
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 24 May 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.867252
 Federico Raveglia1†
Federico Raveglia1† Marco Scarci1†
Marco Scarci1† Arianna Rimessi1
Arianna Rimessi1 Riccardo Orlandi1
Riccardo Orlandi1 Paola Rebora2
Paola Rebora2 Ugo Cioffi3
Ugo Cioffi3 Angelo Guttadauro4
Angelo Guttadauro4 Enrico Ruffini5
Enrico Ruffini5 Mauro Benvenuti6
Mauro Benvenuti6 Giuseppe Cardillo7
Giuseppe Cardillo7 Davide Patrini8
Davide Patrini8 Fernando Vannucci9
Fernando Vannucci9 Nasser Yusuf10
Nasser Yusuf10 Pramoj Jindal11
Pramoj Jindal11 Robert Cerfolio12
Robert Cerfolio12
Objective: Patients with several thoracic complications induced by SARS-CoV-2 infection may benefit from surgery, but its role in this condition is largely unknown, and many surgeons’ advice against any surgical referrals. Our aim is to investigate the efficacy and safety of surgery in COVID-19 patients with thoracic complications requiring surgery.
Methods: We designed a multicenter observational study, involving nine thoracic surgery departments, evaluating patients who developed thoracic complications in hospital, surgically managed from March 1, 2020, to May 31, 2021. An overall 30-day mortality was obtained by using the Kaplan–Meier method. Multivariable Cox regression model and logistic models were applied to identify the variables associated with mortality and postoperative complications.
Results: Among 83 patients, 33 (40%) underwent surgery for complicated pneumothorax, 17 (20.5%) for pleural empyema, 13 (15.5%) for hemothorax, 8 (9.5%) for hemoptysis, 5 patients (6%) for lung abscess, 4 (5%) for infected pneumatoceles, and 3 (3.5%) for other causes. Within 30 days of surgery, 60 patients (72%) survived. At multivariable analysis, age (HR 1.05 [95% CI, 1.01, 1.09], p = 0.022), pulmonary hypertension (HR 3.98 [95% CI, 1.09, 14.5], p = 0.036), renal failure (HR 2.91 [95% CI, 1.19, 7.10], p-value 0.019), thoracotomy (HR 4.90 [95% CI, 1.84, 13.1], p-value 0.001) and infective affections (HR 0.17 [95% CI, 0.05, 0.58], p-value 0.004) were found to be independent prognostic risk factors for 30-day mortality. Age (OR 1.05 [95% CI, 1.01, 1.10], p = 0.023) and thoracotomy (OR 3.85 [95% CI, 1.35, 12.0] p = 0.014) became significant predictors for 30-day morbidity.
Conclusion: Surgical management of COVID-19-related thoracic complications is affected by high mortality and morbidity rates, but a 72% survival rate still seems to be satisfactory with a rescue intent. Younger patients without pulmonary hypertension, without renal insufficiency and undergoing surgery for infectious complications appear to have a better prognosis.
In late 2019, the world was overwhelmed by the rise of a new respiratory infection called SARS-CoV-2, which has since rapidly spread around the world. By November 2021, there had been more than 252,000,000 confirmed cases and over 5,000,000 deaths worldwide (1, 2).
Significant efforts have been made to better understand this infection’s natural history and its optimal management. Mild COVID-19 disease condition can cause symptoms such as fever, cough, and shortness of breath as well as fatigue and muscle aches. However, severe and critical illnesses are characterized by dramatic and life-threatening symptoms (3). Among these, the most common is pneumonia, which rapidly progresses to ARDS (acute respiratory distress syndrome) (4); but pneumatoceles, parenchymal air-filled cysts, pneumothorax and hemotysis seem to occur more likely in COVID-19 patients as well (5).
The second common critical condition is blood hypercoagulability, probably caused by vascular endothelial cell injury, which manifests with a higher incidence of venous and arterial thrombosis and pulmonary embolism requiring prolonged anticoagulant prophylactic treatment; these conditions cause more likely adverse events such as unexpected hematoma and intrapleural bleeding.
Lastly, these patients often develop co-infections, which lead to further complications such as empyema and septic shock.
The above conditions often require a surgical approach, but based on published data reporting high early postoperative morbidity and mortality (6), this is often a huge challenge.
Therefore, the question is whether salvage surgery should be always excluded or should be considered when it represents the only remaining effective option. In the absence of solid data and recommendations, this is a challenging question for thoracic surgeons.
We designed a multicenter study to record the experiences of several worldwide high-volume thoracic departments. Our objective was to investigate the efficacy and safety of surgery in COVID-19 patients who developed thoracic complications requiring operative management. Our first endpoint was to estimate postoperative survival at 30 days; the secondary endpoints were incidence of postoperative complications and prognostic factors for 30 days’ mortality and morbidity.
We have designed an observational retrospective multicenter international study, involving nine experienced thoracic surgery departments, in five different countries:
San Gerardo Hospital, Monza, Italy, (coordinator center);
San Giovanni Battista Molinette Hospital, Turin, Italy;
Spedali Civili Hospital, Brescia, Italy;
San Camillo Forlanini Hospital, Rome, Italy;
University College London Hospitals, London, United Kingdom;
NYU Langone Health, New York, USA;
Hospital Federal do Andaraí, Rio de Janeiro, Brazil;
Chest Hospital, Calicut, India;
Sir Ganga Ram Hospital, New Delhi, India.
