- 1Department of Thoracic Surgery, Daping Hospital, Army Medical University, Chongqing, China
- 2Computer Teaching and Research Office, Army Academy of Artillery and Air Defense, Hefei, China
Purpose: To identify the optimal number of lymph nodes dissected during esophagectomy following neoadjuvant therapy for carcinoma of the esophagus by using the Surveillance, Epidemiology and End Results Registry (SEER) database.
Patients and Methods: Patients who underwent neoadjuvant Chemoradiotherapy (nCRT) plus esophagectomy with EC from 2001–2016 were analyzed retrospectively in the SEER database. We analyzed the correlation between the lymphadenectomy count and nodal stage migration and overall survival (OS) by using a binary logistic regression model and Cox proportional hazards regression. The curves of the odds ratios (ORs) of nodal stage migration and hazard ratios (HRs) of OS were smoothed using the LOWESS technique, and the cutoff points were determined by the Chow test. The OS curves were calculated with the Kaplan-Meier method.
Results: Among the 4,710 patients analyzed in the SEER database, a median of 12 lymph nodes (IQR, 7–19) were harvested. There was a significantly proportional increase in nodal stage migration (OR, 1.017; 95% CI, 1.011 to 1.023; P < 0.001) and serial improvements in OS among node-negative patients (HR, 0.983; 95% CI, 0.977 to 0.988; P < 0.001) with an increased ELN count after adjusting for the T stage. The corresponding cutoff point of the 16 ELNs was calculated for the OR of stage migration by the Chow test. For those with node-negative and node-positive diseases, no significant trend of survival benefit that favored a more extensive lymphadenectomy was demonstrated (HR, 1.001; 95% CI, 0.989 to 1.012; P = 0.906; and HR, 0.996; 95% CI, 0.985 to 1.006; P = 0.405, respectively).
Conclusion: On the basis of these results, we recommend that at least 16 ELNs be removed for accurate nodal staging as well as for obtaining a therapeutic benefit after nCRT for EC. Furthermore, once precise nodal staging has been achieved, patient survival does not improve with additional ELN dissection after nCRT, regardless of pathological nodal staging (negative or positive).
Introduction
Esophageal cancer is ranked seventh in cancer incidence rates (572,000 cases) and sixth for cancer deaths (508,000 cases) worldwide, with a poor prognosis and high mortality rate (1). For patients with potentially curable localized tumors, surgical resection via esophagectomy with two- or three-field lymphadenectomy is still the primary form of treatment, with a 5-year survival rate of 23–41% (2). With regard to the count of the examined lymph nodes, the current National Comprehensive Cancer Network (NCCN) guidelines recommend that at least 15 examined lymph nodes (ELNs) should be achieved in patients undergoing esophagectomy without preoperative treatment for adequate nodal staging (3).
For patients with locally advanced disease, neoadjuvant chemotherapy or chemoradiotherapy prior to surgery has been revealed to be associated with a significant improvement in overall survival (4, 5). Concerning the extent of lymphadenectomy, several previous studies have demonstrated that high lymph node retrieval was associated with accurate staging and excellent outcomes (6–9). Kamel MK and colleagues (10) analyzed the National Cancer Database of 14,503 esophageal cancer patients and revealed that the cutoff number of resected LNs after induction chemoradiation that was associated with the highest survival benefit was 20 nodes. In contrast, another study conducted by Zhan et al. (11) demonstrated that 18, 19, and 28 ELNs could achieve accurate N0, N1, and N2 staging for patients with neoadjuvant therapy. However, the optimal count of ELN dissection post nCRT for carcinoma of the esophagus has not yet been well established. Moreover, the real value of extended lymphadenectomy following neoadjuvant treatment in EC is still elusive and debatable.
The purpose of the present study was to use the Surveillance, Epidemiology, and End Results (SEER) database to investigate the optimal count of ELN dissection and the real value of extended lymphadenectomy among patients who underwent neoadjuvant treatment followed by esophagectomy with lymphadenectomy at a population-based level.
Materials and Methods
Patient Population
All cases were obtained from the National Cancer Institute’s SEER database (http://www.seer.cancer.gov) with the use of SEER*Stat version 8.3.8 software: SEER 18 Regs Custom Data (with additional treatment field), Nov 2018 Sub (1975–2016 varying) database. Using the “Primary Site-labeled” variable, we chose tumor cases from the primary site of the esophagus diagnosed between 2001 and 2016. Only patients who received radiation (with or without chemotherapy) prior to surgery were included in this study. Patients with any of the following criteria were excluded: surgery alone, unknown regional nodes examined or positive, and unknown survival time. Information on the age at diagnosis, sex, race, size of the tumor, histologic type, grade, primary site, American Joint Committee on Cancer (AJCC) stage (6th ed or 7th ed), radiation sequence with surgery, chemotherapy recode (yes or no), regional nodes examined, regional nodes positive, distant metastasis, cause of death, and survival time was collected. Patients were staged according to the seventh edition of the TNM classification.
