- 1Immunosciences Lab. Inc., Los Angeles, CA, United States
- 2Cyrex Labs LLC, Phoeniz, AZ, United States
- 3Ariel University, Ariel, Samaria, Israel
- 4Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel-Aviv University, Ramat Gan, Israel
Introduction
The use of silicone-lubricated syringes for autologous fat injection [AFI] has become very popular in the fields of dermatology and plastic surgery. It has been used successfully in facial rejuvenation, scar contracture repair, and more (1, 2). This procedure aims to improve deep defects in the skin surface through injections of the patient’s own separated fatty tissue (3). AFI is considered by many practitioners as a minimally invasive and safe procedure, with possible complications such as fat necrosis, oil cyst formation, calcification, and infection (4). However, it should be noted that in addition to these non-fatal complications, there have been reports of several cases of fatal cerebral fat embolism and stroke. For example, the injection of fat into the facial/eye orbit may cause a reflux of fat into the ophthalmic or cerebral arteries, resulting not only in fat embolism, but, in very rare cases, even blindness or cerebral infarction (5–8). Although these rare but devastating consequences of what was then called autologous fat grafting [AFG] have been in the literature since the 1980s, two striking cases that were published very recently by Lieu et al. in Frontiers in Medicine have rekindled interest in the possibility of catastrophic embolism following facial AFI (9). The article describes how two women respectively received AFI into the temporal and frontal areas. The first woman underwent decompressive craniectomy, suffered a continuous deterioration of her condition, and died. Imaging examinations showed occlusion of the right external carotid artery. The second woman’s vision had failed to improve at the 3-month follow-up visit. Her examinations showed that multiple retinal arterioles were segmentally occluded. Liu et al. also searched reference lists and identified a total of 26 articles that dealt with similar topics. They concluded that since AFI in healthy adults can lead to such devastating and even fatal complications, the procedure ‘should be performed gently and slowly with low pressure, and blunt needles may be the most appropriate instruments’ (9). The potential mechanisms listed for this cerebral infarction included cerebral artery injury, increased blood coagulation, decreased blood supply, air or fat embolism, and emboli from the aorta. However, the Liu group did not discuss whether additional factors within fat tissue and the syringes used in the process could be responsible for this AFI-induced catastrophic embolism.
Silicone Oil and Its Adjuvant Activity
This possibility was investigated by other research teams who connected these terrible complications of fat injection to the use of silicone oil as a lubricant to coat the barrel of the syringe in order to make it easier to move the plunger with reasonable pressure during the injection (9–15). These syringes are used to inject fat cells, insulin, allergens, vaccines, antigens, monoclonal antibodies (including SARS-CoV-2 spike protein antibodies), anti-vascular endothelial growth factor, anti-cytokines, and more, and due to this lubrication process, thousands, if not millions, of silicone oil droplets are mixed in with the different contents of these syringes (15, 16). It is not unreasonable to posit some consequence from the injection of these silicone oil droplets into our systems. One good example might be the appearance of silicone floaters in the eye as a result of Avastin eye injections. Because silicone is known to be an excellent adjuvant (17), the pharmaceutical industry and regulatory agencies have serious concerns that silicone oil particles (SiOPs) have the potential to increase protein aggregates, or, that by functioning as adjuvants, they might generate anti-protein or anti-haptenic chemical antibodies (18–21). In fact, various recombinant proteins have been shown to bind to SiOPs released from emulsified silicone oil which is usually included in the proteins used as therapeutics (18, 20, 22). For example, IgG1 monoclonal antibody is capable of effector function; if this protein forms aggregates with silicone droplets, the resulting aggregate may mimic immune complexes that trigger antibody-dependent cell-mediated cytotoxicity, which is involved in type II allergic reactions (23). Furthermore, SiOPs-adsorbed antigens may mimic pathogen-associated molecular patterns (PAMPs), which may possibly result in enhanced phagocytosis, cytokine release, and autoinflammatory reaction (24, 25).
