
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 31 March 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.863249
This article is part of the Research Topic Women in Thoracic Surgery View all 6 articles
Background: Dexmedetomidine reduces the occurrence of postoperative nausea and vomiting (PONV); however, the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery remains inconclusive. In addition, the effect of different dexmedetomidine application methods, anesthetics, and surgical procedures on the effects of dexmedetomidine on PONV remains unclear. Therefore, the purpose of this meta-analysis was to study the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery.
Methods: Electronic databases were searched to identify randomized controlled trials studying the effects of dexmedetomidine on nausea and vomiting after thoracic surgery. In total, 12 articles that met the inclusion criteria were obtained. The primary outcome of this comprehensive analysis was the incidence of PONV; secondary outcomes included the incidence of postoperative nausea, the incidence of postoperative vomiting, postoperative visual analog score (VAS), the amount of intraoperative sufentanil, and the number of times postoperative salvage analgesia was administered.
Results: Twelve trials involving 905 participants undergoing thoracic surgery were included. Compared with placebo, dexmedetomidine reduced the incidence of nausea and vomiting after thoracic surgery [12 trials; 905 participants; risk ratio (RR) = 0.32; 95% CI (0.23, 0.44); P < 0.00001, I2 = 0%]. The subgroup analysis revealed that dexmedetomidine reduces the occurrence of PONV in both thoracotomy and thoracoscopic surgery. In addition, both intravenous and local infusion of dexmedetomidine can reduce the occurrence of PONV, and intravenous or inhaled anesthetics do not affect the effect of dexmedetomidine on reducing PONV. Dexmedetomidine can reduce the postoperative resting VAS of patients, and no statistically significant differences in the amount of intraoperative sufentanil and the number of salvage analgesia procedures after surgery were noted.
Conclusion: Compared with placebo, dexmedetomidine can reduce the occurrence of PONV in patients undergoing thoracic surgery, and this effect is not affected by the method of dexmedetomidine administration, use of minimally invasive surgery, and use of a combination of intravenous or inhalation anesthetics.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#myprospero, PROSPERO, identifier: CRD42021269358.
The risk of pulmonary complications after thoracic surgery (19–59%) is relatively high and greater than that note for upper abdominal surgery (16–17%) and lower abdominal surgery (0–5%) (1). Nausea and vomiting are the two most common adverse events after surgery. The incidence rate of postoperative nausea and vomiting (PONV) is 30% in the general population and 80% in the high-risk population (2). PONV is a very painful experience that is obviously related to patient dissatisfaction (3, 4). PONV has a serious impact on the patient's postoperative rehabilitation. It can also cause disorders based on water, electrolyte, and acid-base balance; prolong the hospital stay and increase the economic burden of the patient (5). It leads to reflux and aspiration of the patient, which subsequently increases the incidence of postoperative pulmonary complications and causes death in severe cases (1).
At present, many methods are available to prevent and treat PONV. Among them, drugs are one of the important methods. Numerous drugs can be used to reduce PONV, such as 5-HT3 receptor antagonists, neurokinin 1 (NK1) receptor antagonists, corticosteroids, and anticholinergics. These drugs have different pharmacokinetics, curative effects, and side effects (6). The incidence of PONV varies greatly among different populations. There are many studies on the prevention of PONV, but these studies are limited to specific patient populations (7, 8). Dexmedetomidine is a highly selective adrenergic receptor agonist that inhibits sympathetic nerves and provides analgesic and sedative effects with fewer side effects, such as respiratory depression (9). There are numerous ways to administer dexmedetomidine, such as intravenous, nasal, and local applications. A study by Hu et al. (10) confirmed that intravenous administration of dexmedetomidine can reduce the incidence of PONV in patients undergoing cesarean section (10). Despite statistical heterogeneity, an SRMA found reduced rates of PONV as a secondary outcome in children receiving intranasal dexmedetomidine for separation anxiety compared to intranasal or oral midazolam (11). A randomized controlled trial by Hong et al. (12) found that dexmedetomidine as an adjuvant to TPVB effectively relieves pain and significantly reduces the demand for opioids in VATS (12).
Opioids are closely related to the occurrence of PONV (2). Studies have shown that dexmedetomidine not only reduces the incidence of PONV (13, 14) but also significantly reduces postoperative pain scores and the amount of opioids (15, 16) which may reduce nausea and vomiting associated with opioids.