Approval for the study by the local institutional review board and written consent were not required because it was a public database of clinical research.
Statistical Analysis
Categorical data were compared using Pearson chi-square tests or Fisher’s exact test, and continuous data were compared using ANOVA. A binary logistic regression model and Cox proportional hazards regression were carried out to determine the correlation between ELNs and stage migration (node negative versus node positive) and OS. The survival rate was calculated using the Kaplan-Meier method, and a log-rank test was used to assess the survival differences between groups. Statistical analyses were performed by using IBM SPSS 25.0 software (IBM, Inc.). P values of less than 0.05 were considered statistically significant.
In accordance with the procedures previously described by Liang et al. (12), the curves of the odds ratios (ORs; stage migration) of each ELN count compared with one ELN (as a reference) were smoothed using the locally weighted scatter-plot smoother (LOWESS) technique with a bandwidth of 2/3 (default). The structural break points were determined by the Chow test, which were considered the threshold of clinical impact. Then, the curves of hazard ratios (HRs; OS) of more ELN counts compared with the threshold ELN count (as a reference) were smoothed to characterize the relationship between survival and the extended ELN yield. Both the LOWESS and Chow tests were performed by using Python version 3.7 software (Python Software Foundation, Delaware).
Results
Patient Characteristics
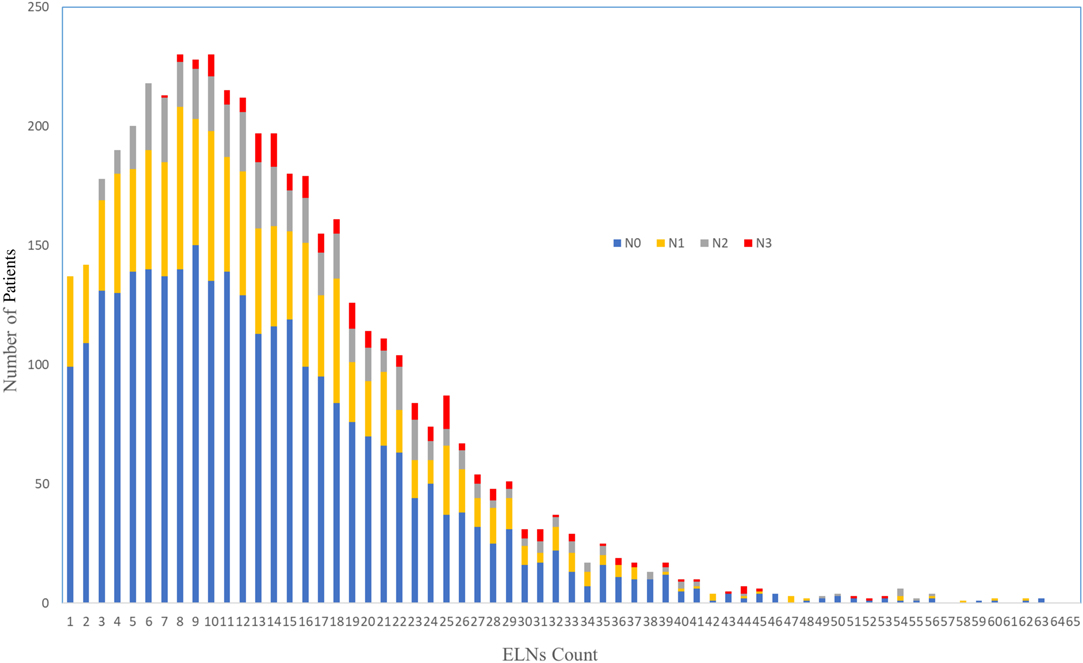
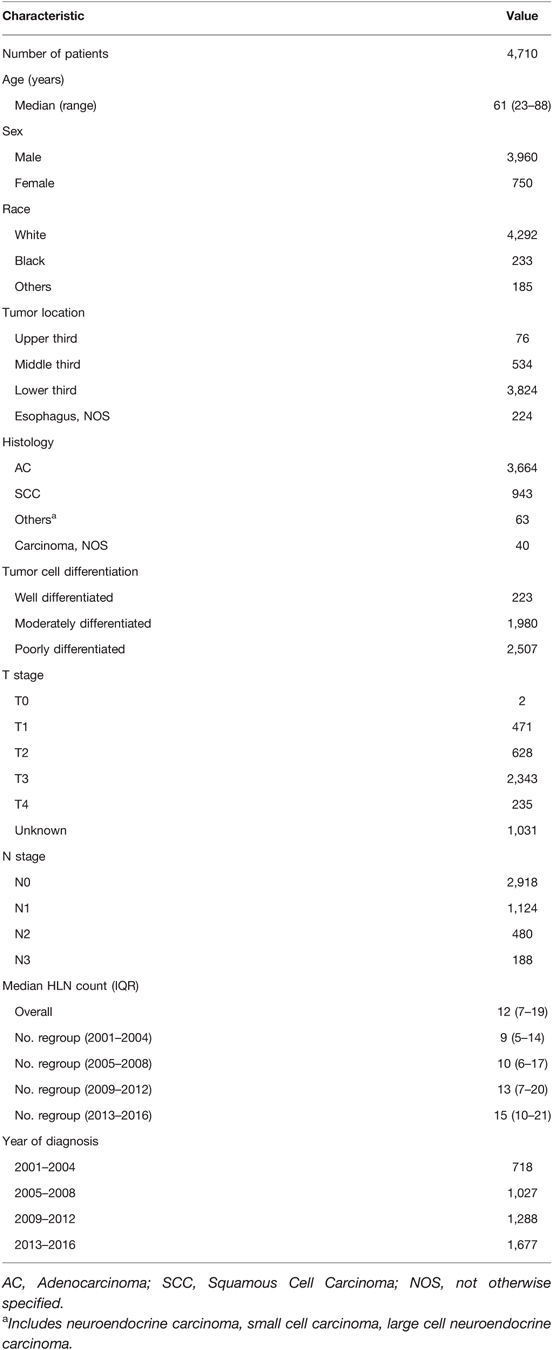
A query of the SEER Database resulted in a total of 4,710 patients who received nCRT followed by esophagectomy that were performed for cancer between 2001 and 2016. The characteristics of the patients are described in Table 1. The study group consisted of 750 women and 3,960 men, with a median age of 61 years (range: 23–88) and a distinct preponderance of adenocarcinoma histology (adenocarcinoma: others, 3.5:1). The distribution of ELNs is shown in Figure 1, with a median number of harvested ELNs of 12 (range: 7–19). Furthermore, the median number of ELNs increased from 9 in 2001 to 2004 to 15 in 2013 to 2016.
