- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Clinical Nutrition, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: The current study aims to explore the outcomes of type 2 diabetes mellitus (T2DM) on gastric cancer patients following gastrectomy through propensity score matching (PSM) analysis.
Methods: A retrospective study of gastric cancer patients following gastrectomy was conducted in a single clinical center from January 2014 to December 2019. The short-term outcomes, overall survival (OS) and disease-free survival (DFS) were analyzed between T2DM group and Non-T2DM group.
Results: A total of 703 patients were enrolled in this study. After 1:1 PSM, 84 patients in T2DM group and 84 patients in Non-T2DM were matched for final analysis. No significant difference was found in terms of operation time, intra-operative blood loss, retrieved lymph nodes, postoperative stay, blood transfusion and complications between T2DM group and Non-T2DM group (p > 0.05). The Kaplan-Meier curve implied that T2DM had no impact on OS or DFS. Cox regression was conducted to identify predictive factors for prognosis. Body mass index (BMI) (p = 0.039 < 0.05, HR = 0.725, 95% CI = 0.534–0.983), pre-operative lymphocyte (p = 0.017 < 0.05, HR = 0.678, 95% CI = 0.493–0.932), pathological tumor node metastasis (pTNM) stage (p = 0.000 < 0.05, HR = 2.619, 95% CI = 2.048–3.349) and complications (p = 0.006 < 0.05, HR = 1.528, 95% CI = 1.132–2.061) were predictive factors for OS, and BMI (p = 0.013 < 0.05, HR = 0.524, 95% CI = 0.315–0.872), pTNM stage (p = 0.000 < 0.05, HR = 2.619, 95% CI = 2.048–3.349) and complications (p = 0.008 < 0.05, HR = 1.892, 95% CI = 1.179–3.036) were independent predictive factors for DFS.
Conclusion: T2DM did not have an impact on gastric cancer patients following gastrectomy in terms of short-term outcomes and prognosis.
Introduction
Gastric cancer is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths (1, 2). More than one million gastric cancer cases were newly diagnosed worldwide each year, of which 44% were diagnosed in China (1, 3). Although the expanding application of multidisciplinary team boomed in gastric cancer, surgery is the only curing method for resectable cases (4, 5).
Type 2 diabetes mellitus (T2DM) is a metabolic disease with a high incidence worldwide, and it is also one of the leading causes of death in the world (6). As was reported, 493 million people were affected by T2DM in 2019, and 700 million people would develop T2DM in 2045 (7, 8).
It has been reported that T2DM could increase the incidence of gastric cancer (9, 10), however, the impact of T2DM on prognosis after gastrectomy remained controversial (11, 12). The propensity score matching (PSM) method could reduce the interference caused by the mismatch of baseline information (13). However, there were few studies using PSM analysis to explore the effect of T2DM on gastrectomy (12, 14). Therefore, the current study aims to explore the outcomes of T2DM on gastric cancer patients following gastrectomy through PSM.
Methods
Patients
A retrospective study of gastric cancer patients following gastrectomy was conducted in a single clinical center from January 2014 to December 2019, which was carried out in accordance with the World Medical Association Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of local hospital (2021-336) and all the patients signed informed consent.
Inclusion and Exclusion Criteria
Gastric cancer patients who underwent gastrectomy were included in this study (n = 855), and the patients were excluded by the following criteria: 1, combined with other malignant tumors (n = 17); 2, palliative gastrectomy (n = 33); 3, remnants of gastric cancer (14); and 4, incomplete medical data (n = 88). Finally, a total of 703 patients were included in this study, and the flow of selection was shown in Figure 1.
Surgery Management
Patients were confirmed gastric cancer pathologically before surgery, and underwent gastrectomy plus D2 lymph node dissection according to the Japanese gastric cancer treatment guidelines (15). All the surgeries were conducted by two surgeons who had more than 10 years' experience in a team. Patients were regularly followed up every 3 months in the first 2 years and every 6 months in the following 3 years.
Definitions
We classified the postoperative complications in accordance with the Clavien-Dindo classification (16). Overall survival (OS) was defined as the time from gastrectomy to death or last follow-up, and disease-free survival (DFS) was defined as the time from gastrectomy to recurrence, death or last follow-up.
The remission of T2DM was divided into three situations: complete remission, partial remission and no remission (17, 18). Complete remission was defined as follows: fasting blood glucose (FBG) returned to a normal range with no requirement for medication. Partial remission was defined as follows: FBG returned to an improved level with reduced medication. No remission was defined as follows: no changes in medication, more medication requirements or aggravation in FBG levels after surgery.
Data Collection
The perioperative information was retrospectively collected through outpatient and inpatient system including baseline information, surgical information and postoperative information. The baseline information included age, sex, body mass index (BMI), T2DM duration, anti-diabetes medication, T2DM status after gastrectomy, neo-adjuvant chemotherapy, pre-operative hemoglobin, pre-operative albumin, pre-operative lymphocyte; The surgical information included type of resection, surgery methods, reconstruction methods, operation time, intra-operative blood loss and retrieved lymph nodes; The postoperative information included pathological stage, complications, blood transfusion and postoperative hospital stay; The follow-up information included OS and DFS which was conducted by inpatient system and telephone interviews.
