- 1Lung Cancer Center, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Thoracic Surgery, West China Hospital of Sichuan University, Chengdu, China
- 3Department of Clinical Lab, Chongqing University Cancer Hospital and Chongqing Cancer Hospital, Chongqing, China
Background: Whether wedge resection or stereotactic body radiation therapy (SBRT) has better effectiveness in treatment of clinical stage I non-small cell lung cancer (NSCLC) patients remains unclear. Here we conducted the first meta-analysis to directly compare the survival outcomes of clinical stage I NSCLCs treated with wedge resection and SBRT.
Methods: We systematically searched studies from PubMed, Embase, and Corchrane Library up to October 1, 2021. Data for analysis mainly included overall survival (OS) and disease-free survival (DFS), which were obtained directly from the text results or calculated from the Kaplan–Meier survival curve. We used the standard random-effect model test (DerSimonian and Laird method) to analyze the pooled hazard ratios (HRs) and 95% confidence intervals (CIs). The Q-test and I2-test were used to assess heterogeneity. The stability of pooled HRs was examined by sensitivity analysis.
Results: Six retrospective studies with a total of 11,813 clinical stage I NSCLCs who received wedge resection or SBRT were included. The results showed that patients receiving wedge resection had a significantly better OS (HR = 1.20, 95% CI = [1.07, 1.34], P = 0.002) than those with SBRT, but no significant difference of DFS (HR 1.53, 95% CI = [0.83–2.83], P = 0.17) was observed. There was no significant heterogeneity during our analysis, but there may be potential publication bias among these studies.
Conclusions: Our meta-analysis showed that clinical stage I NSCLCs treated with wedge resection had superior OS than those treated with SBRT. However, more prospective clinical trials should be well-designed to evaluate the optimal treatment modality of early-stage NSCLCs.
Introduction
Lung cancer causes the most cancer-related deaths worldwide (1). The two major types of lung cancer are non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC), of which the former accounts for about 80% (2). Nowadays, with the routine use of computed tomography (CT), more and more early-stage NSCLCs are being detected (3). Surgery plays an important role in the treatment of early-stage NSCLC and it mainly consists of lobectomy, sleeve lobectomy, sublobar resection (segmentectomy and wedge resection) and so on. Among them, lobectomy with systematic lymph node dissection is still the golden standard of medically operable patients with clinical stage I NSCLC (4). However, when patients are unsuitable for lobectomy or with low grade malignancy peripheral-type NSCLC <2 cm, sublobar resection is recommended (5). Moreover, previous study (6) found that among elderly aged (≥75 years) stage I NSCLC patients, those treated with sublobar resection had similar OS compared with lobectomy, but yielded less postoperative complications, which suggested sublobar resection but not lobectomy may be the optimal treatment for elderly stage I NSCLC patients.
On the other hand, with the progress of medical treatment, stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), is the standard treatment for medically inoperable early-stage NSCLC patients (7). Furthermore, several reports showed SBRT had a good local control in the medically inoperable patients, ranging from 80 to 99% (8–11). However, it's still controversial whether there is still a role of SBRT for operable early-stage NSCLC patients. Although a large number of retrospective observational studies directly compared surgery and SBRT for operable early-stage NSCLCs, they have reported conflicting results. It still has limited information to guide clinicians for decision-making because there have not been a completed prospective trial and several (POSITIVL, VALOR and STABLE-MATES) (12–14) randomized controlled trial (RCTs) are still ongoing.
In our previous meta-analysis (15), we made a comparison between SBRT and surgery in treating stage I NSCLC. In the subgroup analysis, we found that SBRT and sublobar resection yielded similar 3-year survival rate, OS and 3-year loco-regional control (LRC) rate, while lobectomy yielded significantly longer OS than SBRT. However, our sublobar resection subgroup included both wedge resection and segmentectomy, whether wedge resection or segmentectomy resulted in the similar efficacy to SBRT in patients with stage I NSCLC remains unclear. Besides, segmentectomy and wedge resection are different surgical procedures, and previous study showed segmentectomy was associated with better OS and cancer–specific survival (CSS) compared with wedge resection in treating NSCLC <2 cm (16). In our opinion, both wedge resection and SBRT are local therapies, provide better lung function preservation and can be well-tolerated by most of the patients (17). The comparison between wedge resection and SBRT may be more appropriate than other types of surgical resections. Hence, with a growing number of previous similar studies (18–23), we performed the first meta-analysis to investigate whether wedge resection or SBRT had a better effect in clinical stage I NSCLCs.