The study was approved by the research ethics committee at the lead center (San Gerardo Hospital, Monza; registration number 3822, October 19, 2021) and was conducted in accordance with the Declaration of Helsinki. At the other centers, local principal investigators were tasked with getting approval. Given the emergency situation leading to surgical treatment and the need to collect data, the IRB decided that consent could be waived.
The study population consisted of patients who developed in-hospital COVID-19 thoracic complications. The inclusion criteria were as follows: (1) age >18 years old; (2) molecular diagnosis of SARS-CoV-2 infection through nose-pharyngeal swab or bronchoalveolar lavage via real-time PCR analysis; (3) hospital admission because of clinical/radiological diagnosis of pneumonia; (4) onset of thoracic complications during hospitalization or prolonged hospitalization requiring thoracic surgical procedures (we included both life-threatening emergencies and situations in which medical treatment was ineffective); (5) hospital admission from March 1, 2020 to May 31, 2021.
Thoracic complication is defined as any condition involving the thorax, which is a direct or indirect consequence of COVID-19, including either pathology strictly related to the infection or the iatrogenic effects of therapeutic attempts to treat it. Because of the wide span of diagnosis, the novelty of this pathology and the different protocols adopted by participating centers, it was not possible to identify common criteria for surgical indications. A wide variety of surgical procedures were included. Patients who had undergone chest tube placement alone were not included in the study.
Data were retrospectively collected in a dedicated password-protected database. Demographic, anamnestic, clinical, surgical, and outcome-related variables were derived from medical and nursing records, laboratory reports, and radiological findings. Patients were followed up to discharge or to 30 days after surgery if discharge occurred before (7).
Follow-up consisted of an outpatient clinic visit, including physical examination and chest x-ray; patients discharged with long-COVID, who were under quarantine at home, according to the regulations in force, were tele medically evaluated.
The primary endpoint was postoperative survival at 30 days from surgery. The secondary endpoints were postoperative complications. The postoperative complications were graded according to the Thoracic Morbidity and Mortality Classification System from grade I (not needing treatment or intervention) to grade V (leading to death).
The baseline characteristics of the patients were described in terms of median (I and III quartiles) and frequencies (percentage). Cumulative incidence of in-hospital mortality and discharge was estimated by using the Aalen–Johansen estimator. Overall mortality at 30 days was obtained by the Kaplan–Meier method and compared among groups by using the log-rank test. In order to account for time to death, a multivariable Cox regression model was applied to identify the variables associated with mortality 30 days after surgery. A multivariable logistic model was applied to identify the variables associated with postoperative complications—these included clinical parameters (age, sex, and maximum ventilator support)—and the variables associated with outcomes at the unadjusted analyses (renal insufficiency, pulmonary hypertension, and infective affection (e.g., empyema/pneumatoceles) compared with the other ones (e.g., pneumothorax, hemothorax, etc.) and the type of surgery). The results were presented in terms of hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CI). Data were analyzed using R software (Version 4.0.3).
A total of 83 patients were enrolled in the study, drawn from intensive care, pulmonology and internal medicine departments at different levels of care. All of them were still positive for SARS-Cov-2 RT-PCR at the time of surgery. Anamnestic data are reported in Table 1.
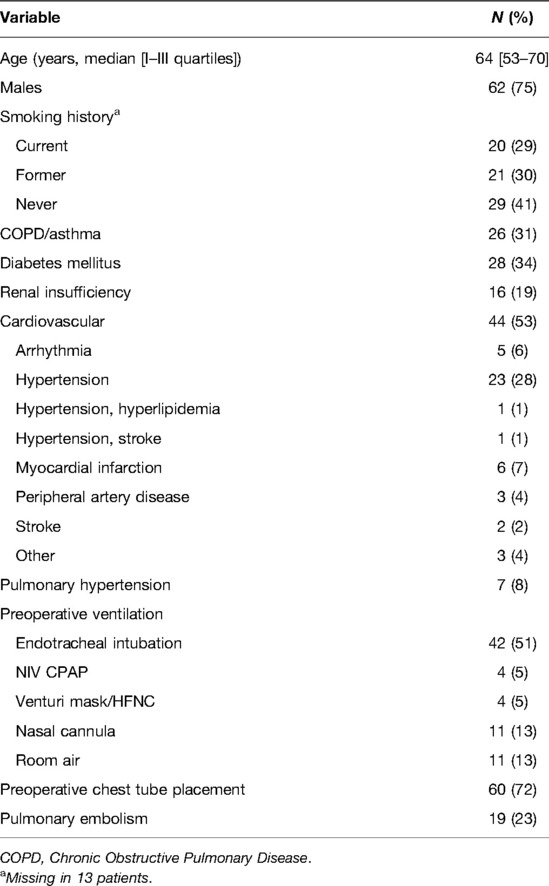
Table 1. Anamnestic data of the whole population from 9 different worldwide thoracic surgery departments.