PSM
To minimize the selection bias of patients, PSM was conducted between T2DM group and Non-T2DM group. Nearest neighbor matching was performed without replacement at a 1:1 ratio and a caliper width with a 0.01 standard deviation was specified. The baseline information was matched including age, sex, BMI, neo-adjuvant chemotherapy, pre-operative hemoglobin, pre-operative albumin, type of resection, surgery methods, reconstruction methods and pathological stage.
Statistical Analysis
Continuous variables are expressed as the mean ± SD, and independent-sample t-test was used to compare the difference between T2DM group and Non-T2DM group. Frequency variables are expressed as n (%), and Chi-square test or Fisher's exact test was used. The Kaplan-Meier curve was conducted to compare T2DM on different pathological stage, and cox regression analyses were performed to identify predictive factors for OS and DFS. Data were analyzed using SPSS (version 25.0) statistical software. A bilateral p-value of < 0.05 was considered statistically significant.
Results
Patient Information Before PSM
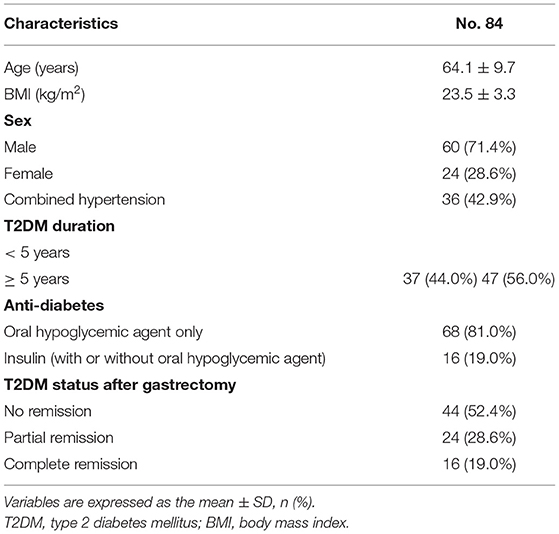
A total of 703 patients were enrolled in the current study according to the inclusion and exclusion criteria. There were 84 T2DM patients and 619 Non-T2DM patients. The clinical characteristics of the 84 T2DM patients including age, BMI, sex, combined hypertension, T2DM duration, anti-diabetes medication and T2DM status after gastrectomy were concluded in Table 1. In this study, 47.6% of patients had T2DM remission after gastrectomy.
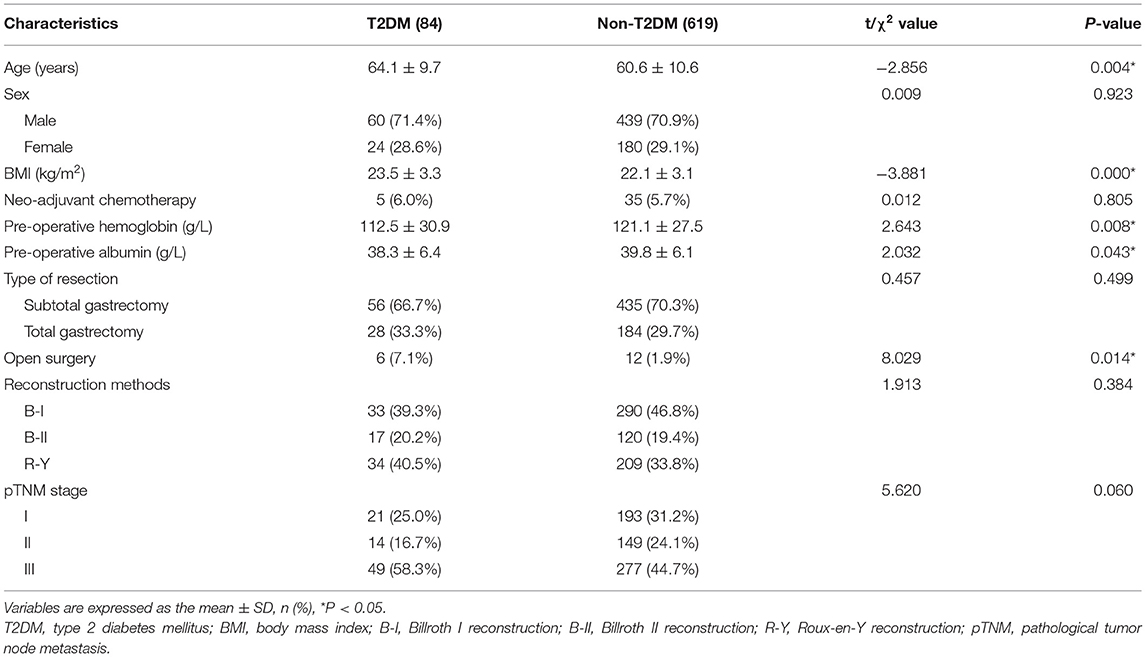
Besides, the patient information including age, sex, BMI, neo-adjuvant chemotherapy, pre-operative hemoglobin, pre-operative albumin, type of resection, surgery methods, reconstruction methods and pathological stage were compared between the two groups. T2DM group had higher age (p = 0.004 < 0.05), BMI (p = 0.000 < 0.05), open surgery (p = 0.014 < 0.05), pre-operative hemoglobin (p = 0.008 < 0.05), and pre-operative albumin (p = 0.043 < 0.05). No significant difference was found in type of resection, reconstruction methods and pathological stage (p > 0.05) (Table 2).