Correlation between ELNs and Stage Migration
The median number of ELNs differed significantly within subgroups of the histologic type (AC: SCC: others: NOS, 14.3:13.2:13.9:10.7; P = 0.01), T stage (T0: T1: T2: T3: T4: TX, 10.0:13.1:13.8:14.8:13.7:13.3; P < 0.01) and N stage (N0: N1: N2: N3, 13.4:13.9:16.0:21.7; P < 0.01). There was a significantly proportional increase in the N stage (from N0 to N1, N2, and N3), with an increasing ELN count after adjusting for the T stage (OR, 1.017; 95% CI, 1.011 to 1.023; P < 0.001; Table 2). However, after stratification by histologic type, only a consistent trend was observed in patients with AC with an increasing ELN count (OR, 1.021; 95% CI, 1.014 to 1.028; P < 0.001).
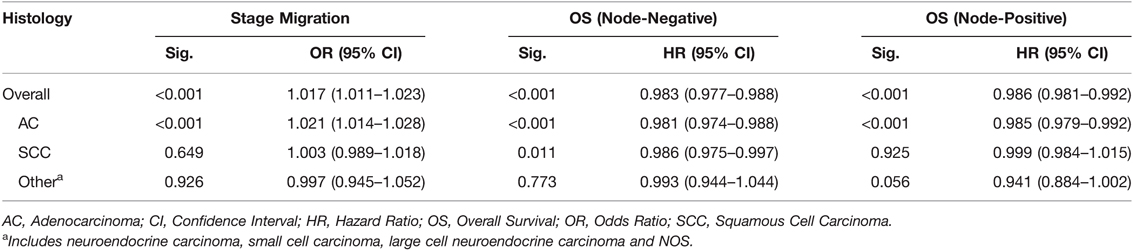
Table 2. Number of ELNs (as a continuous variable) and Stage Migration and OS, Stratified by Histologic Subgroups, Adjusted for T stage.
Correlation between ELNs and OS
After adjusting for the T stage, a greater number of ELNs was significantly associated with a better OS among patients with node-negative (N0) disease (HR, 0.983; 95% CI, 0.977 to 0.988; P < 0.001) and patients with node-positive (N1, N2, and N3) disease (HR, 0.985; 95% CI, 0.979 to 0.992; P < 0.001). However, after stratification by histologic type, only a consistent trend was observed in patients with AC, with the exception of node-negative patients with SCC (HR, 0.986; 95% CI, 0.975 to 0.997; P < 0.001; Table 2).
Cut-Point Analysis for Nodal Stage Migration and Validation
As shown in Figure 2, the fitting curves for the OR of nodal stage migration were smoothed using the LOWESS technique. The corresponding cutoff point of 16 ELNs was calculated for the OR of stage migration by the Chow test.