The median time that elapsed between COVID-19 diagnosis and surgery was 21 days, with 85% of subjects with time lower than 58 days. All of the patients reporting with empyema was a consequence of pneumonia-related complicated pleural effusion.
Indications for surgery, surgical procedures and their combinations are summarized in Table 2. The median post-operative hospital stay was 12 days. A total of 91 surgical procedures was performed on the 83 patients. A total of eight patients (10%) underwent resurgery, all by thoracotomy. One patient was treated for intractable atelectasis (12.5%), managed through pneumonectomy, 1 had empyema (12.5%), treated through decortication and lung resection, 1 had bronchopleural fistula (12.5%), handled through stump repair, 3 patients had hemothorax (37.5%), managed through toilette and hemostasis, 1 had persistent air leak, treated through wedge resection, and 1 had pulmonary infarction, handled through lobectomy.
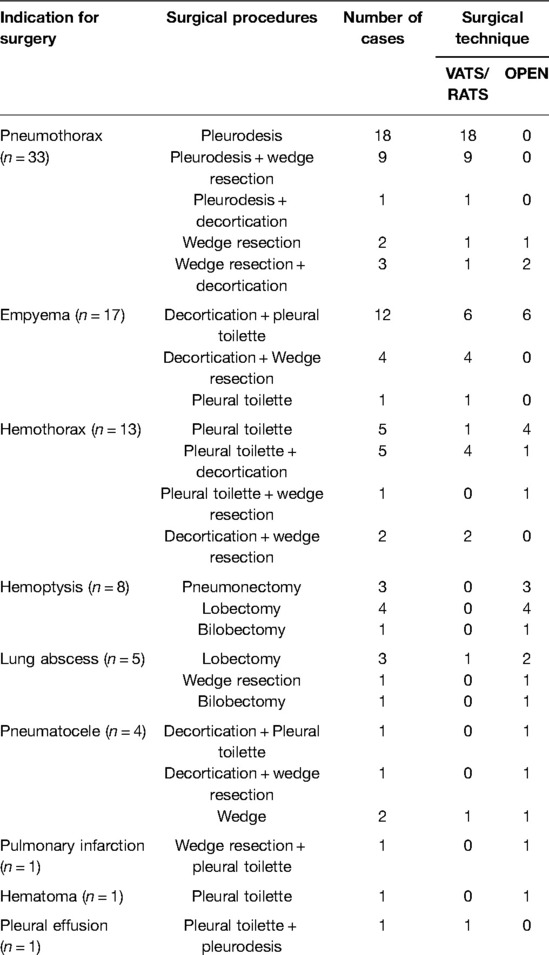
Table 2. Indications for surgery, surgical procedures performed and surgical techniques adopted, in details.
Figure 1 reports in-hospital mortality and discharge among the 83 enrolled patients. At 30 days from surgery, 23 patients (28%, 95% CI, 18–37%) died, 45 (54%, 95% CI, 44–65%) survived and were discharged and the remaining 15 were still recovering in hospital; 5 patients died after 30 days during hospitalization; no discharged patient died at home within 30 days. Among the 23 patients who died within 30 days, 13 (16%) died because of postoperative complications, whereas 10 (12%) died for reasons apparently unrelated to surgery and rather linked to the underlying COVID-19 or the disease already present before intervention (Table 3).
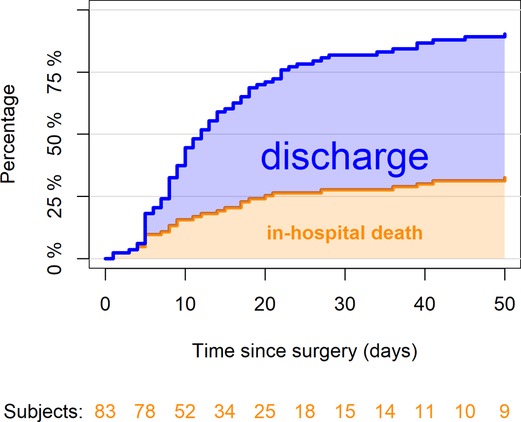
Figure 1. Percentages of patients who died in hospital or who were discharged by the time since surgery (n = 83). At 30 days since surgery, 23 patients (28%, 95% CI, 18–37%) died, 45 (54%, 95% CI, 44–65%) were discharged and the remaining 15 were still recovering in hospital. The confidence limits are explained in a supplementary table (Table 7).
Single pathologic afflictions outcomes are reported in Table 4.
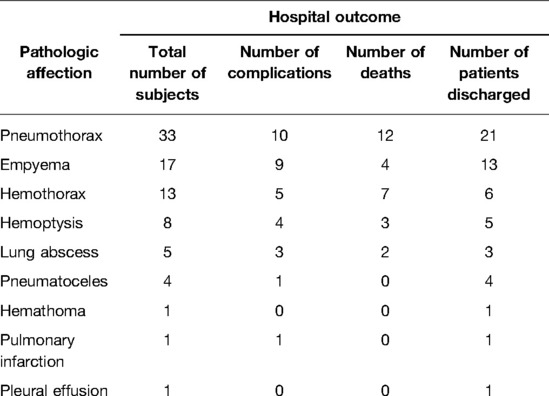
Table 4. Outcomes, in terms of complications and the number of deaths, for every single pathologic affection investigated.