PSM Analysis
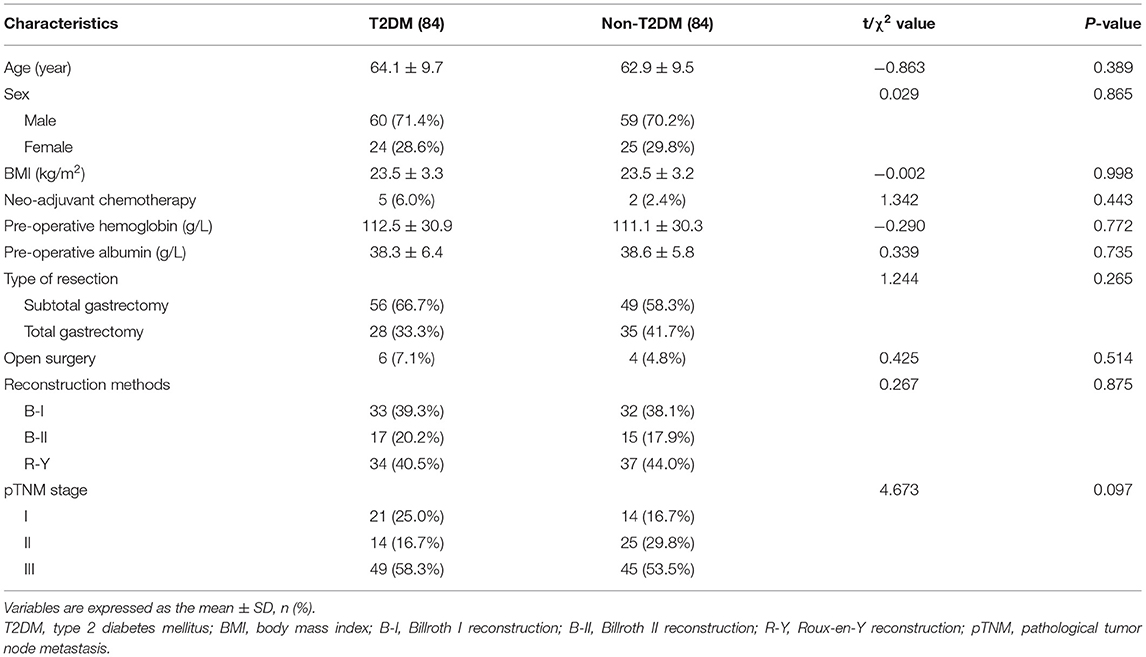
There were differences between T2DM group and Non-T2DM group, therefore, PSM was conducted to minimize the difference. After 1:1 PSM, 84 patients in T2DM group and 84 patients in Non-T2DM were matched for final analysis. There was no difference in terms of age, sex, BMI, neo-adjuvant chemotherapy, pre-operative hemoglobin, pre-operative albumin, type of resection, surgery methods, reconstruction methods and pathological stage (p > 0.05) (Table 3).
Short-Term Outcomes
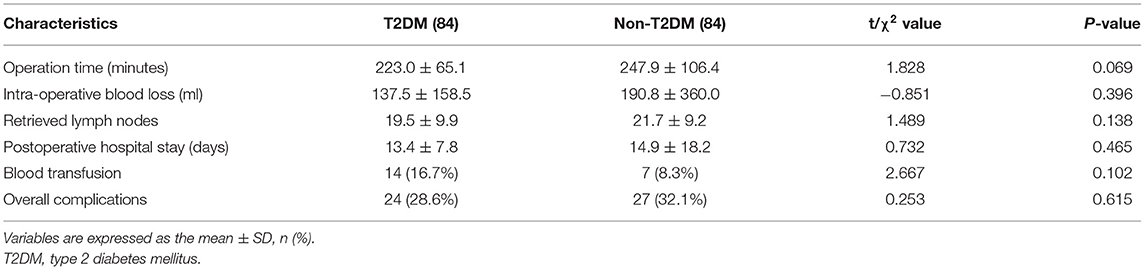
We compared the operation time, intra-operative blood loss, retrieved lymph nodes, postoperative stay, blood transfusion and complications between T2DM group and Non-T2DM group after PSM. However, no significant difference was found (p > 0.05) (Table 4).