The adjuvant activity, enhanced phagocytosis, and induction of protein aggregates by silicone is well-known for the role of all of these in the development of multi-organ system disorders, including rheumatic and neurologic diseases. There are many articles and case reports from the time period 1983–1995 alone (26–47) that address this.
In one of my own articles from back then, my group hypothesized that an immune reaction to silicone breast implants would include host reactivity not just against the silicone but also the macromolecules within the environment of the implant, and that the generated autoantibodies may react with other tissue antigens distal from the site of the implant (46). To test this hypothesis, we obtained sera from 520 symptomatic women with silicone implants who had developed silicone-related immunological disorders, and also from 520 matched controls without implants. We tested these sera for the presence of antibodies against silicone bound to human serum albumin (HSA), HSA alone, and myelin basic protein (MBP). Antibodies against these antigens were detected in about 2% of healthy controls, and in up to 43% of the symptomatic patients with implants.
Based on our findings and the literature available at the time (26–47), we concluded that the silicone breast implant oozes or bleeds small silicone particles, which are then absorbed or bound to macromolecules surrounding the silicone bag. The tissue antigens bound to silicone spheres are presented to macrophages. Cooperation between T-helper cells and B cells produce specific antibodies, which may react specifically to both the silicone and the tissues from which the tissue antigens bound to the silicone originated. You may see Supplementary Figure S1 (in Supplementary Material), which shows the original illustration from our 1994 article; it summarizes the hypothetical mechanism described above and shows how silicone can contribute to autoimmunity.
Possible Association between Silicone Implants and Autoimmunity
It is worth noting that these articles we have cited about silicone breast implants and their association with many autoimmune disorders were published before the introduction of autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome) in 2011 (48). In fact, in 1994 I and two of my colleagues collaborated to write an editorial titled ‘Silicone breast implants and autoimmunity: Causation or myth?’ (45). Studies like the ones we have cited, together with major public outcry, led the Food and Drug Administration (FDA) to ban the use of silicone breast implants for cosmetic purposes in 1992. However, the pressing demand for cosmetic breast implants led to the introduction of saline and other types of breast implants. Today there are four general types of breast implants defined by their filler material: silicone gel, saline solution, structured, and composite filler. No matter what the filler is, the implants use sacs that are still elastomer silicone shells. All of these types of implants are currently on the market and freely available for cosmetic augmentation, including silicone breast implants, because in 2006 the US government ended the 14-year ban on them. A look at the FDA website today will simply reveal general warnings about all types of breast implants. However, there soon came a rising tide of increasing reports not only about specific autoimmune diseases but also autoimmune-related or peripheral disorders that could not easily be categorized. This is what led Shoenfeld and Agmon-Levin to introduce autoimmune/inflammatory syndrome induced by adjuvants, which would later come to be called Shoenfeld’s syndrome (48). In this article published in the Journal of Autoimmunity, the authors found that adjuvants, which are factors normally used to activate or enhance immune response for a positive or beneficial purpose, could also be associated with both defined and non-defined immune-mediated diseases. One of these conditions was siliconosis, with the adjuvant being silicone.
With this kind of history behind silicone and its association with ASIA or Shoenfeld’s syndrome, we should not be surprised that silicone-treated syringes may have a role in the induction of catastrophic embolism, coagulopathy, and anti-phospholipid syndrome after autologous fat injection, as already discussed above. However, we believe that silicone-treated syringes may not be the only perpetrators of this crime of inducing autoimmunity.