At unadjusted analysis, age over 70 (53% vs. 20%, p-value 0.017), anamnestic renal insufficiency (69% vs. 18%, p-value <0.0001) and anamnestic pulmonary hypertension (53% vs. 25%, p-value = 0.031) were positively associated with 30 days’ mortality. Moreover, patients undergoing thoracotomy had worse survival rates than those operated through a minimally invasive approach (47% vs. 16%, p-value 0.007). Finally, the survival rates were lower in patients who experienced postoperative complications than in those who did not (50% vs. 12%, p < 0.001). On the other hand, gender, days until surgery, smoking history, diabetes, ventilation before surgery, surgical indication and surgical procedures were all not statistically associated with 30-day mortality. All investigated variables are described in Table 5A.
At multivariable analysis (Table 5B), age, renal insufficiency, pulmonary hypertension and the thoracotomic approach were found to be independent prognostic risk factors for 30-day mortality. In particular, the older the patient, the higher was the risk of mortality (HR 1.05 [95% CI, 1.01, 1.09], p-value 0.022); renal insufficiency was confirmed as a statistically significant prognostic factor in adjusted analysis. In our series, patients who already suffered kidney failure at the time of surgery and those with impaired renal function presented a higher mortality (HR 2.91 [95% CI, 1.19, 7.10], p-value 0.019). Lastly, pulmonary hypertension (HR 3.98 [95% CI, 1.09, 14.5], p-value 0.036) and thoracotomy (HR 4.90 [95% CI, 1.84, 13.1], p-value 0.001) were also associated with a lower 30-day survival. Preoperative endotracheal intubation failed to reach statistical significance, although showing a clear trend toward a higher 30-day mortality (HR 2.85 [95% CI, 0.74, 11], p-value 0.13). Finally, with regard to the different affections surgically treated, infective pathologies (e.g., empyema/pneumatoceles/lung abscess) compared with others (e.g., pneumothorax, hemothorax, etc.) resulted in a significant protective factor, ensuring a lower 30-day mortality (HR 0.17 [95% CI, 0.05, 0.58], p-value 0.004).
Postoperative complications are reported in Table 6A multivariable adjusted analysis, the only variables statistically significative as predictors for 30-day morbidity were age (adjusted OR 1.05 [95% CI, 1.01, 1.10], p-value 0.023) and thoracotomy (adjusted OR 3.85 [95% CI, 1.35, 12.0] p-value, 0.014) (Table 6B).
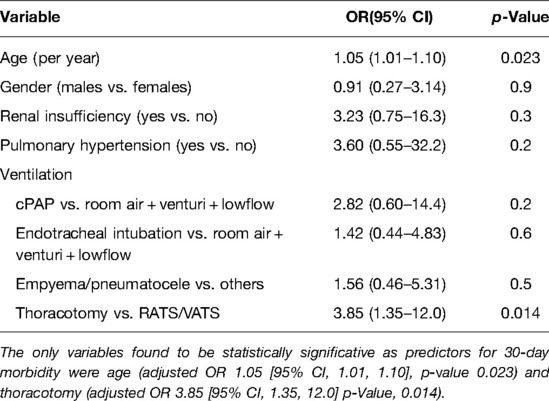
Table 6B. Multivariable logistic regression model on morbidity concerning postoperative complications (n = 83, 34 complications).

Table 7. Cumulative incidence of in-hospital mortality and discharge by time since surgery estimated by using the Aalen–Johansen estimator with 95% confidence intervals.
Many recent studies have focused on patients with SARS-CoV-2 infection undergoing elective or emergency surgical procedures. Reports have shown high morbidity and mortality rates (8–12); therefore, it is commonly believed that surgery should be avoided or postponed, whenever possible. The COVID-Surg Collaboration group (9) set up the largest observational study on this topic, considering an international sample of more than 1,000 SARS-CoV-2 positive patients undergoing emergency and elective surgery. They showed 23.8% (268/1,128) mortality rate and 51.25% (577/1,128) pulmonary morbidity rate; 7.6% of surgical procedures (86/1,128) involved the cardiothoracic district. Knisely et al. (8), considering a sample of 36 patients with SARS-CoV-2 infection who underwent urgent and emergent surgical procedures at 2 hospitals in New York City, reported a perioperative morbidity of 58.3% and postoperative mortality of 16.7%. In a recent review by Nahshon et al. (11) who focused on postoperative outcomes of patients diagnosed with COVID-19 during the perioperative period, 27.5% postoperative mortality rate (14/51) and severe pulmonary complications were reported. De Luca et al. (12), whose study involved 68 SARS-CoV-2 positive patients undergoing cancer and emergency surgery in 20 Italian surgical institutions, recorded a postoperative mortality rate of 14.7% (10/68) and a pulmonary complication rate of 33.8% (26/68).