Prognosis
The medium follow-up time was 29 (1–101) months. The Kaplan-Meier curve was conducted to compare T2DM on different pathological stages. No significant difference was found in all stages (p = 0.682), stage I (p = 0.317), stage II (p = 0.208) or stage III (p = 0.689) in terms of OS, and furthermore, there were no differences in all stages (p = 0.378), stage I (p = 0.317), stage II (p = 0.224) or stage III (p = 0.503) in terms of DFS (Figures 2, 3).
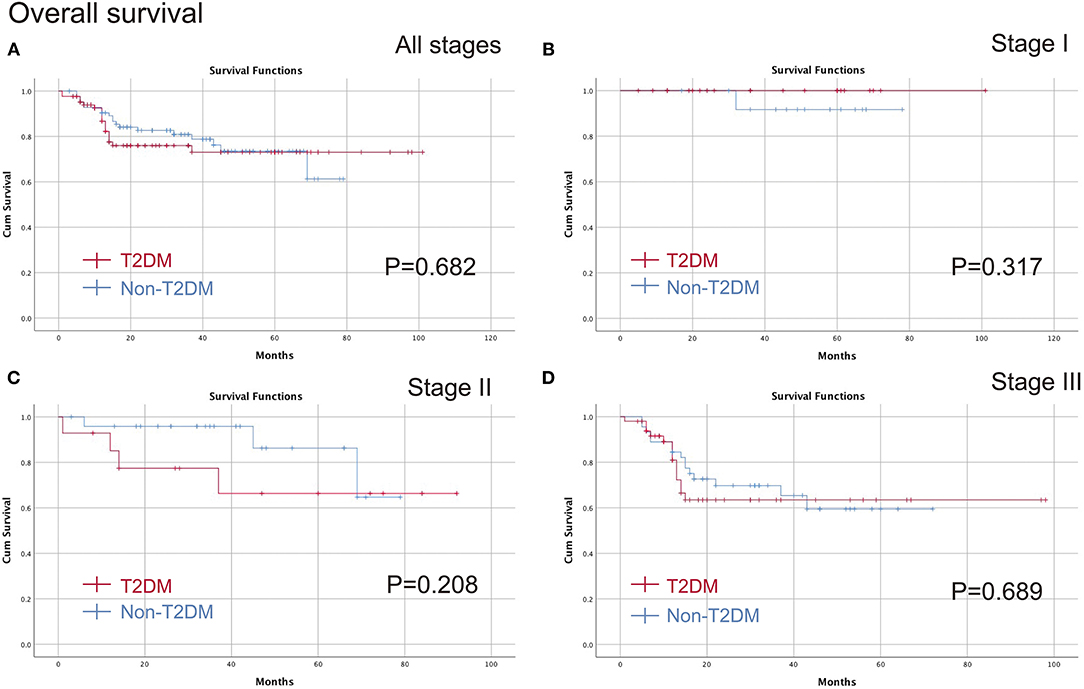
Figure 2. Comparison of the OS between T2DM group and Non-T2DM group. (A) all stages; (B) stage I; (C) stage II; (D) stage III. OS, overall survival; T2DM, type 2 diabetes mellitus.
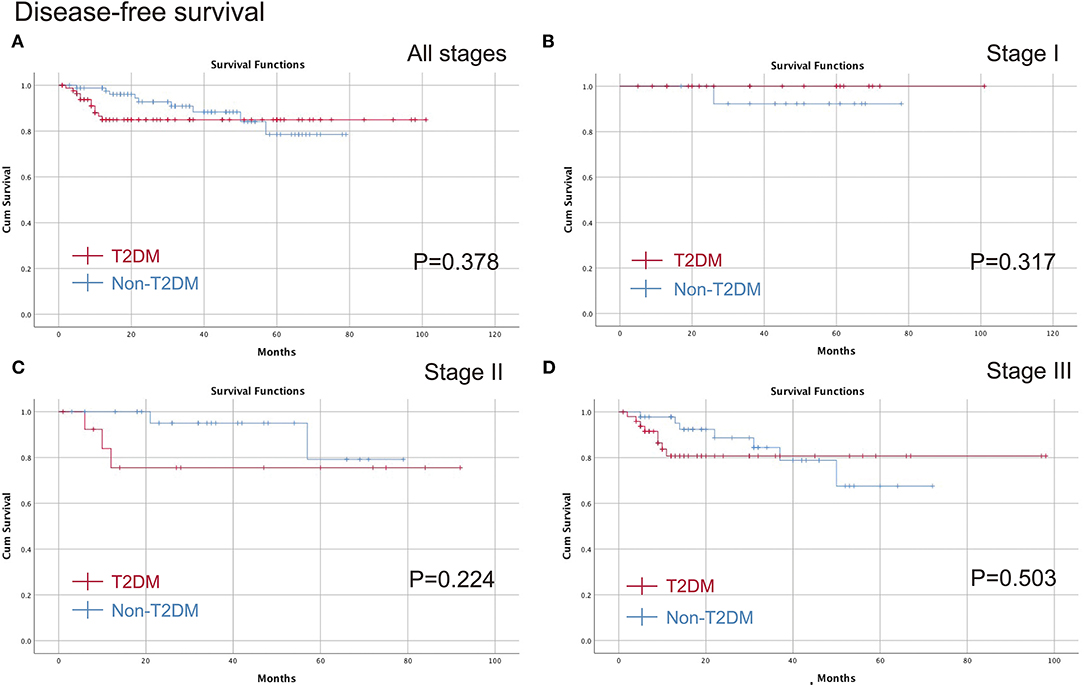
Figure 3. Comparison of the DFS between T2DM group and Non-T2DM group. (A) all stages; (B) stage I; (C) stage II; (D) stage III. DFS, disease free survival; T2DM, type 2 diabetes mellitus.
