic resonance imaging findings of infectious sacroiliitis associated with iliopsoas abscess: a case report in a young male. Reumatismo. (2018) 70:264–7. doi: 10.4081/reumatismo.2018.1071
23. Comegna L, Guidone PI, Prezioso G, Franchini S, Petrosino MI, Di Filippo P, et al. Pyomyositis is not only a tropical pathology: a case series. J Med Case Rep. (2016) 10:372. doi: 10.1186/s13256-016-1158-2
24. Ishibashi H, Oshio T, Sogami T, Nii A, Mori H, Yada K, et al. Iliopsoas abscess in an infant. J Med Invest. (2014) 61:213–6. doi: 10.2152/jmi.61.213
25. Yano T, Takamatsu H, Noguchi H, Tahara H, Kaji T, Saruwatari Y, et al. Iliopsoas abscess in the neonate. J Pediatr Surg. (2004) 39:e13–5. doi: 10.1016/j.jpedsurg.2004.03.080
26. Tuerlinckx D, Bodart E, de Bilderling G, Nisolle JF. Pneumococcal psoas pyomyositis associated with complement deficiency. Pediatr Infect Dis J. (2004) 23:371–3. doi: 10.1097/00006454-200404000-00025
27. Deanehan JK. Point-of-care ultrasound identification of a psoas abscess in a child presenting with hip pain. Pediatr Emerg Care. (2017) 33:437–9. doi: 10.1097/PEC.0000000000001159
28. Atkinson C, Morris SK, Ng V, Friedman JN. A child with fever, hip pain and limp. CMAJ. (2006) 174:924. doi: 10.1503/cmaj.051165
29. Panda PK, Sharawat IK. Acute flaccid paralysis in a child: it is not guillain-barré syndrome always!. Indian Pediatr. (2021) 58:93–4. doi: 10.1007/s13312-021-2115-8
30. Sauer C, Gutgesell M. Ballet dancer with hip and groin pain: crohn disease and psoas abscess. Clin Pediatr (Phila). (2005) 44:731–3. doi: 10.1177/000992280504400814
31. Ladhani S, Phillips SD, Allgrove J. Low back pain at presentation in a newly diagnosed diabetic. Arch Dis Child. (2002) 87:543–4. doi: 10.1136/adc.87.6.543
32. Sharma H, Kondekar SV, Ahmed M, Rathi S. Eosinophilia in an acutely limping child: an easy guess of rare systemic aetiology!. J Clin Diagn Res. (2016) 10:SD03–4. doi: 10.7860/JCDR/2016/20503.8018
33. Venkatesan DK, Chaudhary H, Verma S. Every loin swelling in an infant is not a renal mass: rare presentation of psoas abscess in an infant. BMJ Case Rep. (2020) 13:e237137. doi: 10.1136/bcr-2020-237137
34. Horiuchi A, Kameoka K, Kuwabara J, Watanabe Y, Kawakami S, Tauchi H, et al. Neonatal iliopsoas abscess. Pediatr Int. (2012) 54:712–4. doi: 10.1111/j.1442-200X.2012.03593.x
35. Chintakrinda AK, Das B, Dogra S, Mitra D. Primary iliopsoas abscess in an infant: a case report. J Indian Assoc Pediatr Surg. (2018) 23:222–4. doi: 10.4103/jiaps.JIAPS_215_17
36. Vastyan AM, MacKinnon EA. Primary psoas abscess in a neonate. Am J Perinatol. (2006) 23:253–4. doi: 10.1055/s-2006-939532
37. Maines E, Franceschi R, Cauvin V, d’Annunzio G, Pini Prato A, Castagnola E, et al. Iliopsoas abscess in adolescents with type 1 diabetes mellitus. Clin Case Rep. (2015) 3:638–42. doi: 10.1002/ccr3.267
38. Khalid M, Junejo S, Mir F. Invasive community acquired methicillin-resistant staphylococcal aureus (CA-MRSA) infections in children. J Coll Physicians Surg Pak. (2018) 28:S174–7. doi: 10.29271/jcpsp.2018.09.S174
39. Akın MŞ, Ağaçkıran D, Ünal E, Yavuz ÖÖ, Yiğit Ş. Neonatal iliopsoas abscess presenting with transient cyanosis of a single extremity: a case report and review of the literature. Turk J Pediatr. (2020) 62:160–4. doi: 10.24953/turkjped.2020.01.025
40. Ovadia D, Ezra E, Ben-Sira L, Kessler A, Bickels J, Keret D, et al. Primary pyomyositis in children: a retrospective analysis of 11 cases. J Pediatr Orthop B. (2007) 16:153–9. doi: 10.1097/BPB.0b013e3280140548