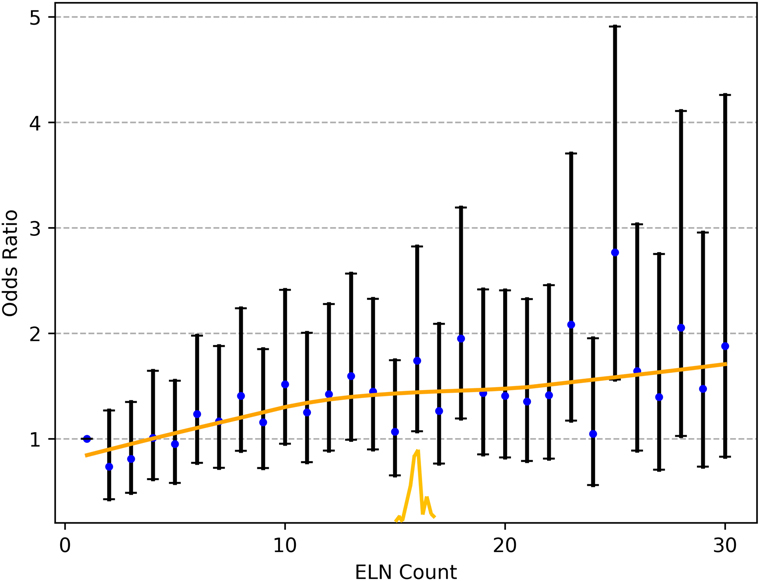
Figure 2. The fitting curves for the OR of nodal stage migration were smoothed using the LOWESS technique, and the corresponding cutoff point of the ELNs was calculated by the Chow test. ELN, examined lymph node. OR, odds ratio.
At the time of analysis, the median follow-up was 62.0 months. For node-negative patients, the five-year survival rates and median survival time were 59.2% and 72.0 months (95% confidence interval, 61.5 to 79.5 months), respectively, among patients with ELN count ≥16 and 52.9% and 48.6 months (95% confidence interval, 40.0 to 52.5 months), respectively, among those with ELN count <16 (P < 0.001; Figure 3). With regard to node-positive patients, the five-year survival rates and median survival time were 32.2% and 29.0 months (95% confidence interval, 25.7 to 32.2 months), respectively, among patients with ELN count ≥16 and 24.2% and 21.0 months (95% confidence interval, 18.8 to 23.1 months), respectively, among those with ELN count < 16 (P < 0.001; Figure 4).
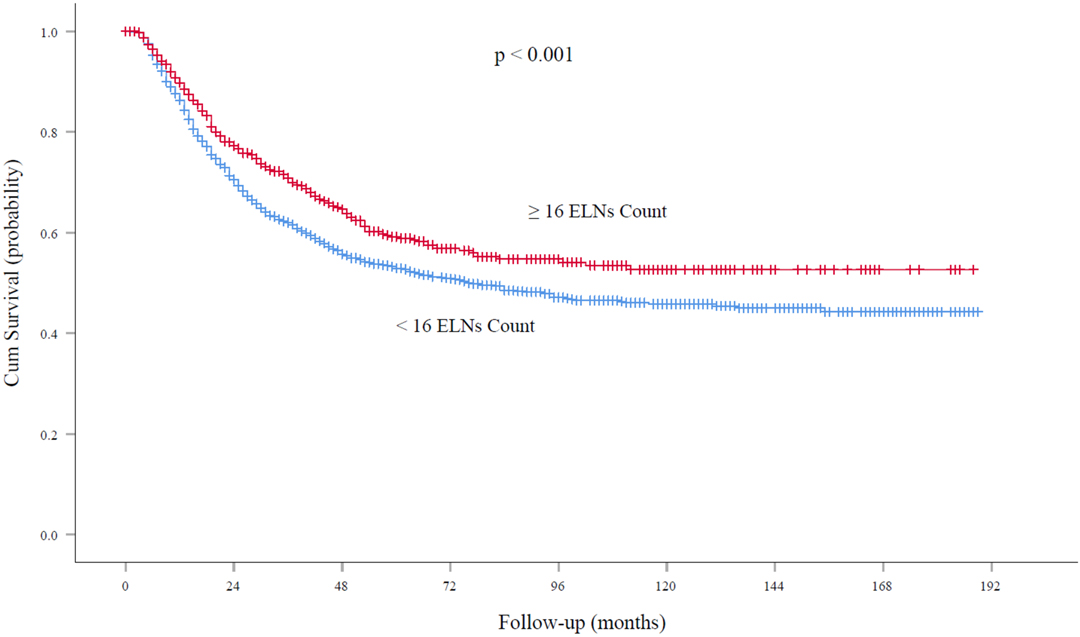
Figure 3. Overall survival rates among patients with node-negative EC at the cutoff point of 16 ELNs. ELN, examined lymph node. EC, esophageal cancer.