Cox Regression
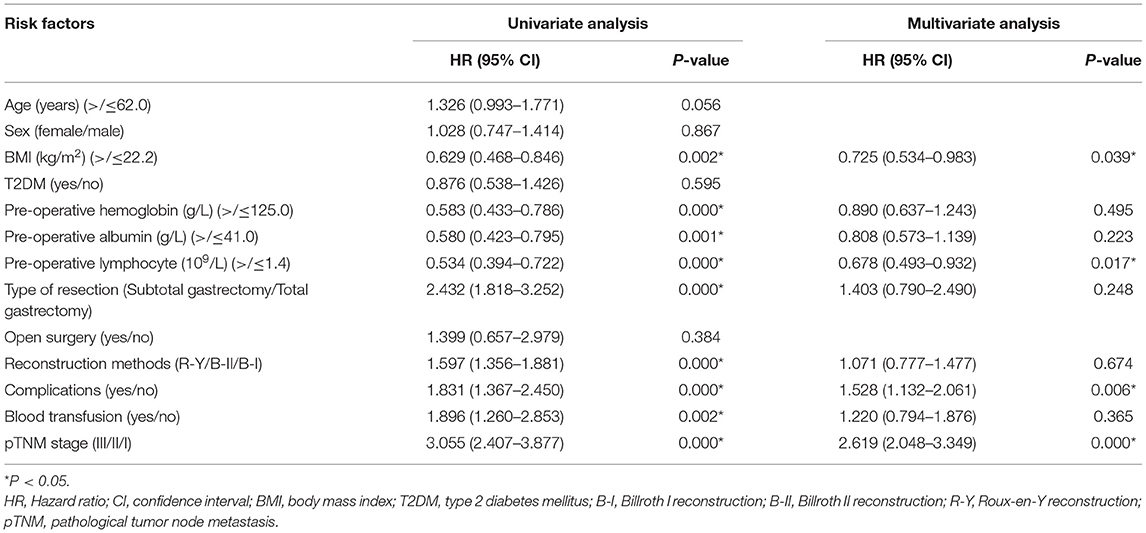
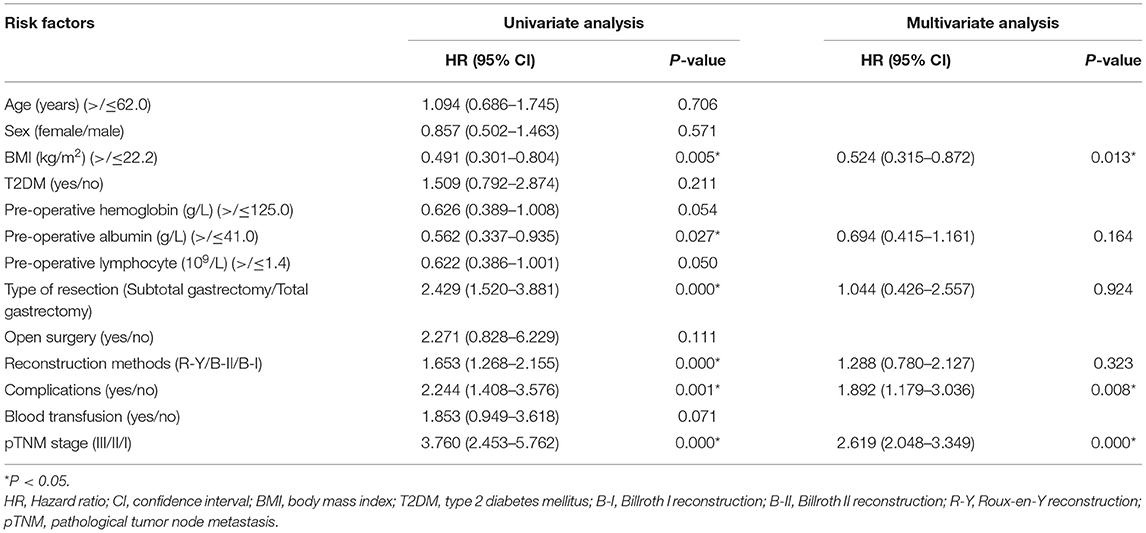
Cox regression was conducted to identify predictive factors for prognosis. In terms of OS, BMI (p = 0.039 < 0.05, HR = 0.725, 95% CI = 0.534–0.983), pre-operative lymphocyte (p = 0.017 < 0.05, HR = 0.678, 95% CI = 0.493–0.932), pathological tumor node metastasis (pTNM) stage (p = 0.000 < 0.05, HR = 2.619, 95% CI = 2.048–3.349) and complications (p = 0.006 < 0.05, HR = 1.528, 95% CI = 1.132–2.061) were predictive factors. In terms of DFS, BMI (p = 0.013 < 0.05, HR = 0.524, 95% CI = 0.315–0.872), pTNM stage (p = 0.000 < 0.05, HR = 2.619, 95% CI = 2.048-3.349) and complications (p = 0.008 < 0.05, HR = 1.892, 95% CI = 1.179–3.036) were independent prognostic factors. In addition, T2DM was not the predictive factor for OS (p = 0.595 > 0.05, HR = 0.876, 95% CI = 0.538–1.426) or DFS (p = 0.211 > 0.05, HR = 1.509, 95% CI = 0.792–2.874) (Tables 5, 6).