Methods
Our meta-analysis was conducted in accordance to the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24). All the data were collected from published papers and ethical approval was waived from our hospital. The present meta-analysis was registered in the PROSPERO database.
Search Strategy
We used the following searching strategy: (“lung carcinoma” OR “lung cancer” OR “lung neoplasms”) AND (“wedge ” OR “wedge resection” OR “sublobar resection”) AND (“stereotactic body radiotherapy” OR “SBRT” OR “Stereotactic body radiation therapy” OR “SABR” OR “Stereotactic ablative radiotherapy”) to search the electronic database of PubMed, Embase, and Cochrane Library from inception until October 1, 2021. Only papers published in English were evaluated.
Inclusion Criteria and Exclusion Criteria
Before we searched eligible literatures to conduct this meta-analysis, the following inclusion criteria were set, (1) RCT or observational study, (2) comparison between stage I NSCLC patients treated with wedge resection and SBRT, (3) study provided sufficient survival data [OS or disease-free survival (DFS)] for analysis. We also made the following criteria for study exclusion: (1) study written in non-English language; (2) review, case report, conference abstract, and editorial material and letters; (3) study did not report OS or DFS.
Data Extraction and Quality Assessment
Two authors (Peng and Huang) screened the titles and abstracts to find potentially available ones according to the inclusion and exclusion criteria. And another author (Deng) would solve the disagreement if there was. After browsing and evaluating the full text that met the inclusion criteria, the two authors independently extracted the following information: (1) language, author, publication date and study type; (2) patients' quantity of each group, median age, median follow-up time and radiation therapy strategy. If primary reports didn't directly record the data, we calculated HRs with 95% CIs with spreadsheet according to the survival curves with the published methodology by analyzing Kaplan–Meier curves of the included studies with Engauge Digitizer version 2.11. Subsequently, for observational studies, we used Newcastle–Ottawa Scale (NOS) (25) to evaluate the risk of bias, which contained three factors: (1) selection of study group, (2) comparability of groups, and (3) assessment of outcomes. Papers with a quality score >6 were considered high-quality. For RCTs, we used Jadad Scale (26) to assess the methodological quality, and if the score >4, the paper was defined as high-quality paper.
Statistical Analysis
We performed a meta-analysis of wedge resection vs. SBRT and two subgroup meta-analyses (one meta-analysis of wedge resection vs. SBRT with propensity-matched (PSM) cohort and another without PSM cohort). We used the software Review Manager (RevMan) version 5.4 (the Cochrane Collaboration, Oxford, England) and STATA (StataCorp;College Station, TX, USA) 12.0 package to perform all statistical analysis based on the PRISMA guidelines (24). Quantitative synthesis of the 5-year OS and 5-year DFS was conducted by using HRs with 95% CIs, and it was considered high heterogeneity if an I2 ≥ 50% or P ≤ 0.10 was detected. When we encountered high heterogeneity, the random-effect model test (DerSimonian and Laird method) was performed for statistical analysis. Otherwise, the standard fixed-effect model test (Mantel-Haenszel method) was available. The sensitivity analysis was performed to estimate the stability of quantitative synthesis results, and the reliability of the meta-analysis results were robustly confirmed if the results of the meta-analysis did not change significantly. To evaluate publication bias, a funnel plot with Begger's and Egger's tests was used.
Results
Study Selection
A total of 456 papers were identified from the three main sources including Pubmed, Embase and Cochrane Library. After we deleted 141 duplicates. By browsing titles and abstracts, we excluded 42 reviews, 19 conference abstracts, and 241 obviously irrelevant papers. In the remaining 13 papers, we further excluded another seven papers by full-text assessment and finally, six studies (18–23) were included in the final meta-analysis (Figure 1).