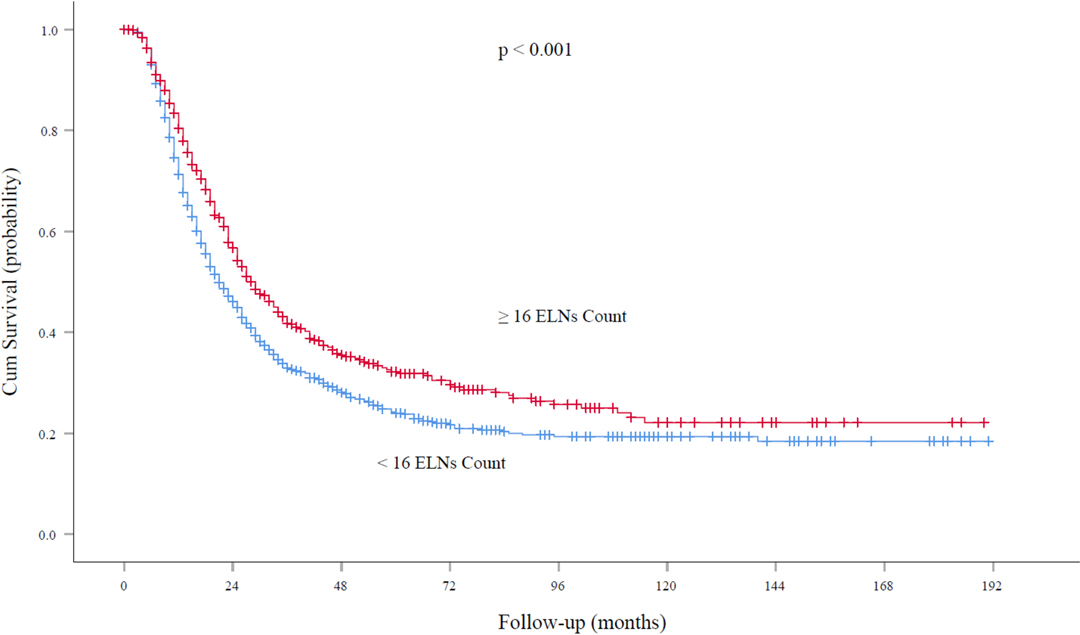
Figure 4. Overall survival rates among patients with node-positive EC at the cutoff point of 16 ELNs. ELN, examined lymph node. EC, esophageal cancer.
Impact of Extended Lymphadenectomy
For those with node-negative and node-positive diseases, the curves of the hazard ratios (HRs; OS) of each ELN count (>16 nodes) compared with 16 ELNs (as a reference) were smoothed using the LOWESS technique and are shown in Figure 5 and Figure 6, respectively; however, they failed to demonstrate a significant trend of survival benefit that favored a more extensive lymphadenectomy (HR, 1.001; 95% CI, 0.989 to 1.012; P = 0.906; and HR, 0.996; 95% CI, 0.985 to 1.006; P = 0.405, respectively).
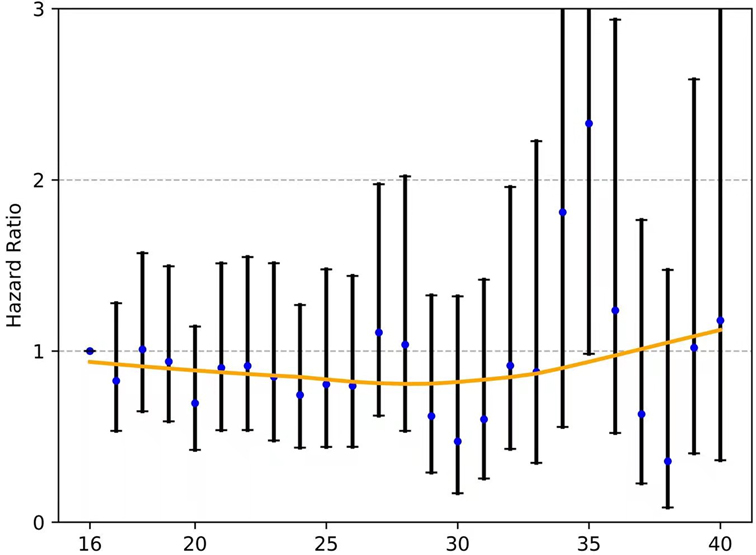
Figure 5. The fitting curves for the HR of each ELN count (>16 nodes) compared with 16 ELNs (as a reference) among patients with node-negative EC were smoothed using the LOWESS technique. ELN, examined lymph node. EC, esophageal cancer. OR, odds ratio.
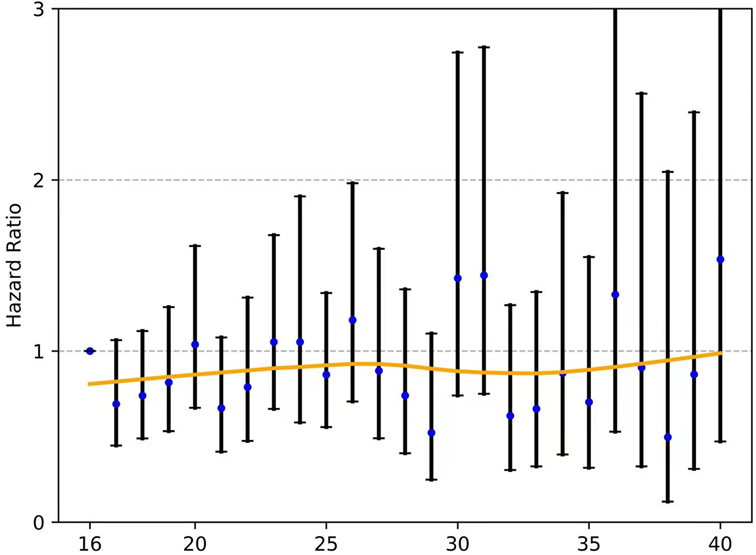
Figure 6. The fitting curves for the HR of each ELN count (>16 nodes) compared with 16 ELNs (as a reference) among patients with node-positive EC were smoothed using the LOWESS technique. ELN, examined lymph node. EC, esophageal cancer. OR, odds ratio.
