
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 13 May 2022
Sec. Vascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.864846
 Eliza Russu1,2†
Eliza Russu1,2† Adrian Vasile Mureșan1,2†
Adrian Vasile Mureșan1,2† Reka Kaller1
Reka Kaller1 Lucian Toma1
Lucian Toma1 Cătălin Mircea Coșarcă1
Cătălin Mircea Coșarcă1 Călin Bogdan Chibelean3
Călin Bogdan Chibelean3 Emil Marian Arbănași1*
Emil Marian Arbănași1* Eliza Mihaela Arbănași4
Eliza Mihaela Arbănași4
We present the case of a 56-year-old patient admitted to the vascular unit of the Targu Mures County Emergency Clinical Hospital after a computed tomography angiography performed for critical limb ischemia showed a tumor of the right kidney of 11.3/12/11 cm anteroposterior/later-lateral/craniocaudal, accompanied by an abdominal aortic aneurysm (AAA) (3 cm diameter) and right iliac artery occlusion. An interdisciplinary team formed of urological and vascular surgeons decided and performed a one-step operation. The right kidney was removed, and the limb revascularization was achieved by performing a bypass that used the right renal arterial stump as an inflow artery, thus called a reno-femoral bypass. The AAA had no indication for reconstruction. The final pathology interpretation of the kidney tumor revealed a clear cell renal cell carcinoma, excised with oncological safety margins. A short-term follow-up found the patient without ischemic symptomatology and a fully functional graft.
Peripheral artery disease (PAD) morbidity has seen a significant increase in the last 20 years, currently affecting 1,466 people out of 100,000 worldwide, compared with 1,229 out of 100,000 in 2010. The countries with the highest incidence are Denmark, with 5,330 people out of 100,000, and the United States, with 3,799 people out of 100,000 (1).
Chronic limb-threatening ischemia (CTLI) is a severe stage of PAD and corresponds to stages III–IV Leriche-Fontaine (2), or 4–6 Rutherford (3), and is associated with a high rate of amputation and mortality (4, 5). CTLI is characterized by rest pain, tissue damage, or tissue necrosis (6, 7). Striving for endovascular or surgical revascularization is mandatory to preserve the viability and function of the affected limb (6).
PAD’s concomitance with malignancy is well known and heavily studied. The disease has the same risk factors, such as smoking, obesity, and diabetes, and some similarities regarding the physiopathological mechanisms (8). In a manuscript published by El Sakka et al., the authors could signal malignancy in 22 (11.5%) out of the 192 studied critical limb ischemia patients (9). Also worth citing is the paper of Nicolajsen et al., which reported 1 out of 5 acute limb ischemia patients having a malignancy (10).
In terms of incidence rate, kidney tumors occupy the fourteenth place worldwide as newly diagnosed cases in 2020, accounting for 431,288 patients. They are in the fifteenth place regarding mortality rates, accounting for 179,368 fatalities (11).
Abdominal aortic aneurysm (AAA) and malignancy concomitance were also studied in detail, casting a shadow on the dispute over the one-stage vs. two-stage therapeutical approach (12). The 2019 European Society for Vascular Surgery Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac artery Aneurysms have a specific set of references about the issue of concomitant malignant disease. Still, these are recommendations for managing an aneurysm with a particular diameter (over 5.5 cm) (13). AAA has a high mortality rate and may evolve toward retroperitoneal rupture, aorto-enteric fistula formation, or spontaneous thrombosis (14, 15).
This paper aims to present an innovative surgical technique of using the remaining renal artery, after nephrectomy, as an inflow artery for the revascularization of the critical ischemic leg, in a patient presenting with an infrarenal AAA and the occlusion of the common and external right iliac artery.