Different types of surgery exhibit different incidences of PONV. Laparoscopy, bariatric surgery, gynecological surgery, and cholecystectomy may be associated with an increased risk of PONV (8). In total, 25–30% of surgical patients will experience PONV, so it is a problem that anesthesiologists are concerned about (17, 18). Wang et al. (19) reported that dexmedetomidine reduces the incidence of PONV in thoracic surgery, while in a study by Cai et al. (20), dexmedetomidine exhibited no significant difference in reducing PONV compared with normal saline in thoracic surgery. A consensus on the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery is lacking. Therefore, the purpose of this meta-analysis was to comprehensively explore the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery.
In this meta-analysis, we comprehensively analyzed 12 randomized controlled trials assessing the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery to explore the effect of dexmedetomidine on PONV in thoracic patients.
The meta-analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (21) and is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (22). This study was prospectively registered in the PROSPERO registry (CRD42021269358), https://www.crd.york.ac.uk/prospero/#myprospero.
Types of trials: randomized controlled trials (RCT).
Types of participants: patients undergoing thoracic surgery.
Types of interventions: dexmedetomidine.
Types of outcome measures: the incidence of PONV, the incidence of postoperative nausea, the incidence of postoperative vomiting, postoperative visual analog score (VAS) score, the amount of intraoperative sufentanil, and the number of postoperative salvage analgesia procedures.
Animal experiments, published in non-English languages.
One reviewer searched for studies reported in PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials through July 1, 2021, without any restrictions. Controlled vocabulary (MeSH in PubMed and Emtree in Embase) and keywords were used. Search terms included those related to PONV, dexmedetomidine, thoracic surgery, and their variants. The complete search strategy is available in additional file 1. Two reviewers hand-checked the reference lists of eligible trials and previous reviews.
The Cochrane Collaboration's tool (23) was used to assess the risk of bias and to evaluate the methodology of the included studies. We reviewed each trial and scored them as high, low, or unclear in terms of their risk involving the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Disagreements were resolved by discussion with a third reviewer.
After the records were imported into EndNote reference management software (Clarivate Analytics), duplicate records were removed. Two reviewers screened the titles and abstracts for relevance and labeled records as included, excluded, or uncertain. In the case of uncertainty, the full-text articles were retrieved to assess eligibility. Disagreements were resolved by discussion with other reviewers.
Two reviewers independently extracted the data using a standardized form. We collected information on the trial characteristics (year of publication, number of patients), patient characteristics (age), intervention characteristics (Methods of administration of anesthetics, types of compound anesthetics), and data about the primary and secondary outcomes. Disagreements were resolved by discussion with other reviewers.
The data are expressed as the mean ± SD. If the study provided the median and interquartile range instead of the mean and SD, we calculated the mean and SD using the method developed by McGrath et al. (24).
Review Manager (RevMan 5.3; Copenhagen: Nordic Cochrane Center Collaboration, 2014) was used for analysis. Differences are expressed as relative risks (RRs) with 95% CIs for dichotomous outcomes and standardized mean differences (SMDs) with 95% CIs for continuous outcomes. P < 0.05 indicates that the difference is statistically significant. Heterogeneity testing was performed using the Z score and X2 statistical analysis; P < 0.1 was considered to indicate heterogeneity. When P > 0.1 and I2 < 50%, the heterogeneity was ignored, and a fixed-effects model was used when I2 > 50%; if the heterogeneity was not easily explained, a random-effects model was selected, subgroup analysis was performed, the effect index was changed, or sensitivity analysis was conducted. Subgroup analysis was conducted based on the surgical methods, methods of dexmedetomidine administration, and types of combined anesthetics. Potential publication bias was analyzed using a “funnel plot” by the Review Manager (RevMan 5.3; Copenhagen: Nordic Cochrane Center Collaboration, 2014).
The study flow diagram is shown in Figure 1. The initial search yielded 475 records, after removing duplicates and screening the titles and abstracts, 43 articles were deemed potentially eligible. After reviewing the full-text articles, 12 trials (19, 20, 25–34) were included in the final analysis.
The characteristics of the included trials are summarized in Table 1. The 12 included trials were published from 2016 to 2020 with sample sizes ranging from 41 to 143 subjects and a total of 905 subjects. Details of the risk of bias are presented in Figure 2.