Discussion
The importance of the number of ELNs during esophagectomy alone for staging accuracy and long-term survival has been well documented and acknowledged (13–18). Accordingly, the NCCN guidelines recommend that at least 15 lymph nodes (LNs) should be removed in patients undergoing esophagectomy without preoperative treatment for adequate nodal staging (3). However, the impact of neoadjuvant treatment on the dissemination and the pattern of lymph node distribution has not been well established. Moreover, the potential effect of extensive lymphadenectomy on survival after surgery following neoadjuvant therapy remains controversial. The optimum lymphadenectomy during esophagectomy in patients after receiving neoadjuvant treatment has not yet been well identified. The study by Castoro and associates (19) on 402 patients with cancer of the esophagus or esophagogastric junction demonstrated that not only did the frequency of lymph node metastases decrease after neoadjuvant chemoradiotherapy (nCRT) but also the nodal localization and pattern were significantly modified. Moreover, in the Dutch CROSS trial, the median (interquartile range) number of resected nodes for patients who underwent surgery alone and received multimodality treatment was 18 (12–27) and 14 (9–21), with 2 (1–6) and 0 (0–1) resected positive nodes, respectively (20). Similarly, randomized clinical trials published in the New England Journal of Medicine have also revealed that there was a significant trend toward less advanced nodal disease in the perioperative chemotherapy group than in the surgery group (84.4% vs. 70.5%, P = 0.01) (4). Therefore, the question arises whether suboptimal lymphadenectomy affects the accuracy of nodal stage or long-term outcome after nCRT in patients with EC.
A limited number of LNs harvested in EC patients after nCRT may lead to inaccurate nodal staging, particularly with regard to those with nodal upstaging. As Meguid et al. (21) reported, almost seventy percent of EC patients are partial responders and nonresponders to nCRT. In the current study, the US SEER Database was analyzed and the data clearly indicated that a larger number of LNs dissection was correlated with a higher proportion of more advanced nodal stage cases in SCC and AC post neoadjuvant treatment. Furthermore, our results suggested that at least 16 nodes should be removed to avoid nodal staging migration during esophagectomy for patients undergoing nCRT, which is mainly consistent with the NCCN guideline recommendations for those undergoing surgery alone (15 ELNs). Similarly, Stiles et al. (7) revealed that optimal lymphadenectomy, as defined by the WECC, may also be applicable to cases of esophagectomy following neoadjuvant therapy, particularly those who were not downstaged by pathological tumor depth classification and those with persistent nodal metastases.
A second question concerning long-term effects is whether overall survival may be improved by extended lymphadenectomy for patients treated with nCRT. Some investigators believe that resecting more LNs is helpful for accurate nodal staging and decreasing the chance of undiscovered positive LNs. For patients with node-positive disease (not declared as node-negative disease due to fewer ELNs), adjuvant treatment after surgery could improve the OS. In addition, extended nodal resection would benefit the clearance of micrometastases and residual lesions after neoadjuvant therapy and reduce the risk of local recurrence. For example, Solomon and colleagues (22) analyzed the SEER database of 4,224 patients and showed that there is a cooperative survival benefit for neoadjuvant radiation and adequate lymphadenectomy (ELNs ≥18) in patients with node-positive esophageal adenocarcinoma. Furthermore, a meta-analysis conducted by Visser et al. (8) demonstrated a survival benefit of high lymph node yield on overall survival (HR = 0.82; 95% CI = 0.73–0.92; P < 0.01). In contrast, however, in the present study, our results showed that once precise nodal staging (>16 nodes) had been achieved, patient survival did not improve with additional LNs dissection after nCRT, regardless of nodal stage (negative or positive). As stated by, the total number of ELNs was significantly associated with survival for patients in the surgery-alone arm (P = 0.007) but not in the nCRT arm (P = 0.98). Similarly, Shridhar et al. (23) noted that the number of LNs harvested during esophagectomy after nCRT does not impact OS or disease-free survival (DFS). Furthermore, Noordman BJ et al. (24) revealed that compared to surgery alone, the addition of nCRT may reduce the need for extended lymphadenectomy to improve long-term survival in patients with esophageal adenocarcinoma. This difference in effect of lymphadenectomy might be partially explained by the sterilization of micrometastases after nCRT and high local tumor control rate of radiotherapy on the regional lymph nodes. It has been reported that the pathological complete response rate was 19–40% in neoadjuvant CRT (25, 26). Moreover, several previous studies (24, 25) revealed that the number of resected LNs and the number of resected positive LNs were significantly decreased in patients treated with nCRT than in those treated with surgery alone, suggesting that the clinical importance of extended lymphadenectomy to improve the locoregional control rate may differ between surgery after nCRT and surgery alone. In addition, As Patti et al. (27) demonstrated, it is not the extent of lymphadenectomy that dictates outcomes but rather the tumor biology and the stage of the tumor during the surgery. In addition, a more extensive lymphadenectomy does have the disadvantage of greater surgical morbidity, which confers a large negative impact on survival after esophagectomy for cancer (8, 28). Further studies with larger study populations or other methods are required to better elucidate the role of extended lymphadenectomy following neoadjuvant treatment.