Silicone in the syringes may not be the only perpetrators of autoimmunity induction. To find other suspects, we turn from the silicone in the syringe to the actual fat or adipose tissue used in AFI. In reviewing the structure of the fat cell or adipocyte, we note that like any other cell it contains a cell membrane, cytoplasm, Golgi apparatus, nucleus, mitochondrion, and more. However, a very large portion of the adipocyte’s cytoplasm is occupied by the fat reservoir. This fat reservoir serves as the storage compartment for lipophilic persistent organic pollutants (POPs). The size of these cells depends on the amount of chemicals stored within them. Due to their molecular structure, POPs can bind to the membrane, particularly to the phospholipids, endoplasmic reticulum, and even to the mitochondrion. Overall, the accumulation of pollutants, especially lipophilic POPs, in adipocytes increases the total body burden of toxic chemicals (49–51).
Here’s what you should know about POPs:
• Highly toxic chemicals
- Pesticides
- Industrial chemicals
- Unwanted industrial by-products harmful to humans and the environment
• An estimated 400 million tons are produced annually worldwide
• The dirty dozen: aldrin, chlordane, DDT, dieldrin, dioxins, furans, endrin, HCB, heptachlor, mirex, PCBs, toxaphene
• Stored in fat, persistent
• Each one of us, depending on our body weight and percentage of adipose tissue, may carry a few grams of each of the above chemicals in our fat
• The main routes of exposure to these toxic chemicals are seafood (salmon, eel, shellfish, fish liver, fish oil), animal fat (meat, poultry), cow’s milk (butter, dairy products) and other foods such as vegetables, cereals and fruit.
Discussion
Considering that various interactions between adipose tissue and POPs have been reported (52, 53), and increased exposure to these toxic chemicals results in greater body burden within the liver and particularly in adipocytes, any interference with the physiological environment of adipocytes may result in the release of these pollutants into the environment of the tissue, and/or, the adipocytes may undergo apoptosis or necrosis (see Figure 1). How does all this relate to autologous fat injection?
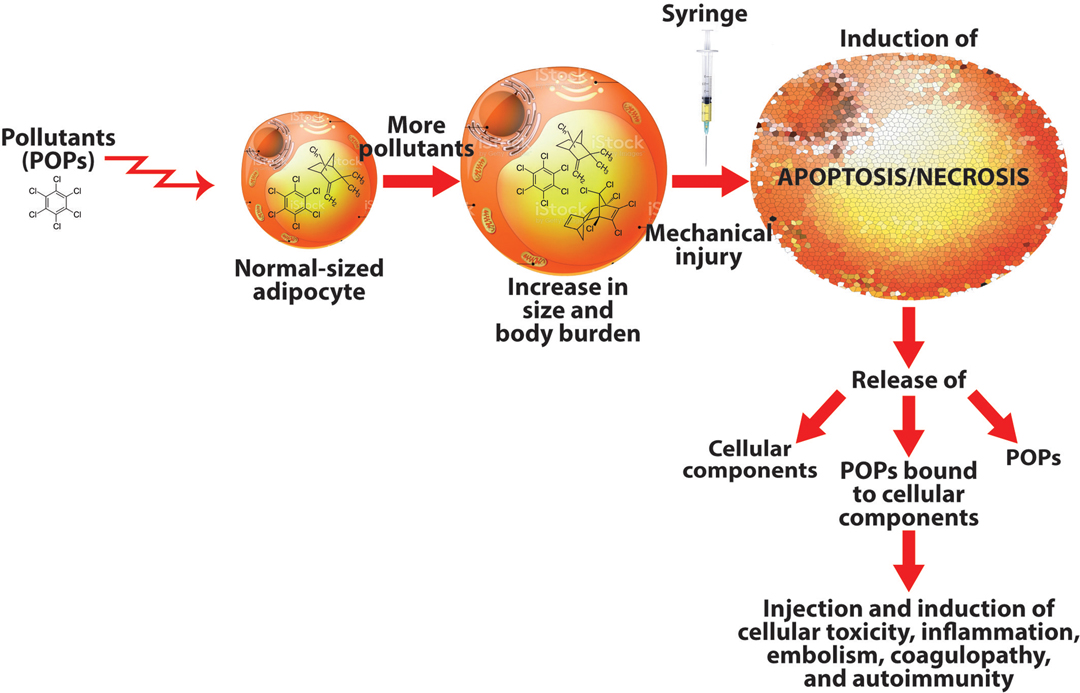
Figure 1. How pollutants stored in adipocytes may lead to autoimmunity. Adipocytes filled with stored persistent organic pollutants (POPs) may burst upon extraction, releasing the POPs, cellular components, and POPs bound to cellular components into the syringe, which will inject all these materials into the patient, possibly inducing immune-mediated disorders.