Discussion
A total of 703 patients were included in this study. After 1:1 PSM, 84 patients in T2DM group and 84 patients in Non-T2DM group were matched for final analysis. No significant difference was found in terms of operation time, intra-operative blood loss, retrieved lymph nodes, postoperative stay, blood transfusion, complications, OS or DFS between T2DM group and Non-T2DM group. Furthermore, BMI, pre-operative lymphocyte, pTNM stage and complications were predictive factors for OS, and BMI, pTNM stage and complications were predictive factors for DFS.
T2DM is a metabolic disease, which is related to the onset of digestive tumors including gastric cancer, colorectal cancer, and esophageal cancer (9, 19, 20). In addition to the influence on tumor onset, T2DM might also affect the prognosis of patients, including OS, DFS, and cancer-specific survival (12, 14). A study reported that the OS of gastric cancer patients could be improved by metformin (21). Among patients with gastric cancer, there was also another study reporting that the remission of T2DM would affect the prognosis as well (22). There were few studies concerning about the postoperative outcomes and prognosis through PSM, therefore, the current study aims to explore the outcomes of T2DM on gastric cancer patients following gastrectomy through PSM.
A recent study reported that T2DM was associated with increased infection and readmission rate (23). Lee et al. (24) reported T2DM could increase the postoperative complications, postoperative stay, combined with other diseases and the status of hyperglycemia might contribute to the complications. However, in our study, it was found no statistical difference in terms of postoperative stay or complications, which was consistent with previous studies (25).
The mechanism between T2DM and gastric cancer remained unclear, some potential mechanism might contribute to the tumor genesis: 1. The status of hyperglycemia which promoted the tumor cells proliferation (26); 2. In addition, T2DM together with obesity is a chronic low-grade inflammatory disease which could result in malignant tumors (27); and 3. The mitosis and metastasis of tumor cells were enhanced in hyperinsulinemia (28).
Chen et al. (12) reported T2DM could decrease the progress-free survival. In another study, Sheng et al. (14) reported T2DM could apparently worsen the OS of gastric cancer patients undergoing gastrectomy. However, in the PSM study, it was not statistically significant in terms of OS or DFS. Therefore, large-scale prospective studies should be conducted to determine the exact results of T2DM on prognosis.
In this study, BMI, pre-operative lymphocyte, tumor stage and complications were predictive factors of OS. Besides, BMI, pTNM stage and complications were predictive factors for DFS. The results were consisting with previous studies (29, 30). Pre-operative lymphocyte was closely associated with human's immune function, and lower level of lymphocyte indicated worse OS in this study (31, 32). Therefore, perioperative management should be careful to conduct to improve the outcomes of gastric patients.
Few studies reported the prognosis of T2DM on gastric cancer patients following gastrectomy (12, 14). To our knowledge, this is the first study to analyze the short-term outcomes of T2DM on gastric cancer patients using PSM. Inconsistent with the previous studies, T2DM had no effect on short-term outcomes or prognosis in this study.
Furthermore, there was an interesting phenomenon that FBG level improved after gastrectomy in some patients concurrent with gastric cancer and T2DM, and the conclusion was proved previously (11, 33, 34). The remission rate of T2DM in our single center was 47.6%, which was approximately consistent with the previous studies (17, 18, 35–37). Gastrectomy, regarded as the onco-metabolic surgery, could control metabolic diseases including hypertension and T2DM (38, 39). This phenomenon was found in CRC patients as well (19, 40). However, the influence on remission of T2DM after gastrectomy needed more prospective studies to prove in the future.
There were several limitations in this present study as well. First, it could bring some selection bias due to the retrospective single-center study with a small amount of cases; Second, the medium follow-up time was relatively short; Third, we were lacking the data related diabetes severity, such as glycated hemoglobin, C-peptide and degree of diabetic complication; Last, some nutritional or immunological factors including neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, CD4+/ CD8+, IL-18 and IL-1β (41–44) were lacking for further analysis. Therefore, some large scale and multi-center prospective randomized controlled trials with more comprehensive data should be conducted in the following experiments.