41. Oosthuizen GV, Harrower JE, Hadley GP. Psoas abscess in children: making the diagnosis. Trop Doct. (2006) 36:246–7. doi: 10.1258/004947506778604760
42. Neves MT, Livani B, Belangero WD, Tresoldi AT, Pereira RM. Psoas abscesses caused by Paracoccidioides brasiliensis in an adolescent. Mycopathologia. (2009) 167:89–93. doi: 10.1007/s11046-008-9152-x
43. Ogata H, Nagasawa K, Takeuchi N, Hagiwara S, Sawada D, Umimura T, et al. Psoitis and multiple venous thromboses caused by Panton Valentine Leukocidin-positive methicillin-sensitive Staphylococcus aureus in a 12-year-old girl: a case report. J Infect Chemother. (2019) 25:630–4. doi: 10.1016/j.jiac.2019.02.019
44. Nogueira H, Pereira J, Couto A, Alves J, Lopes D, Freitas J, et al. Pyogenic sacroiliitis in a pediatric patient: a rare case of infection by streptococcus intermedius. J Am Acad Orthop Surg Glob Res Rev. (2018) 2:e052. doi: 10.5435/JAAOSGlobal-D-17-00052
45. Kei S, Chan C, Yuen M. Chan J: Pyogenic infection of the sacroiliac joint complicated by iliacus abscess in a paediatric patient. J HK Coll Radiol. (2007) 10:70–73.
46. Le Doare K, Brooker E, Ladhani S. Travel-associated salmonella mbandaka sacroiliac osteomyelitis in a healthy adolescent. Case Rep Infect Dis. (2013) 2013:543147. doi: 10.1155/2013/543147
47. Latawiec-Mazurkiewicz I, Juszkiewicz P, Pacanowski J, Kwas A, Rybkiewicz M, Rudnicki J, et al. Tumour-like inflammatory abdominal conditions in children. Eur J Pediatr Surg. (2005) 15:38–43. doi: 10.1055/s-2004-830544
48. Wu SY, Wei TS, Chen YC, Huang SW. Vertebral osteomyelitis complicated by iliopsoas muscle abscess in an immunocompetent adolescent: successful conservative treatment. Orthopedics. (2012) 35:e1576–80. doi: 10.3928/01477447-20120919-34
49. Sobaih B, Sobaih L, Zamil FA. Iliopsoas abscess. Pak J Med Sci. (2021) 37(2):605–7. doi: 10.12669/pjms.37.2.3816
50. Pérez-López LM, Vara-Patudo I, Torner-Rubies F, Moreno-Romo D, Cabo LS, Fortuny C, et al. Pediatric psoas abscess, early diagnosis of a challenging condition. J Acute Med. (2017) 7:158–66. doi: 10.6705/j.jacme.2017.0704.004
51. Gruenwald I, Abrahamson J, Cohen O. Psoas abscess: case report and review of the literature. J Urol. (1992) 147(6):1624–6. doi: 10.1016/s0022-5347(17)37650-4
52. Ibrahim FMF, El-Rady AEMA. Transverse process osteotomy for surgical drainage of primary iliopsoas abscess and secondary cases combined with spondylodiscitis. Int Orthop. (2021) 45:165–71. doi: 10.1007/s00264-020-04732-5
53. Yacoub WN, Sohn HJ, Chan S, Petrosyan M, Vermaire HM, Kelso RL, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. (2008) 196:223–7. doi: 10.1016/j.amjsurg.2007.07.032
54. Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg. (1986) 10:834–43. doi: 10.1007/BF01655254
55. Toren A, Ganel A, Lotan D, Graif M. Delayed diagnosis of a primary psoas abscess mimicking septic arthritis of the hip. J Pediatr Surg. (1989) 24:227–8. doi: 10.1016/s0022-3468(89)80259-3
56. Kang M, Gupta S, Gulati M, Suri S. Ilio-psoas abscess in the paediatric population: treatment by US-guided percutaneous drainage. Pediatr Radiol. (1998) 28:478–81. doi: 10.1007/s002470050389
57. Kleiner O, Cohen Z, Barki Y, Mares AJ. Unusual presentation of psoas abscess in a child. J Pediatr Surg. (2001) 36:1859–60. doi: 10.1053/jpsu.2001.28870
58. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Primary pyomyositis. J Bone Joint Surg Am. (2002) 84:2277–86. doi: 10.2106/00004623-200212000-00024
59. Yu CW, Hsiao JK, Hsu CY, Shih TT. Bacterial pyomyositis: MRI and clinical correlation. Magn Reson Imaging. (2004) 22:1233–41. doi: 10.1016/j.mri.2004.08.005