The collection of fat tissue using syringe, mechanical or enzymatic treatment may result in the induction of the apoptosis/necrosis program in some adipocytes. When these cells die/disintegrate, they will release their cellular contents (including the POPs and POPs bound to the adipocyte’s cellular components) into the syringe. The injection of these factors released from damaged adipocytes along with many healthy cells into genetically susceptible individuals may result in fat embolism, coagulopathy, and autoimmune reactivity against various autoantigens. It behooves us, then, whether patient or practitioner, that whenever we see someone poised with a syringe in hand about to inject its contents into a living human being, we should reflect carefully on the many items that may be included in those contents. You may see Supplementary Figure S2 (in Supplementary Material), which shows some of these contents related to this matter.
While we do not have solid experimental evidence for the contributions of POPs in adipocytes to the development of these disorders, we hope that our work will generate further investigation towards identifying other suspects aside from silicone-treated syringes for the crime of specific and non-specific immune disorders through the modus operandus of autologous fat injection.
Author Contributions
AV and YYS wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank Joel Bautista for the figures and the preparation of this manuscript for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fsurg.2022.867994/full#supplementary-material.
Conflict of Interest
AV is the CEO and co-owner of Immunosciences Lab., Inc. in Los Angeles, CA, and as a consultant is the Chief Science Officer for Cyrex Labs, LLC in Phoenix, AZ. YYS is the President of ARIEL University in Israel and the founder and head of Zabludowicz Center for Autoimmune Diseases.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abu-Ghname A, Perdanasari AT, Reece EM. Principles and applications of fat grafting in plastic surgery. Semin Plast Surg. (2019) 33:147–54. doi: 10.1055/s-0039-1693438
2. Li FC, Chen B, Cheng L. Breast augmentation with autologous fat injection: a report of 105 cases. Ann Plast Surg. (2014) 73:S37–S42. doi: 10.1097/SAP.0000000000000271
3. Chajchir A. Fat injection: long-term follow-up. Aesthetic Plast Surg. (1996) 20:291–6. doi: 10.1007/BF00228458
4. Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg. (2007) 119:775–85. doi: 10.1097/01.prs.0000252001.59162.c9
5. Wang C, Wang X. Cerebral hemorrhage after cosmetic facial injection. Plast Reconstr Surg Glob Open. (2019) 7:e2397. doi: 10.1097/GOX.0000000000002397
6. Wang C, Wang X, Huang J, Yu N, Long X. Severe fat embolism after analogous fat grafting in vaginal tightening and breast augmentation surgery. J Int Med Res. (2020) 48:7441288. doi: 10.1177/0300060520949109
7. Renard D, Charavel P, Dahmani L, Freitag C. Cerebral fat embolism after autologous fat injection for reconstructive eye surgery. Rev Neurol (Paris). (2019) 175:94–5. doi: 10.1016/j.neurol.2018.01.382
8. Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat metabolism induced by facial fat injection. Plast Reconstr Surg. (2004) 113:2235–6. doi: 10.1097/01.PRS.0000123627.33690.9E
9. Liu C, Cai Z, Zhang L, Zhou M, He L. Case report and literature review: catastrophic embolism following cosmetic injection of autologous fat in the face. Front Med (Lausanne). (2021) 8:646657. doi: 10.3389/fmed.2021.646657