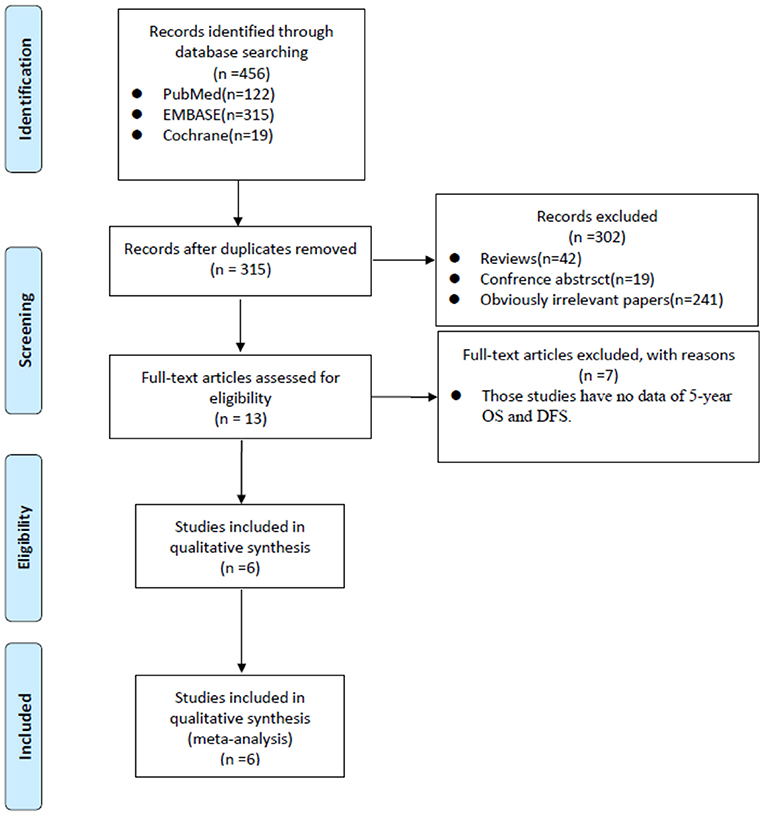
Figure 1. PRISMA flow diagram showed the process of relevant studies evaluation. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; OS, overall survival; DFS, disease-free survival.
Characteristics of Eligible Studies
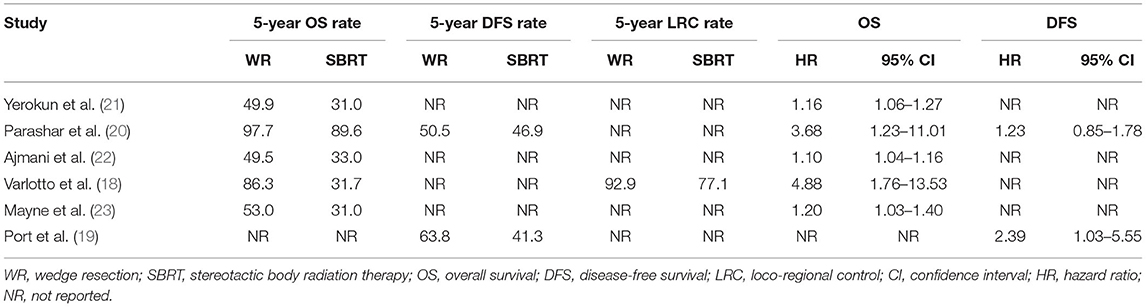
In summary, all of the six retrospective observational studies contained 11,813 clinical stage I NSCLC patients, and the publication year ranged from 2013 to 2020. One study did not report follow-up time, and the others' median follow-up time ranged from 17.5 to 66 months. Three studies did not report radiation dose and cycles, and the others' radiation dose ranged from 30–60, 3–5 cycles. The NOS scores ranged from 7 to 9, which meant our meta-analysis had a low risk of bias. Detailed characteristics of the included studies were shown in Table 1.
Meta-Analysis of Overall Survival Analysis
Four studies directly reported 5-year OS rates of wedge resection vs. SBRT. One study showed the OS rate in a Kaplan-Meier survival curve. And therefore, Engauge Digitizer version 2.11 and HR calculation spreadsheet were used to calculate HRs and 95% CIs of OS. Five studies were included to calculate the HR and 95% CI of OS (Table 2). The heterogeneity test showed that there was high heterogeneity among these studies (I2 = 72%, P = 0.007). Therefore, we use the random-effects model to perform this meta-analysis, and the results showed that stage I NSCLC patients treated with wedge resection had a better OS compared to SBRT (HR = 1.19, 95% CI = [1.05, 1.36], P = 0.008) (Figure 2). In our subgroup analysis, four studies with propensity score-matched analysis including 11,494 patients were analyzed. We found that patients treated with wedge resection still yielded a significantly higher OS rate than SBRT (random-effects model: (HR = 1.16, 95% CI = [1.04, 1.30], P = 0.007, I2 = 69%) (Figure 2). Because only one study reported 5-year LCR rate, we did not perform further meta-analysis based on 5-year LRC rate.