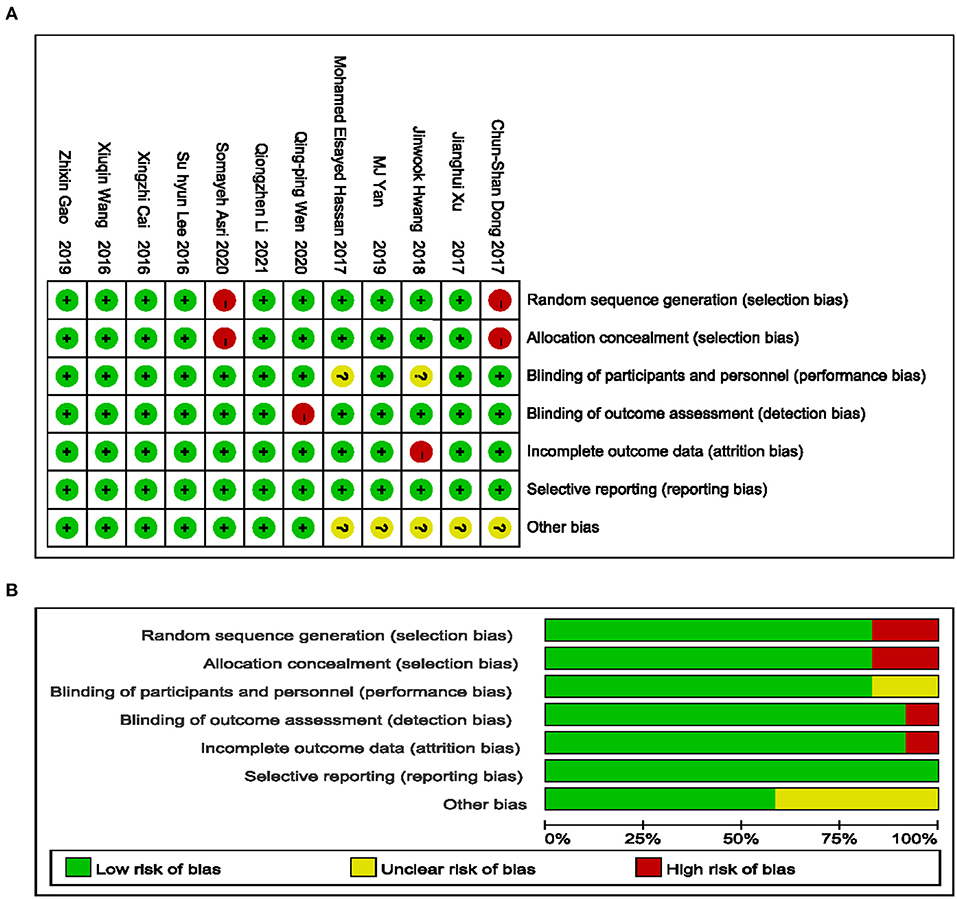
Figure 2. Risk of bias summary. Green low risk, yellow unclear risk, red high risk (A); Green low risk of bias, yellow unclear risk of bias, red high risk of bias (B).
Participants in 10 trials included men and women. One study (20) exclusively enrolled women, and one study (27) exclusively focused on men. Participants underwent thoracoscopy in seven trials and thoracotomy in three trials. Dexmedetomidine was infused intravenously in 8 trials and locally in 4 trials. In 4 trials, dexmedetomidine was infused locally in combination with intravenous anesthetics. In six trials, dexmedetomidine was infused in combination with inhaled anesthetics.
The heterogeneity of the 12 trials included in this meta-analysis was assessed (I2 = 0%), and the value was less than the critical point of 50%. Here, the Q test P = 0.83 >0.1, indicating that the heterogeneity between the studies included in this meta-analysis was not statistically significant. A fixed-effects model was selected for meta-analysis.
Summary results were obtained from 12 studies [RR = 0.32 95%CI (0.23, 0.44)], and the result was statistically significant (Z = 7.02 P < 0.00001 <0.05). These results suggest that the risk of PONV was lower in the dexmedetomidine group compared with the control group. The specific results are shown in Figure 3A.