10. Mojsiewicz-Pienkowska K, Jamrogiewicz M, Szymkowska K, Krenczkowska D. Direct human contact with siloxanes (silicones) – safety or risk Part 1. Characteristics of siloxanes (silicones). Front Pharmacol. (2016) 7:132. doi: 10.3389/fphar.2016.00132
11. Joh NH, Thomas L, Christian TR, Verlinsky A, Jiao N, Allotta N, et al. Silicone oil particles in prefilled syringes with human monoclonal antibody, representative of real-world drug products, did not increase immunogenicity in in vivo and in vitro model systems. J Pharm Sci. (2020) 109:845–53. doi: 10.1016/j.xphs.2019.09.026
12. Abrahams C, Melo GB, Wambier CG. Silicone-rich syringes can cause granuloma-rich reactions in platelet-rich plasma injections. JAAD Case Rep. (2020) 6:751–2. doi: 10.1016/j.jdcr.2020.06.020
13. Melo GB, da Cruz NFS, Emerson GG, Rezende FA, Meyer CH, Uchiyama S, et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog Retin Eye Res. (2020) 80:100862. doi: 10.1016/j.preteyeres.2020.100862
14. Wambier CG, de Andrade EA, Cruz LS, Lemes BM, Carey WD, Moura BPS, et al. Flush technique to minimize adverse reactions from syringe lubricant (silicone oil). JAAD Case Rep. (2019) 81:e169–e171. doi: 10.1016/j.jaad.2018.12.014
15. Melo GB, Emerson GG, Dias Jr CS, Morais FB, Filho ASL, Ota S, et al. Release of silicone oil and the off-label use of syringes in ophthalmology. Br J Ophthalmol. (2019) 104:291–6. doi: 10.1136/bjophthalmol-2019-313823
16. Melo GB, Dias Jr CS, Carvalho MR, Cardoso AL, Morais FB, Figueira ACM, et al. Release of silicone oil droplets from syringes. Int J Retin Vitr. (2019) 5:1. doi: 10.1186/s40942-018-0153-8
17. Naim JO, Lanzafame RJ, van Oss CJ. The adjuvant effect of silicone gel on antibody formation in rats. Immunol Invest. (1993) 22:151–61. doi: 10.3109/08820139309063397
18. Ludwig DB, Carpenter JF, Hamel JB, Randolph TW. Protein adsorption and excipient effects on kinetic stability of silicone oil emulsions. J Pharm Sci. (2010) 99:1721–33. doi: 10.1002/jps.21982
19. Ludwig DB, Trotter JT, Gabrielson JP, Carpenter JF, Randolph TW. Flow cytometry: a promising technique for the study of silicone oil-induced particulate formation in protein formulations. Anal Biochem. (2011) 410:191–9. doi: 10.1016/j.ab.2010.12.008
20. Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga K, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. (2014) 16:658–73. doi: 10.1208/s12248-014-9599-2
21. Krayukhina E, Tsumoto K, Uchiyama S, Fukui K. Effects of syringe material and silicone oil lubrication on the stability of pharmaceutical proteins. J Pharm Sci. (2015) 104:527–35. doi: 10.1002/jps.24184
22. Krayukhina E, Yokoyama M, Hayashihara KK, Maruno T, Noda M, Watanabe H, et al. An assessment of the ability of submicron- and micron-size silicone oil droplets in dropped prefillable syringes to invoke early- and late-stage immune responses. J Pharm Sci. (2019) 108:2278–87. doi: 10.1016/j.xphs.2019.02.002
23. Luo Y, Lu Z, Raso SW, Entrican C, Tangarone B. Dimers and multimers of monoclonal IgG1 exhibit higher in vitro binding affinities to Fcg receptors. MAbs. (2009) 1:491–504. doi: 10.4161/mabs.1.5.9631
24. Clair JBS, Detanico T, Aviszus K, Kirchenbaum GA, Christie M, Carpenter JF, et al. Immunogenicity of isogenic IgG in aggregates and immune complexes. PLoS One. (2017) 12:e0170556. doi: 10.1371/journal.pone.0170556
25. Moussa EM, Panchal JP, Moorthy BS. Immunogenicity of therapeutic protein aggregates. J Pharm Sci. (2016) 105:417–30. doi: 10.1016/j.xphs.2015.11.002