60. Scaglia M, Lugani G, Cassini M, Ambrosini C, Magnan B. Delayed diagnosis and treatment of a psoas abscess as a link between spondylodiscitis and septic necrosis of the femoral head: a case report. Acta Biomed. (2020) 91:241–7. doi: 10.23750/abm.v91i4-S.9627
61. Xu BY, Vasanwala FF, Low SG. A case report of an atypical presentation of pyogenic iliopsoas abscess. BMC Infect Dis. (2019) 19:58. doi: 10.1186/s12879-019-3675-2
62. Hoffer FA, Shamberger RC, Teele RL. Ilio-psoas abscess: diagnosis and management. Pediatr Radiol. (1987) 17:23–7. doi: 10.1007/BF02386590
63. Le Coz J, Orlandini S, Titomanlio L, Rinaldi VE. Point of care ultrasonography in the pediatric emergency department. Ital J Pediatr. (2018) 44:87. doi: 10.1186/s13052-018-0520-y
64. Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J. (2004) 80:459–62. doi: 10.1136/pgmj.2003.017665
65. Hu SY, Hsieh MS, Chang YT, Huang CC, Tsai CA, Tsai CL, et al. Clinical features, management, and outcome of iliopsoas abscess associated with cardiovascular disorders: a hospital-based observational case series study. BMC Musculoskelet Disord. (2019) 20:474. doi: 10.1186/s12891-019-2798-3
66. Habeych ME, Trinh T, Crum-Cianflone NF. Purulent infectious myositis (formerly tropical pyomyositis). J Neurol Sci. (2020) 413:116767. doi: 10.1016/j.jns.2020.116767
67. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. (2013) 23:45–8. doi: 10.1097/SLE.0b013e31826e0ac9
68. Dib M, Bedu A, Garel C, Mazda K, Philippe-Chomette P, Rajguru M, et al. Ilio-psoas abscess in neonates: treatment by ultrasound-guided percutaneous drainage. Pediatr Radiol. (2000) 30:677–80. doi: 10.1007/s002470000309
69. Liu XL, Li L, Li SL, Liu L, Li LX. One case of right iliacus abscess treated by extraperitoneal tube drainage under single-port laparoscopic monitoring. J Development Med. (2019) 7:229–230, (in Chinese). doi: 10.3969/j.issn.2095-5340.2019.03.014
70. Wu TL, Huang CH, Hwang DY, Lai JH, Su RY. Primary pyogenic abscess of the psoas muscle. Int Orthop. (1998) 22:41–3. doi: 10.1007/s002640050205
71. Malhotra R, Singh KD, Bhan S, Dave PK. Primary pyogenic abscess of the psoas muscle. J Bone Joint Surg Am. (1992) 74:278–84. PMID: 1541621
72. Afaq A, Jain BK, Dargan P, Bhattacharya SK, Rauniyar RK, Kukreti R. Surgical drainage of primary iliopsoas abscess-safe and cost-effective treatment. Trop Doct. (2002) 32:133–5. doi: 10.1177/004947550203200304
73. Fujioka M, Yoshida S, Kitamura R, Matsuoka Y. Iliopsoas muscle abscess secondary to sacral pressure ulcer treated with computed tomography-guided aspiration and continuous irrigation: a case report. Ostomy Wound Manage. (2008) 54:44–8. PMID: 18716341
74. Procaccino JA, Lavery IC, Fazio VW, Oakley JR. Psoas abscess: difficulties encountered. Dis Colon Rectum. (1991) 34:784–9. doi: 10.1007/BF02051071
75. Froiio C, Bernardi D, Lovece A, Bonavina G, Manzo CA, Asti E, et al. Retroperitoneoscopic drainage of psoas abscess: a systematic review. Surg Laparosc Endosc Percutan Tech. (2020) 31:241–6. doi: 10.1097/SLE.0000000000000879
76. Hong CH, Hong YC, Bae SH, Son MW, Won SH, Ryu A, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single-center case series and literature review. Medicine (Baltimore). (2020) 99:e19640. doi: 10.1097/MD.0000000000019640
77. Chern CH, Hu SC, Kao WF, Tsai J, Yen D, Lee CH. Psoas abscess: making an early diagnosis in the ED. Am J Emerg Med. (1997) 5:83–8. doi: 10.1016/s0735-6757(97)90057-7