A 56-year-old male patient presented to the vascular unit of the Targu Mures County Emergency Clinical Hospital complaining of severe pain in the right leg, without any relief after taking common analgesic drugs. The patient had no right femoral pulse, with the other clinical signs of an ischemic leg being the absence of hair growth on the right calf, right big toe recurrent mycosis, and a positive Buerger and Ratschow test. He had rest pain, and the measured ankle-branchial index (ABI) was 0.4. One month prior, he was referred to an angiologist, having had claudication in the right leg, and was advised toward smoking cessation and exercise rehabilitation, and was put on first-line medication (cilostazol), in addition to his chronic antihypertension drugs. The laboratory findings revealed slight anemia (Hgb 9.97 g/dl). Doppler examination and computed tomography (CT) angiography jointly diagnosed right common and external iliac artery occlusion type D transAtlantic inter-society consensus (TASC), an infrarenal AAA with a 3- cm diameter with intraluminal thrombus, and a right renal tumor of 11.3/12/11 cm (anteroposterior/later-lateral/craniocaudal), with central necrosis (Figure 1).
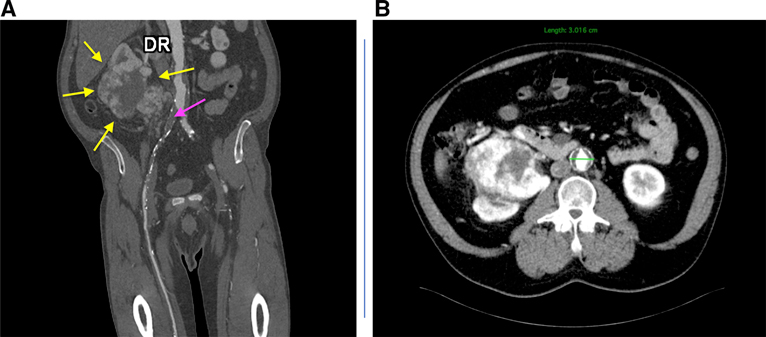
Figure 1. Computed tomography angiography before surgery: coronal section, yellow arrows: kidney tumor; purple arrow: occlusion of the right common and external iliac arteries (A) and axial section, AAA with intraluminal thrombus; renal tumoral mass with central necrosis (B).
The patient also had hypertension and a body mass index of 30.67. Echocardiography showed an ejection fraction of 48% and mild hypokinesia of the lateral wall of the left ventricle. All the kidney function parameters were normal: blood urea nitrogen (BUN) and creatinine. He had an average glomerular filtration rate.
After a senior urologist analyzed the CT angiography, the kidney tumor presented high malignancy characteristics, and nephrectomy was elected as the procedure of choice. After discussing the case in a multidisciplinary meeting, a one-stage open repair was selected, forming an interdisciplinary team with the Urology Department. Endovascular repair (EVAR) was ruled out due to occlusion of the right common and external iliac arteries and atherosclerotic lesions affecting the controlateral iliofemoral axis. The aneurysmal neck was also right below the origin of the renal arteries, rendering the local situation anatomically hostile and leaving little space for clamping or a potential anastomosis. A lateral clamping would have engaged the aneurysmal wall and the intraluminal thrombus. A potential clamping above the renal arteries would have exposed the left (remaining) kidney to further ischemic injuries. Given a young and active patient with a reasonable life expectancy, the femoral–femoral crossover bypass was also ruled out, as it had the potential to harm the donor artery leg. A decision was then taken to leave a right renal artery stump of cca 3 cm, which would allow a safe clamping and a further “noli-me-tangere” of the aortic aneurysm, leaving it, in the future, to be possibly solved by an EVAR with a left femoral approach. After a complete exclusion of all the other scenarios, the decision to perform a reno-femoral bypass was made, considering that the right renal artery was not included in the tumoral mass. Senior vascular and urology surgeons were to perform the surgical procedure in the Vascular Unit Operating Room within the Târgu-Mureș County Emergency Clinical Hospital. The patient was also inclined to favor the one-stage surgery after presenting the options.