26. Baldwin CM Jr, Kaplan EN. Silicone-induced human adjuvant disease? Ann Plast Surg. (1983) 10:270–3. doi: 10.1097/00000637-198304000-00002
27. Kumagai Y, Shiokawa Y, Medsger TA Jr, Rodnan GP. Clinical spectrum of connective tissue disease after cosmetic surgery. Arthritis Rheum. (1984) 27:1–12. doi: 10.1002/art.1780270101
28. Endo LP, Edwards B, Longly S, Corman LC, Panush RS. Silicone and rheumatic disease. Semin Arthritis Rheum. (1987) 17:112–8. doi: 10.1016/0049-0172(87)90033-3
29. Brozena SJ, Fenske NA, Cruse CW, Espinoza CG, Vasey FB, Germain BF, et al. Human adjuvant disease following augmentation mammoplasty. Arch Dermatol. (1988) 124:1383–6. doi: 10.1001/archderm.1988.01670090039008
30. Spiera H. Scleredema after silicone augmentation mammoplasty. JAMA. (1988) 260:236–8. doi: 10.1001/jama.1988.03410020102037
31. Weisman MH, Vecchione RR, Albert D, Moore LT, Mueller MR. Connective tissue disease following breast augmentation: a preliminary test of the human adjuvant disease hypothesis. Plast Reconstr Surg. (1988) 82:626–30. doi: 10.1097/00006534-198810000-00011
32. Varga J, Schumacher HR, Jimenez SA. Systemic sclerosis after augmentation mammoplasty with silicone implants. Ann Intern Med. (1989) 111:377–83. doi: 10.7326/0003-4819-111-5-377
33. Weiner SR, Clements PJ, Paulus HH. Connective tissue disease after augmentation mammoplasty. Arthritis Rheum. (1989) 23:23–24.
34. Varga J, Jimenez S. Augmentation mammoplasty and scleroderma. Arch Dermatol. (1990) 126:1220–2. doi: 10.1001/archderm.1990.01670330100017
35. Gutierrez FJ, Espinoza LR. Progressive systemic sclerosis complicated by severe hypertension; reversal after silicone implant removal. Am J Med. (1990) 89(3):390–2. doi: 10.1016/0002-9343(90)90359-l
36. Sann EE, Garen PD, Silver RM, Maize JC. Sclerodema following augmentation mammoplasty. Arch Dermatol. (1990) 126:1198–202. doi: 10.1001/archderm.1990.01670330078011
37. Press RI, Peebles CL, Kumagai Y, Ochs RL, Tan NM. Antinuclear autoantibodies in women with silicone breast implants. Lancet. (1992) 340:1304–7. doi: 10.1016/0140-6736(92)92491-W
38. Goldblum RM, Pelley RP, O’Donell AA, Pyron D, Heggers JP. Antibodies to silicone elastomers and reactions to ventriculoperitoneal shunts. Lancet. (1992) 340:510–3. doi: 10.1016/0140-6736(92)91710-P
39. Vojdani A, Campbell A, Brautbar N. Immune functional impairment in patients with clinical abnormalities and silicone breast implants. Toxicol Ind Health. (1992) 8:415–29. doi: 10.1177/074823379200800606
40. Wolf LE, Lappe M, Peterson RD, Ezrailson EG. Human immune response to polydimethylsiloxane [silicone]: screening studies in a breast implant population. J Faseb. (1993) 7:1265–8. doi: 10.1096/fasebj.7.13.8405812
41. Teuber SS, Rowley MJ, Yoshida SH, Ansari AA, Gershwin ME. Anticollagen autoantibodies are found in women with silicone breast implants. J Autoimmunity. (1993) 6:367–77. doi: 10.1006/jaut.1993.1031
42. Kossovsky N, Zeidler Z, Chun G, Papasian N, Nguyen A, Rajguru S, et al. Surface dependent antigens identified by high binding avidity of serum antibodies in a subpopulation of patients with breast prostheses. J Appl Biomater. (1993) 4:281–8. doi: 10.1002/jab.770040402
43. Bridges AJ, Connelly C, Wong G, Burns DE, Vassey FB. A clinical and immunological evaluation of women with silicone breast implants and symptoms of rheumatic disease. Ann. Intern Med. (1993) 118:929–36. doi: 10.7326/0003-4819-118-12-199306150-00003