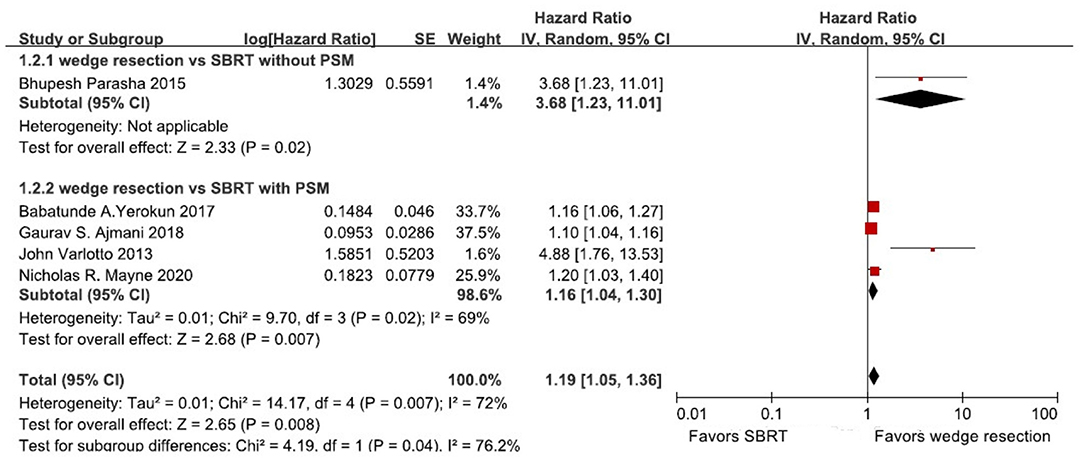
Figure 2. Forest plot of 5-year OS in patients treated with wedge resection compared with SBRT; OS, overall survival; CI, confidence interval; PSM: propensity score-matched.
Meta-Analysis of Disease-Free Survival Analysis
One study provided the 5-year DFS rate of wedge resection vs. SBRT, and one study reported 3-year DFS rate, as it showed the DFS rate in a Kaplan-Meier survival curve. Therefore, Engauge Digitizer version 2.11 and HR calculation spreadsheet were used to calculate HRs and 95% CIs of DFS (Table 2). Because the heterogeneity was high (I2 = 50%, P = 0.16), and a random-effects model was used for analysis. The results indicated that there was no significant differences of DFS between patients treated with wedge resection and SBRT (HR 1.53, 95% CI = [0.83–2.83], P = 0.17) (Figure 3).
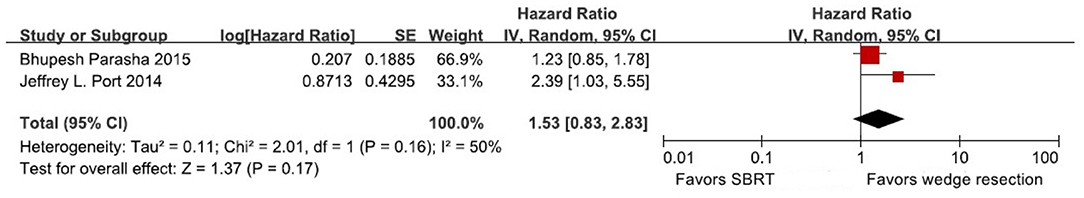
Figure 3. Forest plot of 5-year DFS in patients treated with wedge resection compared with SBRT. CI, confidence interval; DFS, disease-free survival.
Sensitivity Analysis and Publication Bias
We conducted the sensitivity analysis to assess the stability of our meta-analysis results. We sequentially removed each study, and the results showed the overall results of OS did not change, which meant our meta-analysis results had high stability (Supplementary Figure 1). However, when we performed a funnel plot to evaluate publication bias, it showed there was potential publication bias among the studies, as the appearance of funnel plot was asymmetry, and Begger's and Egger's tests of OS (P = 0.003, Supplementary Figure 2) was statistical significance.