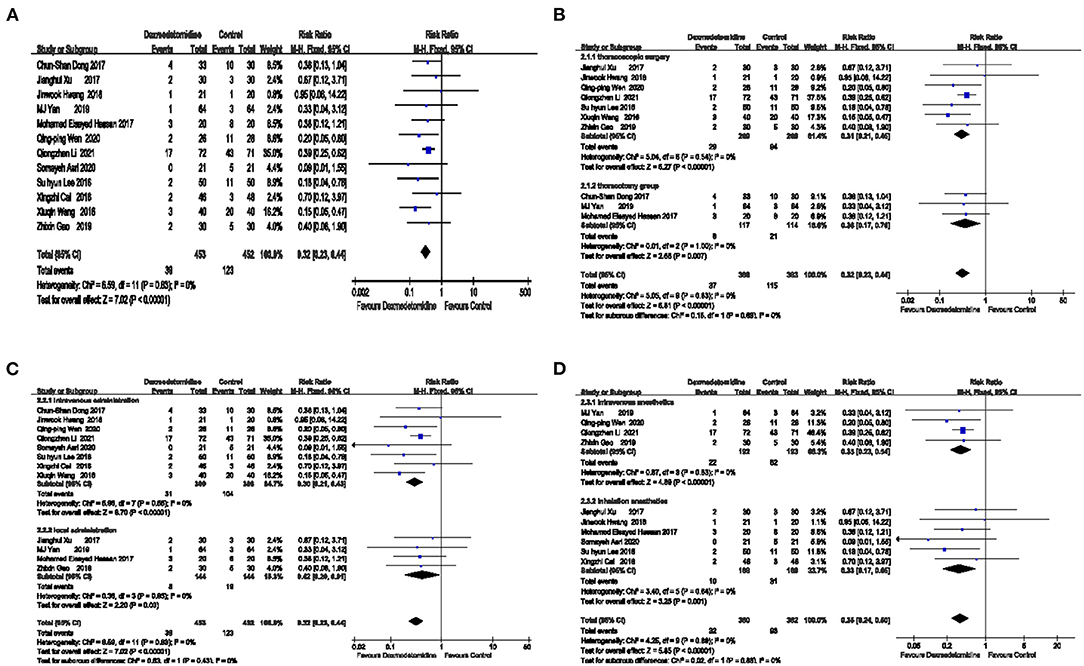
Figure 3. The effect of dexmedetomidine on the incidence of postoperative nausea and vomiting (PONV). The risk of PONV was lower in the dexmedetomidine group compared with the control group (A). Dexmedetomidine reduced the occurrence of PONV in both thoracoscopic surgery and thoracotomy (B). Dexmedetomidine reduced the occurrence of PONV regardless of whether it was intravenously or locally administered (C). Dexmedetomidine reduced the occurrence of PONV regardless of whether it was combined with intravenous anesthetics or inhalation anesthetics (D).
Bias test: A funnel chart is used to investigate whether publication bias exists in this study. The symmetry of the funnel chart indicates no publication bias, suggesting that the publication bias of this outcome is relatively small. The specific results are shown in Supplementary Figure 1A.
The minimally invasive surgery, the types of combined anesthetics, and the method of dexmedetomidine administration were different among the 12 included articles. To explore whether these factors may affect the effect of dexmedetomidine on PONV, three subgroup analyses were performed separately (Figures 3B–D).
Regardless of whether the operation was minimally invasive, the effect of dexmedetomidine on reducing PONV was not affected, as shown in Figure 3B.
According to the different surgical methods, the patients were divided into a thoracoscopy group and a thoracotomy group. In the thoracoscopy group, the combined effect was RR = 0.31 [95% CI (0.21, 0.45)], and the effect was statistically significant (Z = 6.27, P < 0.00001 <0.05), suggesting that the risk of PONV in the dexmedetomidine group during thoracoscopic surgery was 31% of that in the placebo group. In the thoracotomy group, the combined effect was RR = 0.36 [95% CI (0.17, 0.76)], and the effect was statistically significant (Z = 2.68 P = 0.007 <0.05), suggesting that the risk of PONV in the dexmedetomidine group during thoracotomy surgery was 36% of that in the placebo group.
Based on the method of dexmedetomidine administration, the patients were divided into an intravenous administration group and a local administration group, as shown in Figure 3C.
In the intravenous administration group, the combined effect was RR = 0.30 [95% CI (0.21, 0.43)], and the effect was statistically significant (Z = 6.70, P < 0.00001 <0.05), suggesting that the risk of PONV in the dexmedetomidine group was 30% of that in the placebo group. In the local administration group, the combined effect was RR = 0.42 [95% CI (0.20, 0.91)], and the effect was statistically significant (Z = 2.20, P = 0.03 <0.05), suggesting that the risk of PONV in the dexmedetomidine group was 42% of that in the placebo group.
According to the type of compound anesthetics, the patients were divided into an intravenous anesthetics group and an inhalation anesthetics group, as shown in Figure 3D.
The combined effect of the intravenous anesthetics group was RR = 0.35 [95%CI (0.23, 0.54)], and the effect is statistically significant (Z = 4.89, P < 0.00001 <0.05), indicating that the risk of PONV in the dexmedetomidine group was 35% of that in the placebo group when using intravenous anesthetics. In the inhaled anesthetic group, the combined effect was RR = 0.33 [95% CI (0.17, 0.65)], and the effect was statistically significant (Z = 3.25, P = 0.001 <0.05), suggesting that the risk of PONV in the dexmedetomidine group was 33% of that in the placebo group.