In conclusion, T2DM did not have an impact on gastric cancer patients following gastrectomy in terms of short-term outcomes and prognosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, 2021-336. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WT, BK, X-YL, CY, and BZ: data extraction. DP: data analysis. DP, Y-XC, and WT: quality assessments. Y-XC: writing—original draft. DP, Y-XC, WT, BK, X-YL, CY, and BZ: writing—review and editing. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Dr. Zhuozhi Shen, Chongqing Center for Disease Control and Prevention, for the substantial work in the statistical methods.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Tao W, Cheng YX, Zou YY, Peng D, Zhang W. Aorta calcification increases the risk of anastomotic leakage after gastrectomy in gastric cancer patients. Cancer Manag Res. (2021) 13:3857–65. doi: 10.2147/CMAR.S306942
3. Peng D, Zou YY, Cheng YX, Tao W, Zhang W. Effect of time (season, surgical starting time, waiting time) on patients with gastric cancer. Risk Manag Healthc Policy. (2021) 14:1327–33. doi: 10.2147/RMHP.S294141
4. Wu L, Feng Y, Wu Z, Xu H, Zhang C, Ning J, et al. Survival outcomes of adjuvant taxanes, platinum plus fluoropyrimidines versus platinum and fluoropyrimidines for gastric cancer patients after D2 gastrectomy: a retrospective propensity score-matched analysis. World J Surg Oncol. (2021) 19:272. doi: 10.1186/s12957-021-02390-4
5. Peng D, Cheng YX, Tao W, Zou YY, Qian K, Zhang W. Onco-metabolic surgery: a combined approach to gastric cancer and hypertension. Cancer Manag Res. (2020) 12:7867–73. doi: 10.2147/CMAR.S260147
6. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
7. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
8. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
9. Yang HJ, Kang D, Chang Y, Ahn J, Ryu S, Cho J, et al. Diabetes mellitus is associated with an increased risk of gastric cancer: a cohort study. Gastric Cancer. (2020) 23:382–90. doi: 10.1007/s10120-019-01033-8
10. Miao ZF, Xu H, Xu YY, Wang ZN, Zhao TT, Song YX, et al. Diabetes mellitus and the risk of gastric cancer: a meta-analysis of cohort studies. Oncotarget. (2017) 8:44881–92. doi: 10.18632/oncotarget.16487
11. Wei ZW Li JL, Wu Y, Xia GK, Schwarz RE, He YL, et al. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: a retrospective cohort study. Dig Dis Sci. (2014) 59:1017–24. doi: 10.1007/s10620-013-2965-6
12. Chen X, Chen Y, Li T, Jun L, Lin T, Hu Y, et al. Impact of diabetes on prognosis of gastric cancer patients performed with gastrectomy. Chin J Cancer Res. (2020) 32:631–44. doi: 10.21147/j.issn.1000-9604.2020.05.08
13. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. (1996) 52:249–64. doi: 10.2307/2533160
14. Sheng L, Peng H, Pan Y, Wang C, Zhu Y. Evaluating the effect of diabetes on the prognosis of gastric cancer using a propensity score matching method. J Gastrointest Oncol. (2020) 11:999–1008. doi: 10.21037/jgo-20-375
15. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines (2010) (ver. 3). Gastric Cancer. (2011). 14:113–23. doi: 10.1007/s10120-011-0042-4
16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
17. An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. (2013) 19:9410–7. doi: 10.3748/wjg.v19.i48.9410
18. Wang KC, Huang KH, Lan YT, Fang WL, Lo SS Li AF, et al. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J Surg. (2014) 38:431–8. doi: 10.1007/s00268-013-2291-3
19. Peng D, Liu XY, Cheng YX, Tao W, Cheng Y. Improvement of diabetes mellitus after colorectal cancer surgery: a retrospective study of predictive factors for type 2 diabetes mellitus remission and overall survival. Front Oncol. (2021) 11:694997. doi: 10.3389/fonc.2021.694997
20. Xu B, Zhou X, Li X, Liu C, Yang C. Diabetes mellitus carries a risk of esophageal cancer: a meta-analysis. Medicine. (2017) 96:e7944. doi: 10.1097/MD.0000000000007944
21. Tseng CH. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: an extensive review of the literature. Biomolecules. (2021) 11:1022. doi: 10.3390/biom11071022
22. Peng D, Cheng YX, Zhang W. Does Roux-en-Y construction really bring benefit of type 2 diabetes mellitus remission after gastrectomy in patients with gastric cancer? a systematic review and meta-analysis. Diabetes Ther. (2020) 11:2863–72. doi: 10.1007/s13300-020-00934-7