Only a few authors have discussed the burden of COVID-19 in thoracic surgery (13–15) and even fewer have specifically examined thoracic surgical procedures in the management of COVID-19 complications, confining themselves to single case reports (16–20) or limited case series (21, 22); in fact, the general consensus is that surgery in these patients is not advisable. Nevertheless, it is well known that SARS-CoV-2 can lead to dramatic thoracic complications, some of which exclusively need surgical management. Chang et al. (23), describing 13 patients with COVID-19 who underwent surgery for thoracic complications, registered a 30-day mortality rate of 23% and a 30-day morbidity rate of 61%. We assessed the efficacy and safety of surgery in the largest international cohort of patients with COVID-19-related thoracic complications requiring an operative management.
We recorded a 30-day mortality rate of 27.7% and a 30-day morbidity rate of 49%. A rough comparison with outcomes in thoracic surgery before the advent of the pandemic shows dramatic results. Seely et al. (7), based on a dataset of 1,260 thoracic surgical procedures, reported a mortality rate of 2.2% and a morbidity rate of 29.3%, including all types of surgeries performed on all types of patients. These data confirm that surgical management of patients with COVID-19-related thoracic complications is a high-risk procedure and, therefore, should hardly be recommended. However, some other considerations deserve to be drawn.
We compared our results with the literature concerning the same pathologies in patients without SARS-CoV-2 infection, in order to ascertain whether the outcomes similarly worsened for each single affection addressed.
Patients affected by pneumothorax with persistent air leaks secondary to COVID-19 seem to fare worse than those with pneumothorax secondary to interstitial non-infectious lung disease, such as COPD or PFD, for which the in-hospital mortality rate ranges from 1.4% (24) to 5% (25) and from 15% (26) to 21.4% (24), respectively. Nevertheless, these types of patients seem to have a better prognosis than those with pneumothorax secondary to granulomatosis with polyangiitis, where a mortality rate of 40% (27) has been reported.
With regard to the prognosis of patients with pleural empyema, in the last expert consensus statement for surgical management (28), a 30-day mortality rate ranging from 0% to 16.1% was reported. In a recent review, Godfrey et al. (29) suggested that the high variability of these data could be attributed to confused patient selection criteria; for example, the 30-day mortality rate appears to be higher in population-based studies than in single institutions performing up-front VATS decortication in younger patients, less acute and ill patients and patients with fewer comorbidities. Therefore, the prognosis was slightly worse in our series than in patients with unrelated COVID-19 empyema.
Focusing on the 13 patients operated for hemothorax, the registered 30-day mortality rate of 46% seems to be hardly comparable to that reported in the literature concerning patients without COVID-19. In fact, hemothorax is usually a consequence of postoperative or post-traumatic bleeding: the latter usually has a good prognosis with extremely low mortality rates (30) when patients are reoperated early through careful hemostasis, while the former has a wide span of the prognosis depending on the kind of trauma that occurred. Mortality is closely related to traumatic damage and to the vessel involved in bleeding rather than hemothorax itself. In our series, hemothorax was related to iatrogenic factors such as chest tube placement, thoracentesis or previous surgery in 11 patients. The remaining two patients underwent spontaneuos hemothorax or, more likely, it was due to mechanical ventilation, as already described in the literature (31).
The 30-day mortality rate in patients with lung abscess was 40%. In a series of 28 patients with lung abscess undergoing wedge resection, lobectomy or pneumonectomy, Lee and colleagues reported a perioperative mortality rate of 14.3% (32). Huang et al. (33), focusing on a sample of 22 surgical patients, presented a mortality rate of 23%. In both case studies, the mortality rates were clearly lower than those that emerged from our series.
With regard to the causes of death, 13 out of 23 patients died from surgical complications (Clavien V), while 10 probably died from causes apparently not related to surgery but to the underlying COVID-19. Data reveal that this surgery is very demanding but also suggest that prognosis is largely influenced by variables linked to preoperative prognostic factors rather than to the surgery itself.
Notably, age was an independent variable for 30-day mortality, increasing the risk of death within 30 days from surgery by 4%. HR related to advanced age was 1.05. That may apparently seem of irrelevant clinical significance, especially since from the COVIDsurg collaborative study (9) emerged an OR of 2.30 for patients aged 70 years and older. However, while that research included all surgical patients having positivity to SARS-CoV-2 without distinguishing the severity of the disease, we included only patients affected by COVID-19, attenuating the influence of age on the prognosis.
Pulmonary hypertension was found to significantly increase the 30-day mortality risk by more than four times in our population. This variable has not been investigated in previous studies, but we think it could be of primary importance, based on the pathophysiology of COVID-19. Indeed, the latest research has focused attention on the production of self-antibodies against Annexin-A2 as a leading mechanism on the basis of the severe form of this disease (34, 35), which should be explained by the critical role of this phospholipid-binding protein in lung elasticity, fibrinolysis and integrity of the pulmonary vascular system (36), converging toward an increase in pulmonary artery pressure. In our series, pulmonary hypertension, when recorded, was an anamnestic event already present before COVID-19 onset. All cases were studied with cardiac ultrasound, but unfortunately, we did not have sufficient data for making a comparison with the prehospitalization situation. We strongly suggest assessing the pulmonary artery pressure, possibly through non-invasive echocardiography, before surgery.