Discussion
The incidence of NSCLC is increasing and has become a global health problem (1). With the application of multidisciplinary treatment model in treating lung cancer, the 38survival rate of NSCLC patients has improved (27). Traditionally, surgery is the first choice of operable early-stage NSCLC, and lobectomy with systematic lymph node dissection has become the acceptable treatment of stage I NSCLC since 1962 (28). While about 20 to 25% of early-stage NSCLC patients are not the right candidates for lobar resection because of the poor physical conditions (27), some other patients also refuse surgery. For this part of patients, SBRT has been developed as an alternative treatment during the past few years (7, 29). However, whether surgery or SBRT plays an important role on the treating for early-stage NSCLC patients, still has no consensus since numerous non-randomized data have reported different results (30–33). The only two independent RCTs (STARS and ROSEL) comparing surgery and SBRT in operable stage I NSCLC patients were closed due to low accrual, and the two trials' pooled analysis results were limited because of the small sample size and short follow-up time (34), unfortunately. Therefore, based on the lacked evidence, we performed this meta-analysis to directly compare the effect of wedge resection and SBRT in treating early-stage NSCLC for the first time.
In our meta-analysis, six retrospective studies, with a total of 11,813 clinical stage I NSCLC patients treated with wedge resection and SBRT, were included. We chose the 5-year DFS and OS as the survival outcomes and found that wedge resection yielded a significantly better OS (HR = 1.20, 95% CI = [1.07, 1.34], P = 0.002) than SBRT, but there was no significant difference of DFS (HR 1.53, 95% CI = [0.83–2.83], P = 0.17) between wedge resection and SBRT. Our meta-analysis showed similar results with previous researches (18, 21–23). Moreover, considering potential selection bias, we also conducted subgroup analysis based on PSM, we found that survival advantages of wedge resection over SBRT still held true (HR = 1.16, 95% CI = [1.04, 1.30], P = 0.007). Therefore, our study proved that wedge resection had better OS than SBRT in clinical stage I NSCLC patients. In our view, three major factors contributed to the results. First, of note, surgery group innately had better physical condition than SBRT group in these retrospective studies (21). Although we strove to minimize selection bias by conducting subgroup analysis as previously described, it could not be denied that patients who underwent wedge resection generally had less underlying comorbidities than patients undergoing SBRT. For example, patients in the SBRT group were older (Table 1), and they always had more previous surgical histories, serious cerebral diseases, cardiovascular diseases, and diabetic complications (20). Second, biopsy confirmation was strongly recommended before the treatment of SBRT (35), but many patients did not receive biopsy confirmation because of the risk of developing complications. What's more, Ajmani et al. (22) found high-quality wedge resection (negative margins with resected lymph nodes >5) showed improved outcomes compared to lower-quality resection (positive margins or negative margins with resected lymph nodes <5). As a result, wedge resection could remove more lymph nodes, which might improve the prognosis of these patients. Therefore, high-quality wedge resection provided a more accurate pathologic diagnosis, which could guide clinicians to make proper decisions for systemic therapy (36). Third, SBRT evaluated negative preoperative staging through imaging and was lack of pathologic lymph node assessment, but wedge resection with lymph nodal sampling might remove potential metastatic lymph node that could not be recognized by pretreatment imaging, and these upstaging cases received subsequent adjuvant treatment, who might yield better LRC (18) (Table 2) and survival (37).