Finally, it should be noted that there are several other factors that may influence the long-term outcomes in patients undergoing lymphadenectomy post nCRT, including ELN location and ratio and extracapsular ELN involvement. In detail, a study by Phillips (6) concluded that omitting lymphatic dissection of hepatic, celiac, and splenic nodes may lead to a very limited number of extra cancer-related deaths, and thus, the extent of lymphadenectomy post neoadjuvant chemotherapy must be correlated with the node location. With regard to the ELN ratio, Mariette and colleagues (29) demonstrated that the ratio between metastatic and examined lymph nodes is an important predictor of a poor prognosis, especially for inadequately staged EC patients following nCRT. In addition, D’Journo et al. (30) found that extension of tumor cells through the nodal capsule into the fatty tissue seems to be an independent negative prognostic factor affecting survival in patients with locally advanced EC treated with nCRT and surgery.
This study has several limitations. First, it was retrospective, and some important patient clinicopathological features were rather limited, such as the variations in radiation doses and range over the study period, the usage of different combinations of chemotherapeutic agents, various therapeutic modalities given following surgery, various degrees of pathologic tumor response to neoadjuvant therapy, the incidence of postoperative complications, the lack of standardized staging due to missing data of clinical TNM stage, and resection margin status (R0, R1 or R2), all of which have the potential to modulate the treatment effect. Additionally, owing to the limitations of the database, we did not further associate the number of ELNs with their locations (cervical, mediastinal or abdominal), surgical approach (transthoracic or transhiatal), or surgeon/hospital esophagectomy volume, which might affect the ELN status and long-term outcomes as previously reported. We suggest a future prospective randomized trial with a larger study population or other methods to validate our results.
In conclusion, our data suggest that at least 16 ELNs should be achieved for accurate nodal staging as well as for obtaining a therapeutic benefit for patients undergoing nCRT. Furthermore, once precise nodal staging has been achieved, patient survival does not improve with additional LNs dissection after nCRT, regardless of the pathological nodal staging (negative or positive). These findings have important clinical implications and should be considered when performing esophagectomy for patients post nCRT.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Approval for the study by the local institutional review board and written consent were not required because it was a public database of clinical research that only contains de-identified patient data.
Author Contributions
TB and WG conceived and designed the experiments. TB and LB collected the data and draw the graphs. LB contributed analysis tools. LB and TB analyzed the data. TB wrote the manuscript. TB and LB contributed equally to this work and are co-first authors.
Acknowledgments
We acknowledged the SEER database for the provided data for the research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. (2020) 159(1):335–49.e15. doi: 10.1053/j.gastro.2020.02.068
3. NCCN Clinical Practice Guidelines in Oncology- Esophageal and Esophagogastric Junction Cancers (2018 Version I). Available at: http://www.nccn.org (Accessed March 16, 2018).
4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
5. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
6. Phillips AW, Lagarde SM, Navidi M, Disep B, Griffin SM. Impact of extent of lymphadenectomy on survival, post neoadjuvant chemotherapy and transthoracic esophagectomy. Ann Surg. (2017) 265(4):750–6. doi: 10.1097/SLA.0000000000001737
7. Stiles BM, Nasar A, Mirza FA, Lee PC, Paul S, Port JL, et al. Worldwide Oesophageal Cancer Collaboration guidelines for lymphadenectomy predict survival following neoadjuvant therapy. Eur J Cardiothorac Surg. (2012) 42(4):659–64. doi: 10.1093/ejcts/ezs105
8. Visser E, Markar SR, Ruurda JP, Hanna GB, van Hillegersberg R. Prognostic value of lymph node yield on overall survival in esophageal cancer patients: a systematic review and meta-analysis. Ann Surg. (2019) 269(2):261–8. doi: 10.1097/SLA.0000000000002824
9. Markar SR, Noordman BJ, Mackenzie H, Findlay JM, Boshier PR, Ni M, et al. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol. (2017) 28(3):519–27. doi: 10.1093/annonc/mdw560
10. Kamel MK, Harrison S, Lee B, Port JL, Stiles BM, Altorki NK. Extended lymphadenectomy improves survival after induction chemoradiation for esophageal cancer: a propensity matched analysis of the national cancer database. Ann Surg. (2021). doi: 10.1097/SLA.0000000000005197. Epub ahead of print.