Renal failure, showing a trend toward an increasing risk of death of almost three times, was confirmed as a critical prognostic factor. This is not surprising, as acute kidney failure is known to be associated with high morbidity and mortality.
Endotracheal intubation failed to achieve statistical significance; however, in our opinion, it is a prognostic factor worthy of consideration. Indeed, patients intubated before surgery had a postoperative risk of death of almost three times. This finding could be related to the fact that patients deserving intubation before surgery were already in an advanced stage of disease at the peak of cytokines storm, thus conditioning surgical outcomes. The lack of statistical significance could be explained by the low sample size and number of events. Therefore, we suggest being extremely careful in operating these patients, adopting whenever possible alternative therapies that could decrease the inflammatory response before proceeding to surgery.
On the other hand, in our multivariable analysis, patients who underwent surgery for infective affections (empyema/infected pneumatoceles/lung abscess) had a significant lower risk of death, with a reduction in mortality risk of about 83%, suggesting that it can be considered an independent protective factor. This seems quite surprising and could be justified by the minimal invasive approach most often used in the first group. However, no homogeneous preoperative general conditions in the two groups could represent a bias.
We also studied the risk factors for 30-day morbidity. On multivariate logistic analysis, age was confirmed to be also an independent prognostic factor for postoperative morbidity, increasing the risk of complications to 5%. We explain the slight difference behind the mortality rate, but the statistical significance of this variable strengthens the validity of our study. Interestingly, thoracotomy was found to be an independent 30-day prognostic factor for mortality and morbidity as well, with 5-fold and 4-fold increases of the risk of death and complications, respectively, compared with minimally invasive techniques such as VATS or RATS. The advantages of minimally invasive procedures over the classic ones performed through thoracotomy are now well established in thoracic surgery (37), but we think that our result is biased by patient selection, since VATS and RATS were reserved only for less critically ill patients, and, hence, they were less prone to develop postoperative complications and deaths.
Our series has several limitations. First of all, given the retrospective nature of this study, intrinsic patient selection bias could have occurred. Secondly, the sample size, although consistent, remained small, with a limited follow-up period, which only allowed us to investigate early outcomes. Unlikely, we could not determine the total number of COVID-19 patients hospitalized in each participating center, so we were unable to provide the percentage of patients requiring surgery over the total number. Moreover, we could not find adequate controls to be compared with our patients, such as those with COVID-19-related complications not surgically managed, thus weakening our results and constraining us from drawing conclusions over which is the best treatment. Finally, while the choice of conducting a multicenter international study allowed us to generalize our findings, such an exercise could be biased by the absence of standardized indications for surgery and for technical procedures. In particular, the lack of homogeneous data made it impossible to determine how “serious” was COVID-19 infection at the time of surgery.
Furthermore, our study covers a period between the beginning of the vaccination campaign and the ongoing exercise, enrolling both vaccinated and unvaccinated patients. This may be a confounding factor on outcomes.
Another bias concerns the lack of information about the use of vv-ECMO before or during surgery; this a hot topic, as it is commonly suggested that COVID-19 patients who develop complications like empyema or hematothorax on vvECMO-support are particularly challenging to manage. The role of ECMO support in the treatment of severe COVID-19 has been addressed by many authors in the last 2 years, concluding that it should be considered as a rescue therapy for patients with refractory hypoxemia, despite lung-protective ventilation. The criteria for optimizing outcomes have been well described by Hong and co-workers (38). Unfortunately, we have not been able to collect data on ECMO in our series of cases, but one patient underwent surgery on ECMO support at our hospital. This patient underwent surgery for pleural empyema, and intraoperative ECMO support was given because of severe respiratory distress on mono-pulmonary ventilation.
However, to our knowledge, this is the largest study on thoracic surgery in COVID-19 patients to date.
Prognosis for COVID-19-related thoracic complications requiring surgical management is poor and surgery is affected by much higher mortality and morbidity rates than those conventionally accepted in patients without COVID-19. Nevertheless, the reported 30-day survival rate seems to be still satisfactory for ruling out preventive surgery, mainly because mortality is largely influenced by events that are linked to baseline pathological conditions rather than to the surgery itself. Surgery should be considered only in selected patients, with a salvage intent. In particular, prognosis seems to be better for infectious complications such as pleural empyema or infected pneumatoceles than for bleeding complications such as hemothorax or hemoptysis. Moreover, younger individuals without pulmonary hypertension or renal failure appear to have a better prognosis. We suggest a careful management of patients needing preoperative intubation. We strongly recommend a multidisciplinary approach to produce tailored solutions.
This article can be distributed in accordance with the Creative Commons Attribution No Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial See: http://creativecommons.org/licenses/by-nc/4.0/.
The studies involving human participants were reviewed and approved by IRB ASST-MONZA. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors contributed to the article and approved the submitted version.