The effect of dexmedetomidine on postoperative nausea is shown in Figure 4A. Compared with placebo, dexmedetomidine significantly lowered the incidence of postoperative nausea [5 trials RR = 0.29 95% CI (0.20, 0.43) P < 0.00001 I2 = 13% Figure 4A], and the publication bias of this outcome was relatively small, as shown in Supplementary Figure 1B.
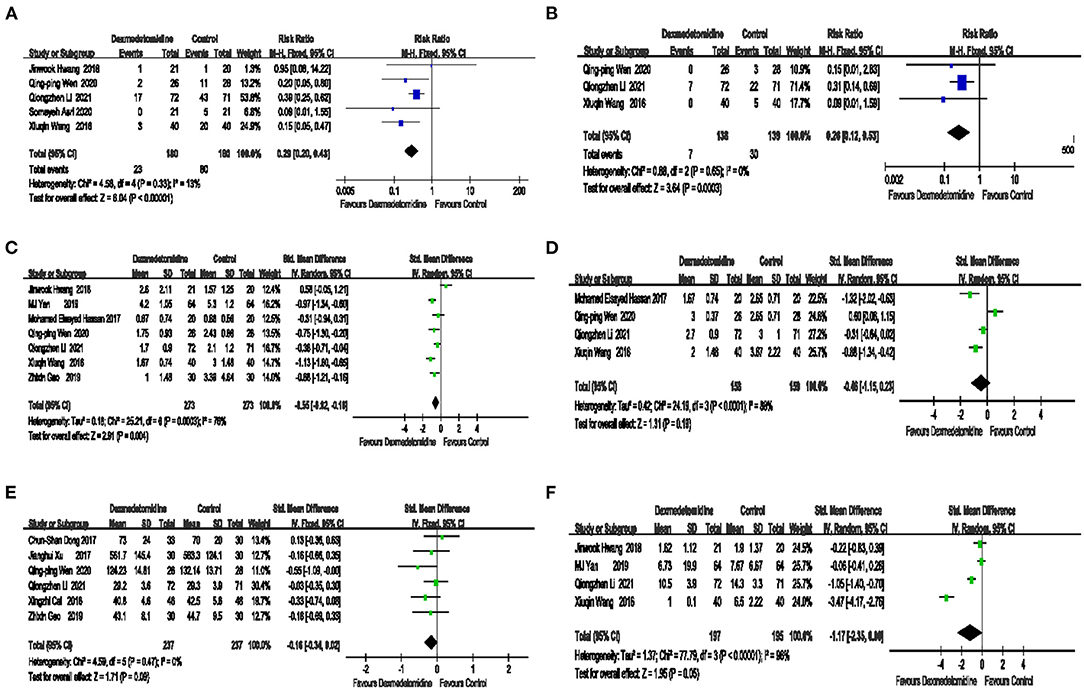
Figure 4. The effect of dexmedetomidine on postoperative nausea, vomiting, and pain control. Compared with placebo, dexmedetomidine significantly reduced the incidence of postoperative nausea (A). The risk of postoperative vomiting in the dexmedetomidine group was significantly lower than that in the control group (B). Compared with placebo, dexmedetomidine significantly reduced the postoperative visual analog score (VAS) score at rest (C). No significant difference in the postoperative VAS score for coughing was noted between dexmedetomidine and placebo (D). No significant difference in the amount of intraoperative sufentanil was noted between dexmedetomidine and placebo (E). The number of postoperative salvage angles in procedures significantly differed between dexmedetomidine and placebo (F).
The effect of dexmedetomidine on postoperative vomiting is shown in Figure 4B. The risk of postoperative vomiting, in the dexmedetomidine group was significantly lower than that in the control group [3 trials RR = 0.26, 95% CI (0.12, 0.53), P = 0.0003, I2 = 0%, Figure 4B], and the publication bias of this outcome was relatively small. These data are presented in Supplementary Figure 1C.
The effect of dexmedetomidine on the postoperative VAS score at rest is shown in Figure 4C. Compared with placebo, dexmedetomidine significantly reduced the postoperative VAS score at rest [7 trials, SMD = −0.55, 95% CI (−0.92, −0.18), P = 0.004, I2 = 76%, Figure 4C].