Using a Jalaguier modified incision, the right kidney was first removed, guided by oncological safety principles, after a biopsy was collected and sent to the morphopathology compartment. The result showed malignancy. The whole procedure took place in a retroperitoneal manner. The vascular team distally prepared, clamped, and heparinized the right common femoral artery. A right renal artery stump of 3 cm was clamped safely, leaving enough space for an end-to-end anastomosis to an externally ringed polytetrafluoroethylene graft (7- mm diameter), performed using a continuous suture with Surgipro™ Monofilament Polypropylene 5-0. The team chose an externally ringed graft for safety reasons, as predicting an evolution free from a local tumoral recurrence was problematic, although perirenal fat was excised and radical lymphadenectomy performed. In this scenario, the best-performing graft against external compression had to be the reasonable option. The graft was then tunneled retroperitoneally in a straight, non-kinked position, taking care of the angles of the proximal anastomosis (Figure 2).
The distal anastomosis was performed end-to-side to the common femoral artery, using a continuous suture with Surgipro™ Monofilament Polypropylene 5-0. The back-bleeding from the profunda femoris artery was vigorous, and no thromboendarterectomy of the common femoral artery was needed.
Early postoperatively, anticoagulation was achieved by administering heparin intravenously, 2,500 IU (international units) every 3 h. We monitored the activated partial thromboplastin time values.
The results were satisfactory postoperatively: palpable femoral, popliteal, distal pulses, and an ABI of 0.9. Ultrasound examination showed triphasic Doppler waveforms from the common femoral artery to the ankle. The patient was discharged without any complications on the 7th postoperatory day, with the anemia corrected and the graft patent. Oral anticoagulants, coumarin-like, were prescribed, as well as clopidogrel. BUN and creatinine were elevated postoperatively, but they began to decrease. The patient had a creatinine value of 3.15 mg/dL at discharge. He did not require a urinary catheter. He was instructed to monitor BUN and creatinine values and the Index Normalized Ratio to explore the coagulation status and was referred to a nephrology specialist.
The final pathology result was Clear Cell Renal Cell Carcinoma of the right kidney, International Society of Urological Pathology grade 2, Fuhrman grade 2, infiltrating the perirenal fat, tumor stage: pT3a. The margins of the excision–renal artery section were negative. The patient was referred to an oncologist and started chemotherapy.
One-month follow-up found a patent graft, palpable pedal pulses, a lack of symptomatology, and an ABI of 0.9. Three-month postop control CT angiography showed a fully functional graft (Figure 3).
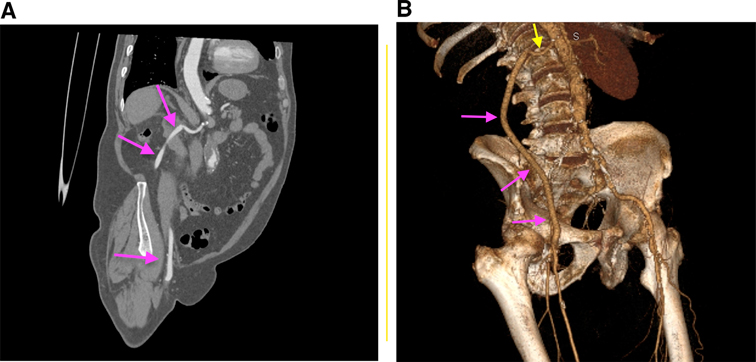
Figure 3. CT angiographic after surgery: coronal section, purple arrows: reno-femoral permeable bypass (A) and 3D reconstruction, yellow arrow: right renal artery; purple arrows: reno-femoral permeable bypass (B).
Tumoral active status patients have a high risk of thromboembolic complications, with an up to 70% increase of the occurred episodes up to 5 months before the oncological diagnostic. The percentages remain high in the first 6 months after (16–18).