We thank Dr Gerardo Cioffi, native speaker, for reviewing the English language.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg (2020) 76:71–6. doi: 10.1016/j.ijsu.2020.02.034
3. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med (2020) 35(5):1545–9. doi: 10.1007/s11606-020-05762-w
4. McGuinness G, Zhan C, Rosenberg N, Azour L, Wickstrom M, Mason DM, et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology (2020) 297(2):E252–62. doi: 10.1148/radiol.2020202352
5. Hamad AM, El-Saka HA. Post COVID-19 large pneumatocele: clinical and pathological perspectives. Interact Cardiovasc Thorac Surg (2021) 33(2):322–4. doi: 10.1093/icvts/ivab072
6. Doglietto F, Vezzoli M, Gheza F, Lussardi GL, Domenicucci M, Vecchiarelli L, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg (2020) 155(8):691–702. doi: 10.1001/jamasurg.2020.2713
7. Watanabe S, Asamura H, Suzuki K, Tsuchiya R. Recent results of postoperative mortality for surgical resections in lung cancer. Ann Thorac Surg (2004) 78(3):999–1002; discussion 1002–3. doi: 10.1016/j.athoracsur.2004.04.007
8. Knisely A, Zhou ZN, Wu J, Huang Y, Holcomb K, Melamed A, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg. (2021) 273(1):34–40. doi: 10.1097/SLA.0000000000004420
9. COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet (2020) 396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X
10. Giovinazzo F, Magalini S, La Greca A, Frongillo F, Fransvea P, Agnes S, et al. Mortality in patients undergoing surgery with perioperative SARS-CoV-2 infection: an Italian COVID-19 Hub point of view. Eur Rev Med Pharmacol Sci. (2020) 24(22):11471–3. doi: 10.26355/eurrev_202011_23786
11. Nahshon C, Bitterman A, Haddad R, Hazzan D, Lavie O. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. (2020) 44(8):2477–81. doi: 10.1007/s00268-020-05575-2
12. De Luca M, Sartori A, Vitiello A, Piatto G, Noaro G, Olmi S, et al. Complications and mortality in a cohort of patients undergoing emergency and elective surgery with perioperative SARS-CoV-2 infection: an Italian multicenter study. Teachings of Phase 1 to be brought in Phase 2 pandemic. Updates Surg (2021) 73(2):745–52. doi: 10.1007/s13304-020-00909-0
13. Scarci M, Raveglia F, Bortolotti L, Benvenuti M, Merlo L, Petrella L, et al. COVID-19 after lung resection in northern Italy. Semin Thorac Cardiovasc Surg (2021) S1043-0679(21)00186-6. doi: 10.1053/j.semtcvs.2021.03.038
14. Peng S, Huang L, Zhao B, Zhou S, Braithwaite I, Zhang N, et al. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. J Thorac Cardiovasc Surg (2020) 160(2):585–92.e2. doi: 10.1016/j.jtcvs.2020.04.005
15. Gonfiotti A, Gatteschi L, Salvicchi A, Bongiolatti S, Lavorini F, Voltolini L. Clinical courses and outcomes of five patients with primary lung cancer surgically treated while affected by severe acute respiratory syndrome coronavirus 2. Eur J Cardiothorac Surg (2020) 58(3):598–604. doi: 10.1093/ejcts/ezaa233
16. Aiolfi A, Biraghi T, Montisci A, Bonitta G, Micheletto G, Donatelli F, et al. Management of persistent pneumothorax with thoracoscopy and bleb resection in COVID-19 patients. Ann Thorac Surg (2020) 110(5):e413–5. doi: 10.1016/j.athoracsur.2020.04.011
17. Bellini R, Salandini MC, Cuttin S, Mauro S, Scarpazza P, Cotsoglou C. Spontaneous pneumothorax as unusual presenting symptom of COVID-19 pneumonia: surgical management and pathological findings. J Cardiothorac Surg (2020) 15(1):310. doi: 10.1186/s13019-020-01343-4
18. Capleton P, Ricketts W, Lau K, Ellis S, Sheaff M, Giaslakiotis K, et al. Pneumothorax and pneumatocoele formation in a patient with COVID-19: a case report. SN Compr Clin Med (2021) 3(1):269–72. doi: 10.1007/s42399-020-00689-z
19. Salah O, Faisal M, Alshahwani I, Elhiday A. Bilateral hemopneumothorax in COVID-19. Cureus (2020) 12(9):e10314. doi: 10.7759/cureus.10314
20. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pthology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol (2020) 15(5):700–4. doi: 10.1016/j.jtho.2020.02.010
21. Xu Y, Li S, Liu H. Clinical outcomes of pleural drainage on pneumothorax and hydrothorax in critically ill patients with COVID-19: a case series with literature review. Heart Lung (2021) 50(2):213–9. doi: 10.1016/j.hrtlng.2020.12.007
22. Tessitore A, Patella M, Giuliani M, Theologou T, Freguia S, Minerva EM, et al. Surgical treatment of pleural empyema in Coronavirus disease 19 patients: the Southern Switzerland experience. Interact Cardiovasc Thorac Surg (2021) 32(3):367–70. doi: 10.1093/icvts/ivaa269