However, it should be noted there is still a role for SBRT in treating some specific cohorts of early-stage NSCLCs (38). By multiple planar and non-planar beams precisely targeting the tumor, SBRT has good protection of the adjacent normal tissue (7). After decades of development, SBRT technology has been proved as a standard care for early-stage NSCLC patients who are medically inoperable or unwilling to receive surgery. Clinical trial conducted by Timmerman et al. (10) showed inoperable NSCLC patients receiving SBRT had 3-year DFS and OS rates of 48.3 and 55.8%, respectively, and the local tumor control rate were 87.2% with moderate treatment-related morbidity. The results of this trial showed that SBRT was an excellent local therapy in medically inoperable early-stage NSCLCs. Meanwhile, in our meta-analysis, we noticed stage I NSCLC patients treated with SBRT were old people, who always had many comorbidities and poor physical condition, so that patients' follow-up could not be too long, which might explain the reason why the DFS (HR 1.53, 95% CI = [0.83–2.83], P = 0.17) between wedge resection and SBRT was no significantly different. Currently, many data indicated that small-size peripheral early-stage NSCLCs with low malignant characteristics had a low risk of lymph node metastasis (39), so SBRT might provide these patients a good local tumor control (10, 35). Moreover, Peterson et al. (40) found for tumors >5 cm, the use of SBRT resulted in excellent outcome and acceptable toxicity. As for centrally located lesions, although they had higher risk of treatment-related toxicity compared to peripheral lesions, similar dilemma still existed that centrally located tumors also could not tolerate lobectomy or sublobar resection, and therefore, SBRT is still an important choice in treating these patients (41). As a result, in our experience, SBRT is recommended to patients who decline surgery, or these who cannot tolerate surgery because of poor cardio-pulmonary function. However, the detailed criteria (age, comorbidities, physical conditions, and others) for the application of SBRT that can be useful in multidisciplinary decisions is urgently needed in clinical practice (42).
Limitations of the Study
There are some limitations of our study. First, all the collected results were based on retrospective studies, which had low-quality evidence. Second, only two studies including 319 clinical stage I NSCLC patients provided DFS data, and the sample size was relatively small, so that we did not perform sensitivity analysis and publication bias based on DFS. Moreover, some studies didn't directly record the sufficient data, and therefore, we analyze Kaplan–Meier curves with Engauge Digitizer version 2.11, and calculated HRs with 95% CIs with spreadsheet as previously described. Third, due to the limited number of the included studies (only six sudies), the potential publication bias was observed and therefore, our meta-analysis results should be interpreted cautiously. Fourth, because 5-year OS rate of included studies ranged from 49.9 to 97.7%, which meant our meta-analysis might have high heterogeneity, although the sensitivity analysis of our meta-analysis results exhibited high stability. Finally, tumor location, tumor size and pathological subtype that might influenced long-term survival, could not be extracted from these studies to perform further subgroup analysis. Therefore, further RCTs are badly needed to confirm our conclusion.
Conclusion
We performed the first meta-analysis to directly compare the long-term survival after wedge resection and SBRT in the treatment of clinical stage I NSCLCs. Our study notably indicated that wedge resection had better OS than SBRT in treating clinical stage I NSCLC patients, while there was no significant difference of DFS between wedge resection and SBRT. However, because there were no completed prospective RCTs, our conclusion may be interpreted with cautions before further well-designed RCTs become available.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LP, H-YD, Z-KL, and Q-WS collected data and drafted the manuscript. Z-KL, K-LH, Q-QZ, WL, and YW designed the study and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.850276/full#supplementary-material
Supplementary Figure 1. Sensitivity analysis for OS. CI, confidence interval; OS, overall survival.
Supplementary Figure 2. Funnel plots showed the publication bias of the analysis of OS (Egger's test, P = 0.003). HR, hazad ratio; OS, overall survival.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Gadgeel SM, Ramalingam SS, Kalemkerian GP. Treatment of lung cancer. Radiol Clin North Am. (2012) 50:961–74. doi: 10.1016/j.rcl.2012.06.003
3. Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. (2013) 368:1980–91. doi: 10.1056/NEJMoa1209120
4. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2013) 143(5 Suppl):e278S−313S. doi: 10.1378/chest.12-2359
5. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15:504–35. doi: 10.6004/jnccn.2017.0050
6. Tsutani Y, Tsubokawa N, Ito M, Misumi K, Hanaki H, Miyata Y, et al. Postoperative complications and prognosis after lobar resection vs. sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. (2018) 53:366–71. doi: 10.1093/ejcts/ezx296
7. Qiao X, Tullgren O, Lax I, Sirzén F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. (2003) 41:1–11. doi: 10.1016/S0169-5002(03)00152-1
8. Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: 4-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. (2009) 75:677–82. doi: 10.1016/j.ijrobp.2008.11.042
9. Zimmermann FB, Geinitz H, Schill S, Grosu A, Schratzenstaller U, Molls M, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. (2005) 48:107–14. doi: 10.1016/j.lungcan.2004.10.015
10. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. (2010) 303:1070–6. doi: 10.1001/jama.2010.261
11. Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. (2010) 68:72–7. doi: 10.1016/j.lungcan.2009.05.007
12. National Library of Medicine (U.S.). Radical resection vs. ablative stereotactic radiotherapy in patients with operable stage I NSCLC (POS-TILV). Identifier: NCT01753414 (2019). Available online at: https://clinicaltrials.gov/ct2/show/NCT01753414. (accessed May 20, 2020).