The effect of dexmedetomidine on the postoperative VAS score for coughing is shown in Figure 4D. No significant difference in the postoperative VAS score for coughing was noted between dexmedetomidine and placebo [4 trials, SMD = −0.46 95% CI (−1.15, 0.23), P = 0.19, I2 = 88%, Figure 4D].
The effect of dexmedetomidine on the amount of intraoperative sufentanil is shown in Figure 4E. No significant difference in the amount of intraoperative sufentanil was noted between dexmedetomidine and placebo [6 trials, SMD = −0.16, 95% CI (−0.34, 0.02), P = 0.09, I2 = 0%, Figure 4E], and the publication bias of this outcome was relatively small. These data are shown in Supplementary Figure 1D.
The effect of dexmedetomidine on postoperative salvage analgesia is shown in Figure 4F. The number of postoperative salvage analgesia procedures was administered between the dexmedetomidine and placebo groups was significantly different [6 trials, SMD = −1.17, 95% CI (−2.35, 0.00), P = 0.05, I2 = 96%, Figure 4F].
The objective of this meta-analysis, which included 12 articles (905 patients), was to compare the effect of dexmedetomidine on PONV in patients undergoing thoracic surgery. The results of this study show that compared with placebo, dexmedetomidine can reduce the incidence of PONV. The risk of PONV in the dexmedetomidine group was 32% of that in the placebo group.
We used Review Manager (RevMan 5.3; Copenhagen: Nordic Cochrane Center Collaboration, 2014) software for analysis, and the data are expressed as the mean ± SD. Regarding data expressed in the form of the median interquartile range, the method provided by McGrath was used to transform the data into the mean ± SD for analysis (24).
Previous studies have shown that compared with thoracotomy lobectomy, thoracoscopic lobectomy is associated with fewer in-hospital postoperative complications (35). The results of this meta-analysis show that regardless of thoracoscopic surgery or thoracotomy, dexmedetomidine can reduce the occurrence of PONV. Previous studies have shown that compared with inhaled anesthetics, intravenous anesthetics can reduce the occurrence of PONV (36). The results of this meta-analysis show that whether it is combined with inhaled anesthetics or combined with intravenous anesthetics, dexmedetomidine can reduce the occurrence of PONV. Previous studies have shown that intravenous and non-intravenous administration of dexmedetomidine offers the same duration of analgesia (37). The meta-analysis results of this study show that both intravenous administrations of dexmedetomidine and local administration can reduce PONV; thus, the effect of dexmedetomidine to inhibit PONV is not affected by its administration method.
The two groups exhibited relatively large heterogeneity in the VAS score at rest, VAS score at coughing, and the number of postoperative salvage analgesia procedures. We removed each study one by one for sensitivity analysis and found that the results did not change. In addition, the heterogeneity did not significantly change. Thus, our results were relatively stable. Heterogeneity may be due to differences in the quality of the studies included in the meta-analysis, the different analgesic programs used for patients in different studies, and patient differences in tolerance.
The development and treatment of PONV involve multiple receptor systems (38), so combination treatment with multiple drugs is more effective than that noted for a single drug. Our research results show that dexmedetomidine can reduce PONV, but there is no significant difference in the amount of intraoperative sufentanil. We hypothesize that dexmedetomidine does not completely reduce PONV by reducing the amount of sufentanil used during surgery. The potential mechanisms by which dexmedetomidine reduces PONV may include the following: (1) reducing intraoperative and postoperative pain scores, opioid consumption and inhalation anesthetic requirements, which subsequently reduce opioid-related adverse events, including PONV (39); (2) intraoperative DEX decreases noradrenergic activity as a result of binding to alpha-2 presynaptic inhibitory adrenoreceptors in the locus coeruleus, which may result in an antiemetic effect (40); and (3) the overall reduction in sympathetic outflow and catecholamine release caused by DEX. High sympathetic tone and catecholamine release may trigger PONV (17).
This article has the following limitations: (i) Given that the included studies did not administer a uniform dose of dexmedetomidine, the effect of different doses of dexmedetomidine on PONV in patients undergoing thoracic surgery was not explored. (ii) The effect of dexmedetomidine on PONV under a certain type of thoracic surgery could not be analyzed in detail. (iii) Data regarding whether dexmedetomidine was administered intraoperatively or postoperatively for PONV could not be obtained and analyzed.
Dexmedetomidine can reduce the occurrence of PONV in patients undergoing thoracic surgery regardless of the surgical method (minimally invasive or not), the combination of anesthetic agents (intravenous or inhalation anesthesia), and method of dexmedetomidine administration (intravenous or nerve block).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WZ: designed the study. RW and BL: searched and screened relevant literature. YZ and XL: data collection. JY: completed the first draft of the manuscript. All authors have read and approved the final manuscript.