23. Chang SH, Chen D, Paone D, Geraci TC, Scheinerman J, Bizekis C, et al. Thoracic surgery outcomes for patients with coronavirus disease 2019. J Thorac Cardiovasc Surg (2021) 162(6):1654–64. doi: 10.1016/j.jtcvs.2021.01.069
24. Nakajima J, Takamoto S, Murakawa T, Fukami T, Yoshida Y, Kusakabe M. Outcomes of thoracoscopic management of secondary pneumothorax in patients with COPD and interstitial pulmonary fibrosis. Surg Endosc (2009) 23(7):1536–40. doi: 10.1007/s00464-009-0438-y
25. Kawai N, Kawaguchi T, Yasukawa M, Tojo T, Sawabata N, Taniguchi S. Surgical treatment for secondary spontaneous pneumothorax: a risk factor analysis. Surg Today (2021) 51(6):994–1000. doi: 10.1007/s00595-020-02206-0
26. Ichinose J, Nagayama K, Hino H, Nitadori J-i, Anraku M, Murakawa T, et al. Results of surgical treatment for secondary spontaneous pneumothorax according to underlying diseases. Eur J Cardiothorac Surg (2016 49(4):1132–6. doi: 10.1093/ejcts/ezv256
27. Shi X, Zhang Y, Lu Y. Risk factors and treatment of pneumothorax secondary to granulomatosis with polyangiitis: a clinical analysis of 25 cases. J Cardiothorac Surg (2018) 13(1):7. doi: 10.1186/s13019-018-0695-8
28. Scarci M, Abah U, Solli P, Page A, Waller D, van Schil P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg (2015) 48(5):642–53. doi: 10.1093/ejcts/ezv272
29. Godfrey MS, Bramley KT, Detterbeck F. Medical and surgical management of empyema. Semin Respir Crit Care Med (2019) 40(3):361–74. doi: 10.1055/s-0039-1694699
30. Zeiler J, Idell S, Norwood S, Cook A. Hemothorax: a review of the literature. Clin. Pulm. Med. (2020) 27(1):1–12. doi: 10.1097/CPM.0000000000000343
31. Guven BB, Erturk T, Kompe Ö, Ersoy A. Serious complications in COVID-19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous emphysema and haemothorax. Epidemiol Infect (2021) 149:e137. doi: 10.1017/S0950268821001291
32. Lee CH, Liu YH, Lu MS, Hsieh MJ, Wu YC, Ko PJ, et al. Pneumonotomy: an alternative way for managing lung abscess. ANZ J Surg (2007 77(10):852–4. doi: 10.1111/j.1445-2197.2007.04257.x
33. Huang HC, Chen HC, Fang HY, Lin YC, Wu CY, Cheng CY. Lung abscess predicts the surgical outcome in patients with pleural empyema. J Cardiothorac Surg (2010) 5:88. doi: 10.1186/1749-8090-5-88
34. Zuniga M, Gomes C, Carsons SE, Bender MT, Cotzia P, Miao QR, et al. Autoimmunity to annexin A2 predicts mortality among hospitalised COVID-19 patients. Eur Respir J (2021) 58(4):2100918. doi: 10.1183/13993003.00918-2021
35. Fang YT, Lin CF, Liao PC, Kuo YM, Wang S, Yeh TM, et al. Annexin A2 on lung epithelial cell surface is recognized by severe acute respiratory syndrome-associated coronavirus spike domain 2 antibodies. Mol Immunol (2010) 47(5):1000–9. doi: 10.1016/j.molimm.2009.11.019
36. Hajjar KA. The biology of annexin A2: from vascular fibrinolysis to innate immunity. Trans. Am. Clin. Climatol. Assoc. (2015) 126:144–55. PMID: 26330668; PMCID: PMC4530673
37. O'Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact. Cardiovasc Thorac Surg (2019) 28(4):526–34. doi: 10.1093/icvts/ivy315
Keywords: COVID-19, complications, thoracic surgery, mortality, surgery
Citation: Raveglia F, Scarci M, Rimessi A, Orlandi R, Rebora P, Cioffi U, Guttadauro A, Ruffini E, Benvenuti M, Cardillo G, Patrini D, Vannucci F, Yusuf N, Jindal P and Cerfolio R (2022) The Role of Surgery in Patients with COVID-19-Related Thoracic Complications. Front. Surg. 9:867252. doi: 10.3389/fsurg.2022.867252
Received: 31 January 2022; Accepted: 2 May 2022;
Published: 24 May 2022.
Edited by:
Calvin Sze Hang Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Patrick Zardo, Hannover Medical School, GermanyCopyright © 2022 Raveglia, Scarci, Rimessi, Orlandi, Rebora, Cioffi, Guttadauro, Ruffini, Benvenuti, Cardillo, Patrini, Vannucci, Yusuf, Jindal and Cerfolio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Raveglia ZmVkZXJpY28ucmF2ZWdsaWFAYXNzdC1tb256YS5pdA==
†These authors have contributed equally to this work
Specialty section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Abbreviations: COVID-19, COronaVIrus Disease 19; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2); ARDS, Acute Respiratory Distress Syndrome; CI, Confidence Intervals; OR, Odds Ratio; HR, Hazard Ratio; VATS, Video Assisted Thoracic Surgery; RATS, Robotic Assisted Thoracic Surgery; COPD, Chronic Obstructive Pulmonary Disease; LMWH, Low-Molecular-Weight Heparin; DVT, Deep Vein Thrombosis.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.