11. Zhan C, Shi Y, Jiang W, Sun F, Li M, Lu T, et al. How many lymph nodes should be dissected in esophagectomy with or without neoadjuvant therapy to get accurate staging? Dis Esophagus. (2020) 33(1):doz009. doi: 10.1093/dote/doz009
12. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a chinese multi-institutional registry. J Clin Oncol. (2017) 35(11):1162–70. doi: 10.1200/JCO.2016.67.5140
13. Rizk NP, Ishwaran H, Rice TW, Chen LQ, Schipper PH, Kesler KA, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. (2010) 251(1):46–50. doi: 10.1097/SLA.0b013e3181b2f6ee
14. Rizk N, Venkatraman E, Park B, Flores R, Bains MS, et al. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American jJoint Committee on Cancer staging system. J Thorac Cardiovasc Surg. (2006) 132(6):1374–81. doi: 10.1016/j.jtcvs.2006.07.039
15. Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. (2008) 112(6):1239–46. doi: 10.1002/cncr.23309
16. Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg. (2007) 11(11):1384–93. doi: 10.1007/s11605-007-0264-2
17. Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. (2008) 248(4):549–56. doi: 10.1097/SLA.0b013e318188c474
18. Hu Y, Hu C, Zhang H, Ping Y, Chen LQ. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol. (2010) 17(3):784–90. doi: 10.1245/s10434-009-0818-5
19. Castoro C, Scarpa M, Cagol M, Ruol A, Cavallin F, Alfieri R, et al. Nodal metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol. (2011) 18(13):3743–54. doi: 10.1245/s10434-011-1753-9
20. Koen Talsma A, Shapiro J, Looman CW, van Hagen P, Steyerberg EW, van der Gaast A, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg. (2014) 260(5):786–92; discussion 792-3. doi: 10.1097/SLA.0000000000000965
21. Meguid RA, Hooker CM, Taylor JT, Kleinberg LR, Cattaneo SM 2nd, Sussman MS, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. (2009) 138(6):1309–17. doi: 10.1016/j.jtcvs.2009.07.069
22. Solomon N, Zhuge Y, Cheung M, Franceschi D, Koniaris LG. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol. (2010) 17(3):791–803. doi: 10.1245/s10434-009-0819-4
23. Shridhar R, Hoffe SE, Almhanna K, Weber JM, Chuong MD, Karl RC, et al. Lymph node harvest in esophageal cancer after neoadjuvant chemoradiotherapy. Ann Surg Oncol. (2013) 20(9):3038–43. doi: 10.1245/s10434-013-2988-4
24. Noordman BJ, van Klaveren D, van Berge Henegouwen MI, Wijnhoven BPL, Gisbertz SS, Lagarde SM, et al. Impact of surgical approach on long-term survival in esophageal adenocarcinoma patients with or without neoadjuvant chemoradiotherapy. Ann Surg. (2018) 267(5):892–7. doi: 10.1097/SLA.0000000000002240
25. Robb WB, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: post hoc analysis of a randomized controlled trial. Ann Surg. (2015) 261(5):902–8. doi: 10.1097/SLA.0000000000000991
26. Visser E, van Rossum PSN, Ruurda JP, van Hillegersberg R. Impact of lymph node yield on overall survival in patients treated with neoadjuvant chemoradiotherapy followed by esophagectomy for cancer: a population-based cohort study in the Netherlands. Ann Surg. (2017) 266:863–9. doi: 10.1097/SLA.0000000000002389
27. Patti MG. Survival after lymphadenectomy in patients with esophageal cancer: a controversial issue. JAMA Surg. (2016) 151(1):40. doi: 10.1001/jamasurg.2015.2599
28. Booka E, Takeuchi H, Nishi T, Matsuda S, Kaburagi T, Fukuda K, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore). (2015) 94(33):e1369. doi: 10.1097/MD.0000000000001369
29. Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. (2008) 247(2):365–71. doi: 10.1097/SLA.0b013e31815aaadf
Keywords: esophageal carcinoma, esophagectomy, neoadjuvant treatment, lymphadenectomy, survival, SEER
Citation: Bao T, Bao L and Guo W (2022) Impact of Examined Lymph Node Count on Precise Staging and Long-term Survival After Neoadjuvant Therapy for Carcinoma of the Esophagus: A SEER Database Analysis. Front. Surg. 9:864593. doi: 10.3389/fsurg.2022.864593
Received: 28 January 2022; Accepted: 11 April 2022;
Published: 29 April 2022.
Edited by:
Qi Liu, Shanghai Cancer Center, Fudan University, ChinaReviewed by:
Mohamed Kamel, College of Medicine, Central Michigan University, United StatesMatthew Fox, University of Louisville, Louisville, United States
Copyright © 2022 Guo, Bao and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Guo d2VpZ3VvZHB5eUAxNjMuY29t
Speciality section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Tao Bao1
Tao Bao1 Wei Guo
Wei Guo