13. National Library of Medicine (U.S.). (2016-present). Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR). Identifier: NCT02984761. Available online at: https://clinicaltrials.gov/ct2/show/NCT02984761. (accessed May 20, 2020).
14. National Library of Medicine (U.S.). JoLT-Ca Sublobar Resection (SR) vs. Stereotactic Ablative Radiotherapy (SAbR) for Lung Cancer (STABLE-MATES). Identifier: NCT02468024 (2020). Available online at: https://clinical-trials.gov/ct2/show/NCT02468024. (accessed May 20, 2020).
15. Deng HY, Wang YC Ni PZ, Li G, Yang XY, Lin YD, Liu LX. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. (2017) 51:203–10. doi: 10.1093/ejcts/ezw272
16. Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of surgical procedure for patients with non-small-cell lung cancer ≤ 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol. (2016) 34:3175–82. doi: 10.1200/JCO.2015.64.6729
17. Zhang Z, Feng H, Zhao H, Hu J, Liu L, Liu Y, et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis. (2019) 11:1838–48. doi: 10.21037/jtd.2019.05.20
18. Varlotto J, Fakiris A, Flickinger J, Medford-Davis L, Liss A, Shelkey J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer. (2013) 119:2683–91. doi: 10.1002/cncr.28100
19. Port JL, Parashar B, Osakwe N, Nasar A, Lee PC, Paul S, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg. (2014) 98:1152–9. doi: 10.1016/j.athoracsur.2014.04.128
20. Parashar B, Port J, Arora S, Christos P, Trichter S, Nori D, et al. Analysis of stereotactic radiation vs. wedge resection vs. wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy. (2015) 14:648–54. doi: 10.1016/j.brachy.2015.04.001
21. Yerokun BA, Yang CJ, Gulack BC, Li X, Mulvihill MS, Gu L, et al. A national analysis of wedge resection vs. stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. (2017) 154:675–86.e674. doi: 10.1016/j.jtcvs.2017.02.065
22. Ajmani GS, Wang CH, Kim KW, Howington JA, Krantz SB. Surgical quality of wedge resection affects overall survival in patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. (2018) 156:380–91. doi: 10.1016/j.jtcvs.2018.02.095
23. Mayne NR, Lin BK, Darling AJ, Raman V, Patel DC, Liou DZ, et al. Stereotactic body radiotherapy vs. delayed surgery for early-stage non-small-cell lung cancer. Ann Surg. (2020) 272:925–9. doi: 10.1097/SLA.0000000000004363
24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
25. Wells GA, Shea B, O'connell D et al. The Newcastle-Ottawa Scale (NOS) for Assessing the quality of non-randomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed15, January 2016).
26. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
27. Lagerwaard FJ, Verstegen NE, Haasbeek CJ, Slotman BJ, Paul MA, Smit EF, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. (2012) 83:348–53. doi: 10.1016/j.ijrobp.2011.06.2003
28. Shimkin MB, Connelly RR, Marcus SC, Cutler SJ. Pneumonectomy and lobectomy in bronchogenic carcinoma. A comparison of end results of the Overholt and Ochsner clinics. J Thorac Cardiovasc Surg. (1962) 44:503–19. doi: 10.1016/S0022-5223(19)32943-5
29. Zimmermann FB, Bamberg M, Molls M, Jeremic B. Radiation therapy alone in early stage non-small cell lung cancer. Semin Surg Oncol. (2003) 21:91–7. doi: 10.1002/ssu.10026
30. Tamura M, Matsumoto I, Tanaka Y, Saito D, Yoshida S, Kakegawa S, et al. Comparison between stereotactic radiotherapy and sublobar resection for non-small cell lung cancer. Ann Thorac Surg. (2019) 107:1544–50. doi: 10.1016/j.athoracsur.2018.10.015
31. Paul S, Lee PC, Mao J, Isaacs AJ, Sedrakyan A. Long term survival with stereotactic ablative radiotherapy (SABR) vs. thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ. (2016) 354:i3570. doi: 10.1136/bmj.i3570
32. Ezer N, Veluswamy RR, Mhango G, Rosenzweig KE, Powell CA, Wisnivesky JP. Outcomes after stereotactic body radiotherapy vs. limited resection in older patients with early-stage lung cancer. J Thorac Oncol. (2015) 10:1201–6. doi: 10.1097/JTO.0000000000000600
33. Wu J, Bai HX, Chan L, Su C, Zhang PJ, Yang L, et al. Sublobar resection compared with stereotactic body radiation therapy and ablation for early stage non-small cell lung cancer: a national cancer database study. J Thorac Cardiovasc Surg. (2020) 160:1350–7. doi: 10.1016/j.jtcvs.2019.11.132
34. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy vs. lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. (2015) 16:630–7. doi: 10.1016/S1470-2045(15)70168-3
35. Wood DE, Kazerooni E, Baum SL, Dransfield MT, Eapen GA, Ettinger DS, et al. Lung cancer screening, version 1.2015: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. (2015) 13:23–34. doi: 10.6004/jnccn.2015.0006
36. Verma V, Simone CB II, Allen PK, Lin SH. Outcomes of stereotactic body radiotherapy for T1-T2N0 small cell carcinoma according to addition of chemotherapy and prophylactic cranial irradiation: a multicenter analysis. Clin Lung Cancer. (2017) 18:675–81. doi: 10.1016/j.cllc.2017.03.009
37. Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, et al. The IASLC lung cancer staging project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. (2007) 2:593–602. doi: 10.1097/JTO.0b013e31807a2f81
38. Deng HY, Tang X. Is There still a role for stereotactic body radiation therapy in early-stage lung cancer? Ann Thorac Surg. (2021) 111:1092–3. doi: 10.1016/j.athoracsur.2020.05.147
39. Deng HY, Zhou J, Wang RL, Jiang R, Qiu XM, Zhu DX, et al. surgical choice for clinical stage IA non-small cell lung cancer: view from regional lymph node metastasis. Ann Thorac Surg. (2020) 109:1079–85. doi: 10.1016/j.athoracsur.2019.10.056
40. Peterson J, Niles C, Patel A, Boujaoude Z, Abouzgheib W, Goldsmith B, et al. Stereotactic body radiotherapy for large (>5 cm) non-small-cell lung cancer. Clin Lung Cancer. (2017) 18:396–400. doi: 10.1016/j.cllc.2016.11.020
41. Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol. (2011) 6:2036–43. doi: 10.1097/JTO.0b013e31822e71d8
Keywords: non-small cell lung cancer, clinical stage I, wedge resection, SBRT, prognosis
Citation: Peng L, Deng H-Y, Liu Z-K, Shang Q-W, Huang K-L, Zheng Q-Q, Li W and Wang Y (2022) Wedge Resection vs. Stereotactic Body Radiation Therapy for Clinical Stage I Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 9:850276. doi: 10.3389/fsurg.2022.850276
Received: 07 January 2022; Accepted: 21 February 2022;
Published: 17 March 2022.
Edited by:
Alessandro Brunelli, Leeds Teaching Hospitals NHS Trust, United KingdomReviewed by:
Marcelo Jimenez, University of Salamanca, SpainMarco Schiavon, University Hospital of Padua, Italy
Copyright © 2022 Peng, Deng, Liu, Shang, Huang, Zheng, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Yu Deng, aGFueXVkZW5nQHNjdS5lZHUuY24=; Yun Wang, eXVud3dhbmdAeWVhaC5uZXQ=
†These authors have contributed equally to this work and share first authorship
 Lei Peng
Lei Peng Han-Yu Deng
Han-Yu Deng Zhen-Kun Liu1
Zhen-Kun Liu1 Kai-Li Huang
Kai-Li Huang Qiang-Qiang Zheng
Qiang-Qiang Zheng Wen Li
Wen Li Yun Wang
Yun Wang