44. Claman HN, Robertson DL. Antinuclear antibodies and breast implants. West J Med. (1994) 160:225–8.
45. Brautbar N, Vojdani A, Campbell A. Silicone breast implants and autoimmunity: causation or myth? Arch Environ Health. (1994) 49:151–3. doi: 10.1080/00039896.1994.9940373
46. Vojdani A, Brautbar N, Campbell A. Antibodies to silicone and native macromolecules in women with silicone breast implants. Immunopharmacol Immunotoxicol. (1994) 16:497–523. doi: 10.3109/08923979409019737
47. Campbell AW, Brautbar N, Vojdani A. Suppressed natural killer cell activity in patients with silicone implants: reversal upon explantation. Toxicol Ind Health. (1994) 10:149–54. doi: 10.1177/074823379401000304
48. Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. (2011) 36:4–8. doi: 10.1016/j.jaut.2010.07.003
49. La Merrill M, Edmond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, et al. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. (2013) 121:162–9. doi: 10.1289/ehp.1205485
50. Garcia DS, Sjodin M, Hellstrandh M, Norinder U, Nikifrova V, Lindberg J, et al. Cellular accumulation and lipid binding of perfluorinated alkylated substances (PFASs) – A comparison with lysosomotropic drugs. Chem Biol Interact. (2018) 282:1–10. doi: 10.1016/j.cbi.2017.12.021
51. Lamounier-Zepter V, Look C, Schunck WH, Schlottmann I, Woischwill C, Bornstein SR, et al. Interaction of epoxyeicosatrienoic acids and adipocyte fatty ac id-binding protein in the modulation of cardiomyocyte contractility. Int J Obes Lond. (2015) 39:755–61. doi: 10.1038/ijo.2014.193
52. Kim MJ, Marchand P, Hewnegar C, Antignac JP, Alili R, Poitiou C, et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. (2011) 119:377–83. doi: 10.1289/ehp.1002848
Keywords: silicone1, syringe2, AFI3, SiOPs4, embolism5, POPs5
Citation: Vojdani A and Shoenfeld YY (2022) Catastrophic Embolism Following Cosmetic Injection of Autologous Fat: Are Silicone-Treated Syringes the Only Suspects on the Crime Scene?. Front. Surg. 9:867994. doi: 10.3389/fsurg.2022.867994
Received: 1 February 2022; Accepted: 25 April 2022;
Published: 9 May 2022.
Edited by:
Shisan (Bob) Bao, The University of Sydney, AustraliaReviewed by:
Nanze Yu, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2022 Vojdani and Shoenfeld. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aristo Vojdani ZHJhcmlAbXNuLmNvbQ== Yehuda Yulius Shoenfeld WWVodWRhLlNob2VuZmVsZEBzaGViYS5oZWFsdGguZ292Lmls
Speciality section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
 Aristo Vojdani
Aristo Vojdani Yehuda Yulius Shoenfeld
Yehuda Yulius Shoenfeld