This study was supported by grants from the Henan Province Medical Science and Technology Research Project Joint Construction Project (LHGJ20190607). The funding body played roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.863249/full#supplementary-material
Supplementary Figure 1. Publication bias. The publication bias of the effect of dexmedetomidine on PONV is relatively small. As noted in the figure, the left and right sides of the plot are basically symmetrical (A–D).
PONV, postoperative nausea and vomiting; VAS, visual analog score; RR, relative risk; SMD, standardized mean difference; RCT, Randomized controlled trials; CI, confidence interval; NK1, neurokinin 1.
1. Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. (2010) 65:815–8. doi: 10.1136/thx.2009.123083
2. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. (1999) 91:693–700. doi: 10.1097/00000542-199909000-00022
3. Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. (2002) 57:1022–7. doi: 10.1046/j.1365-2044.2002.02822.x
4. Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. (2000) 84:6–10. doi: 10.1093/oxfordjournals.bja.a013383
5. Williams KS. Postoperative nausea and vomiting. Surg Clin North Am. (2005) 85:1229–41. doi: 10.1016/j.suc.2005.09.005
6. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2020) 131:411–48. doi: 10.1213/ANE.0000000000005245
7. McCracken G, Houston P, Lefebvre G. Society of obstetricians and gynecologists of Canada. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. (2008) 30:600–7, 608–16. doi: 10.1016/S1701-2163(16)32895-X
8. Sakellaris G, Georgogianaki P, Astyrakaki E, Michalakis M, Dede O, Alegakis A, et al. Prevention of post-operative nausea and vomiting in children–a prospective randomized double-blind study. Acta Paediatr. (2008) 97:801–4. doi: 10.1111/j.1651-2227.2008.00804.x
9. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. (2000) 93:382–94. doi: 10.1097/00000542-200008000-00016
10. Hu B, Zhou H, Zou X, Shi J, Li X, Tan L, et al. Comparison of dexmedetomidine and midazolam for the prevention of postoperative nausea and vomiting caused by hemabate in cesarean delivery: a randomized controlled trial. Drug Des Devel Ther. (2020) 14:2127–33. doi: 10.2147/DDDT.S251525
11. Jun JH, Kim KN, Kim JY, Song SM. The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis. Can J Anaesth. (2017) 64:947–61. doi: 10.1007/s12630-017-0917-x
12. Hong B, Lim C, Kang H, Eom H, Kim Y, Cho HJ, et al. Thoracic paravertebral block with adjuvant dexmedetomidine in video-assisted thoracoscopic surgery: a randomized, double-blind study. J Clin Med. (2019) 8:352. doi: 10.3390/jcm8030352
13. Kim SH, Oh YJ, Park BW, Sim J, Choi YS. Effects of single-dose dexmedetomidine on the quality of recovery after modified radical mastectomy: a randomised controlled trial. Minerva Anestesiol. (2013) 79:1248–58. doi: 10.4037/ajcc2013610
14. Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM, et al. Balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. (2009) 30:1537–41. doi: 10.1016/j.revmed.2009.09.015
15. Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. (2012) 116:1312–22. doi: 10.1097/ALN.0b013e31825681cb
16. Schnabel A, Meyer-Frießem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trial. Pain. (2013) 154:1140–9. doi: 10.1016/j.pain.2013.03.029
17. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. (1992) 77:162–84. doi: 10.1097/00000542-199207000-00023
18. Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. (2000) 59:213–43. doi: 10.2165/00003495-200059020-00005
19. Wang X, Wang K, Wang B, Jiang T, Xu Z, Wang F, et al. Effect of oxycodone combined with dexmedetomidine for intravenous patient-controlled analgesia after video-assisted thoracoscopic lobectomy. J Cardiothorac Vasc Anesth. (2016) 30:1015–21. doi: 10.1053/j.jvca.2016.03.127
20. Cai X, Zhang P, Lu S, Zhang Z, Yu A, Liu D, et al. Effects of Intraoperative dexmedetomidine on postoperative pain in highly nicotine-dependent patients after thoracic surgery: a prospective, randomized, controlled trial. Medicine. (2016) 95:e3814. doi: 10.1097/MD.0000000000003814
21. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
22. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
23. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D. Cochrane bias methods group. Cochrane statistical methods group the cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 30:962280219889080. doi: 10.1177/0962280219889080
25. Hwang J, Shin JS, Son JH, Min TJ. Non-intubated thoracoscopic bullectomy under sedation is safe and comfortable in the perioperative period. J Thorac Dis. (2018) 10:1703–10. doi: 10.21037/jtd.2018.02.10
26. Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. (2019) 7:668. doi: 10.21037/atm.2019.10.74
27. Li Q, Yao H, Xu M, Wu J. Dexmedetomidine combined with sufentanil and dezocine-based patient-controlled intravenous analgesia increases female patients' global satisfaction degree after thoracoscopic surgery. J Cardiothorac Surg. (2021) 16:102. doi: 10.1186/s13019-021-01472-4
28. Yan MJ, Wang T, Wu XM, Zhang W. Comparison of dexmedetomidine or sufentanil combined with ropivacaine for epidural analgesia after thoracotomy: a randomized controlled study. J Pain Res. (2019) 12:2673–78. doi: 10.2147/JPR.S208014
29. Dong CS, Zhang J, Lu Q, Sun P, Yu JM, Wu C, et al. Effect of dexmedetomidine combined with sufentanil for post- thoracotomy intravenous analgesia:a randomized, controlled clinical study. BMC Anesthesiol. (2017) 17:33. doi: 10.1186/s12871-017-0324-4
30. Miao Z, Wu P, Wang J, Zhou FC, Lin Y, Lu XY, et al. Whole-course application of dexmedetomidine combined with ketorolac in nonnarcotic postoperative analgesia for patients with lung cancer undergoing thoracoscopic surgery: a randomized control trial. Pain Physician. (2020) 23:E185–93.
31. Xu J, Yang X, Hu X, Chen X, Zhang J, Wang Y. Multilevel thoracic paravertebral block using ropivacaine with/without dexmedetomidine in video-assisted thoracoscopic surgery. J Cardiothorac Vasc Anesth. (2018) 32:318–24. doi: 10.1053/j.jvca.2017.06.023
32. Lee SH, Lee CY, Lee JG, Kim N, Lee HM, Oh YJ. Intraoperative dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine. (2016) 95:e2854. doi: 10.1097/MD.0000000000002854
33. Hassan ME, Mahran E. Evaluation of the role of dexmedetomidine in improvement of the analgesic profile of thoracic paravertebral block in thoracic surgeries: a randomised prospective clinical trial. Indian J Anaesth. (2017) 61:826–31. doi: 10.4103/ija.IJA_221_17
34. Asri S, Hosseinzadeh H, Eydi M, Marahem M, Dehghani A, Soleimanpour H. Effect of dexmedetomidine combined with inhalation of isoflurane on oxygenation following one-lung ventilation in thoracic surgery. Anesth Pain Med. (2020) 10:e95287. doi: 10.5812/aapm.95287
35. Paul S, Sedrakyan A, Chiu YL, Nasar A, Port JL, Lee PC, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. (2013) 43:813–7. doi: 10.1093/ejcts/ezs428
36. Schraag S, Pradelli L, Alsaleh AJO, Bellone M, Ghetti G, Chung TL, et al. Propofol vs inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol. (2018) 18:162. doi: 10.1186/s12871-018-0632-3
37. Somsunder RG, Archana NB, Shivkumar G, Krishna K. Comparing efficacy of perineural dexmedetomidine with intravenous dexmedetomidine as adjuvant to levobupivacaine in supraclavicular brachial plexus block. Anesth Essays Res. (2019) 13:441–45. doi: 10.4103/aer.AER_105_19
38. Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. (2011) 107(Suppl. 1):i27–40. doi: 10.1093/bja/aer358
39. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. (2006) 53:646–52. doi: 10.1007/BF03021622
Keywords: dexmedetomidine, postoperative nausea and vomiting, thoracic surgery, meta-analysis, visual analog score
Citation: Zhang W, Wang R, Li B, Zhao Y, Liu X and Yuan J (2022) The Effect of Dexmedetomidine on Postoperative Nausea and Vomiting in Patients Undergoing Thoracic Surgery-A Meta-Analysis of a Randomized Controlled Trial. Front. Surg. 9:863249. doi: 10.3389/fsurg.2022.863249
Received: 27 January 2022; Accepted: 17 February 2022;
Published: 31 March 2022.
Edited by:
Monica Casiraghi, European Institute of Oncology (IEO), ItalyReviewed by:
Pasquale Sansone, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Zhang, Wang, Li, Zhao, Liu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingli Yuan, Mjc1NjM2NTk4OEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.