78. Zhang X, Zhang Z, Zhang Y, Wang J, Lu M, Hu W, et al. Minimally invasive retroperitoneoscopic surgery for psoas abscess with thoracolumbar tuberculosis. Surg Endosc. (2015) 29:2451–5. doi: 10.1007/s00464-014-3913-z
79. Atkin G, Qurashi K, Isla A. Laparoscopic drainage of bilateral tuberculous psoas abscesses. Surg Laparosc Endosc Percutan Tech. (2005) 15:380–2. doi: 10.1097/01.sle.0000191590.92108.c4
80. Froiio C, Bernardi DT, Asti E, Bonavina L. Retroperitoneoscopic drainage of cryptogenic psoas abscess. BMJ Case Rep. (2020) 13:e235579. doi: 10.1136/bcr-2020-235579
81. Seretis C, Seretis F, Yahia S, Gill J, Malik A, Zayyan K. Percutaneous retroperitoneoscopic drainage of complex extraperitoneal abscesses using flexible endoscopy: description of technique and perioperative care. Chirurgia (Bucur). (2020) 115:792–7. doi: 10.21614/chirurgia.115.6.792
82. Kodama K, Takase Y, Motoi I, Mizuno H, Goshima K, Sawaguchi T. Retroperitoneoscopic drainage of bilateral psoas abscesses under intraoperative laparoscopic ultrasound guidance. Asian J Endosc Surg. (2014) 7:179–81. doi: 10.1111/ases.12091
83. Joob B, Wiwanitkit V. Retroperitoneoscopic drainage of bilateral psoas abscesses. Asian J Endosc Surg. (2014) 7(4):345. doi: 10.1111/ases.12117
84. Shields D, Robinson P, Crowley TP. Iliopsoas abscess–a review and update on the literature. Int J Surg. (2012) 10:466–9. doi: 10.1016/j.ijsu.2012.08.016
85. Neola B, D’Ambra M, Capasso S, Russo M, Ferulano GP. Laparoscopic drainage of a recurrent psoas abscess. Ann Ital Chir. (2014) 85(ePub):S2239253X14021847. PMID: 24709630
86. Otowa Y, Sumi Y, Kanaji S, Kanemitsu K, Yamashita K, Imanishi T, et al. Appendicitis with psoas abscess successfully treated by laparoscopic surgery. World J Gastroenterol. (2014) 20:8317–9. doi: 10.3748/wjg.v20.i25.8317
87. Gupta S, Suri S, Gulati M, Singh P. Ilio-psoas abscesses: percutaneous drainage under image guidance. Clin Radiol. (1997) 52:704–7. doi: 10.1016/s0009-9260(97)80036-0
88. Jain AK. The conservative management of acute pyogenic iliopsoas abscess in children. J Bone Joint Surg Br. (1999) 81:182. PMID: 10068035
89. Tong CW, Griffith JF, Lam TP, Cheng JC. The conservative management of acute pyogenic iliopsoas abscess in children. J Bone Joint Surg Br. (1998) 80:83–5. doi: 10.1302/0301-620x.80b1.8005
90. Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg. (2009) 144:946–9. doi: 10.1001/archsurg.2009.144
91. Schwaitzberg SD, Pokorny WJ, Thurston RS, McGill CW, Athey PA, Harberg FJ. Psoas abscess in children. J Pediatr Surg. (1985) 20:339–42. doi: 10.1016/s0022-3468(85)80215-3
92. Čizmić A, Žganjer M, Cigit I, Grmoja T, Žganjer V. Primary psoas abscess in children. Paediatria Croatica. (2011) 55:281–3.
Keywords: staphylococcus aureus, ultrasonography, percutaneous catheter drainage, pediatrics, iliopsoas abscess
Citation: Jiang K, Zhang W, Fu G, Cui G, Li X, Ren S, Fu T and Geng L (2022) Ultrasound-Guided Percutaneous Drainage of Iliopsoas Abscess With Septicemia in an Adolescent: A Case Report and Literature Review. Front. Surg. 9:871292. doi: 10.3389/fsurg.2022.871292
Received: 8 February 2022; Accepted: 30 May 2022;
Published: 27 June 2022.
Edited by:
Jürgen Schleef, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Serghei Covantsev, S.P. Botkin Clinical Hospital, RussiaLuigi Bonavina, University of Milan, Italy
Copyright © 2022 Jiang, Zhang, Fu, Cui, Li, Ren, Fu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingliang Fu ZHJmdXRsQHNpbmEuY29t Lei Geng MzgxODExNDFAcXEuY29t
Specialty section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Surgery
 Kun Jiang1
Kun Jiang1 Tingliang Fu
Tingliang Fu