The staging of the renal cell carcinoma (RCC), evaluated as thoroughly as it can be done before having a biopsy performed, is mandatory. Also, having accidentally found a kidney tumor in a young patient with a reasonable life expectancy, time is crucial. The American Cancer Society reported, in 2018, a 5-year survival rate for RCC stage 2 of 74%, thus establishing RCC treatment as a priority. Still, limb revascularization in a CTLI patient remains an against-the-clock issue.
The synchronous approach for AAA and nonvascular procedures was demonstrated to be safe by Ochsner et al., with low rates of allograft infection if done in a manner of first implanting the graft, carefully closing the retroperitoneum over it, and then managing the nonvascular procedure (19). The recommendation was deemed reasonable by several studies and reviews, such as those of Prusa and Mohandas, as staging the procedure would only lead to complications due to scar tissues and even more difficult anatomy (20). AAA associated with RCC was described in several papers, emphasizing the good outcomes after open aortic repair, being done simultaneously with nephrectomy procedures (21).
Nephron-sparing operations were postulated as the standard of choice, as prolonged clamping time is complication-inducing (22). Intra-operative findings guide the surgeon toward the best clamping site after analyzing the CT angiography 3D reconstruction. In a patient undergoing a nephrectomy, clamping above the renal arteries is not desirable. If below the renal arteries origin, the aortic wall is not suitable, as in highly calcified or aneurysmal, a technical challenge arises, as was our case.
The concomitance of an AAA, a tumor, and diffuse atherosclerotic lesions forming a TASC D pattern affecting the aortoiliac configuration with minimal infrainguinal damage creates a challenge in finding an optimal revascularization solution. As the AAA has no indication for repair due to its diameter, a revascularization solution taken into discussion could be an extraanatomical bypass. Given that the left femoropopliteal axis is also affected by atherosclerotic lesions, performing a femoral–femoral crossover bypass or even a left common iliac to right femoral bypass may lead to the left leg claudication onset, precipitated by the hemodynamic steal syndrome. The axillofemoral extra anatomical bypass could have been the procedure of choice for a two-stage surgery: nephrectomy followed by the bypass, with the same objection remaining—an extraanatomical graft with non-optimal patency in a young, active patient with a reasonable life expectancy given by the radical nephrectomy.
The choice of not touching the AAA and using the renal artery stump makes way for a different therapeutical solution to be contemplated, should the need emerge for a future EVAR procedure with a left femoral approach.
Given a 56-year-old patient who preferred a one-stage operation, the need for radicality in the oncological setting, and the extended atherosclerotic lesions of the iliac artery, forming an interdisciplinary team and using a technical innovation were the best choices to strive for the best solution. As far as we know, this type of revascularization has not been reported previously.
As specific situations require specific solutions, the choice of using the renal stump artery was, given the case at hand, the only logical one. Considering a technical innovation is, in complex cases, better than risking inflicting further complications due to prolonged clamping time.
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Târgu-Mureș Emergency County Hospital. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication; ER and AVM share the first authorship.
This paper was published with the support of the University of Medicine, Pharmacy, Science and Technology “George Emil Palade” of Târgu-Mureș. Also, we express our gratitude to our patient for agreeing to participate in this case report and for contributing to the medical knowledge on this subject.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eid MA, Mehta K, Barnes JA, Wanken Z, Columbo J, Stone DH, et al. Global burden of disease of peripheral artery disease. J Vasc Surg. (2021) 74:e327. doi: 10.1016/j.jvs.2021.07.078
2. Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. (1954) 21:499–33.
3. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. (1997) 26:517–38. doi: 10.1016/s0741-5214(97)70045-4
4. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. (2005) 366:1925–34. doi: 10.1016/S0140-6736(05)67704-5
5. Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. (2004) 15:1197–207. doi: 10.1097/01.rvi.0000137978.15352.c6
6. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2017) 69:1465–508. doi: 10.1016/j.jacc.2016.11.008
7. Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. (2019) 69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016
8. Giza DE, Iliescu G, Hassan S, Marmagkiolis K, Iliescu C. Cancer as a risk factor for cardiovascular disease. Curr Oncol Rep. (2017) 19:39. doi: 10.1007/s11912-017-0601-x
9. El Sakka K, Gambhir RPS, Halawa M, Chong P, Rashid H. Association of malignant disease with critical leg ischaemia. Br J Surg. (2005) 92:1498–501. doi: 10.1002/bjs.5125
10. Nicolajsen CW, Dickenson MH, Budtz-Lilly J, Eldrup N. Frequency of cancer in patients operated on for acute peripheral arterial thrombosis and the impact on prognosis. J Vasc Surg. (2015) 62:1598–606. doi: 10.1016/j.jvs.2015.06.223
11. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
12. Lawrie K, Whitley A, Balaz P. A systematic review and meta-analysis on the management of concomitant abdominal aortic aneurysms and renal tumours. Vascular. (2021):17085381211026828. doi: 10.1177/17085381211026827
13. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57:8–93. doi: 10.1016/j.ejvs.2018.09.020
14. Arbănași E-M, Russu E, Mureșan AV, Arbănași E-M. Late rupture of a thrombosed aortic abdominal aneurysm – a case report. J Cardiovasc Emerg. (2021) 7:84–7. doi: 10.2478/jce-2021-0012
15. Kaller R, Mureșan AV, Popa DG, Arbănași E-M, Russu E. Fatal aortoduodenal fistula caused by a ruptured abdominal aortic aneurysm - a case report. J Cardiovasc Emerg. (2021) 4:129–32. doi: 10.2478/jce-2021-0015
16. Mosarla RC, Vaduganathan M, Qamar A, Moslehi J, Piazza G, Giugliano RP. Anticoagulation strategies in patients with cancer: JACC review topic of the week. J Am Coll Cardiol. (2019) 73:1336–49. doi: 10.1016/j.jacc.2019.01.017
17. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
18. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. (2019) 133:781–9. doi: 10.1182/blood-2018-06-860874
19. Ochsner JL, Cooley DA, De Bakey ME. Associated intra-abdominal lesions encountered during resection of aortic aneurysms surgical considerations. Dis Colon Rectum. (1960) 3(6):485–90. doi: 10.1007/BF02616448
20. Prusa AM, Wolff KS, Sahal M, Polterauer P, Lammer J, Kretschmer G, et al. Abdominal aortic aneurysms and concomitant diseases requiring surgical intervention: simultaneous operation vs staged treatment using endoluminal stent grafting. Arch Surg. (2005) 140(7):686–91.
21. Mohandas S, Malik HT, Syed I. Concomitant abdominal aortic aneurysm and gastrointestinal malignancy: evolution of treatment paradigm in the endovascular era–review article. Int J Surg. (2013) 11(2):112–5. doi: 10.1016/j.ijsu.2012.11.022
Keywords: aortic aneurysm, critical limb ischemia, renal artery, nephrectomy, lower limb revascularization, vascular surgery
Citation: Russu E, Mureșan AV, Kaller R, Toma L, Coșarcă CM, Chibelean CB, Arbănași EM and Arbănași EM (2022) Innovative Technical Solution Using the Renal Artery Stump after Nephrectomy as an Inflow Artery for Lower Limb Revascularization—A Case Report. Front. Surg. 9:864846. doi: 10.3389/fsurg.2022.864846
Received: 28 January 2022; Accepted: 25 April 2022;
Published: 13 May 2022.
Edited by:
Christos Argyriou, Democritus University of Thrace, GreeceReviewed by:
Nicolas J. Mouawad, Mclaren Bay Heart & Vascular, United StatesCopyright © 2022 Russu, Mureșan, Kaller, Toma, Coșarcă, Chibelean, Arbănași and Arbănași. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emil Marian Arbănași ZW1pbGFyYmFuYXNpMUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.