23. Zylla D, Gilmore G, Eklund J, Richter S, Carlson A. Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. J Diabetes Complications. (2019) 33:335–9. doi: 10.1016/j.jdiacomp.2018.12.012
24. Lee KC, Chung KC, Chen HH, Cheng KC, Wu KL, Song LC, et al. The impact of comorbid diabetes on short-term postoperative outcomes in stage I/II colon cancer patients undergoing open colectomy. Biomed Res Int. (2020) 2020:2716395. doi: 10.1155/2020/2716395
25. Li SS, Udelsman BV, Parikh A, Klempner SJ, Clark JW, Roeland EJ, et al. Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J Am Coll Surg. (2020) 230:912–24. doi: 10.1016/j.jamcollsurg.2019.12.038
26. Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev. (2020) 41:bnz014. doi: 10.1210/endrev/bnz014
27. Ohara N, Kobayashi M, Ikeda Y, Hoshi T, Morita S, Kanefuji T, et al. Non-insulin-dependent diabetes mellitus induced by immune checkpoint inhibitor therapy in an insulinoma-associated antigen-2 autoantibody-positive patient with advanced gastric cancer. Intern Med. (2020) 59:551–6. doi: 10.2169/internalmedicine.3208-19
28. Hua F, Yu JJ, Hu ZW. Diabetes and cancer, common threads and missing links. Cancer Lett. (2016) 374:54–61. doi: 10.1016/j.canlet.2016.02.006
29. Chen G, Wang J, Chen K, Kang M, Zhang H, Jin X, et al. Relationship between postoperative complications and the prognosis of gastric carcinoma patients who underwent surgical resection: a systematic review and meta-analysis. Cancer Control. (2021) 28:10732748211011955. doi: 10.1177/10732748211011955
30. Maezawa Y, Aoyama T, Ju M, Komori K, Kano K, Sawazaki S, et al. The impact of severe infectious complications on long-term prognosis for gastric cancer. Anticancer Res. (2020) 40:4067–74. doi: 10.21873/anticanres.14404
31. Li H, Su Q, Li B, Lan L, Wang C, Li W, et al. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J Cell Mol Med. (2020) 24:4452–65. doi: 10.1111/jcmm.15104
32. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18:148. doi: 10.1186/s12876-018-0877-9
33. Choi YY, Noh SH, An JY. A.randomized controlled trial of Roux-en-Y gastrojejunostomy vs. gastroduodenostomy with respect to the improvement of type 2 diabetes mellitus after distal gastrectomy in gastric cancer patients. PLoS ONE. (2017) 12:e0188904. doi: 10.1371/journal.pone.0188904
34. Zhu Z, Shan X, Cheng Y, Xu J, Fu H, Wang W, et al. Clinical course of diabetes after gastrectomy according to type of reconstruction in patients with concurrent gastric cancer and type 2 diabetes. Obes Surg. (2015) 25:673–9. doi: 10.1007/s11695-014-1426-4
35. Ho TW, Wu JM, Yang CY, Lai HS, Lai F, Tien YW. Total gastrectomy improves glucose metabolism on gastric cancer patients: a nationwide population-based study. Surg Obes Relat Dis. (2016) 12:635–41. doi: 10.1016/j.soard.2015.11.024
36. Kwon Y, Kwon JW, Kim D, Ha J, Park SH, Hwang J, et al. Predictors of remission and relapse of diabetes after conventional gastrectomy for gastric cancer: nationwide population-based cohort study. J Am Coll Surg. (2021) 232:973–981.e2. doi: 10.1016/j.jamcollsurg.2021.03.019
37. Kwon Y, Kwon JW, Ha J, Kim D, Cho J, Jeon SM, et al. Remission of type 2 diabetes after gastrectomy for gastric cancer: diabetes prediction score. Gastric Cancer. (2022) 25:265–74. doi: 10.1007/s10120-021-01216-2
38. Cheng YX, Peng D, Tao W, Zhang W. Effect of oncometabolic surgery on gastric cancer: the remission of hypertension, type 2 diabetes mellitus, and beyond. World J Gastrointest Oncol. (2021) 13:1157–63. doi: 10.4251/wjgo.v13.i9.1157
39. Kim WJ, Kwon Y, Lee CM, Lim SH Li Y, Wang J, et al. Oncometabolic surgery: Emergence and legitimacy for investigation. Chin J Cancer Res. (2020) 32:252–62. doi: 10.21147/j.issn.1000-9604.2020.02.12
40. Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, et al. Hypertension remission after colorectal cancer surgery: a single-center retrospective study. Nutr Cancer. (2022) 7:1–7. doi: 10.1080/01635581.2021.2025256
41. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. (2019) 19:672. doi: 10.1186/s12885-019-5903-y
42. Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. (2018) 18:11. doi: 10.1186/s12876-018-0741-y
43. Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim Biophys Acta Rev Cancer. (2019) 1872:1–10. doi: 10.1016/j.bbcan.2019.05.001
Keywords: gastric cancer, type 2 diabetes mellitus, propensity score matching, outcome, prognosis
Citation: Cheng Y-X, Tao W, Kang B, Liu X-Y, Yuan C, Zhang B and Peng D (2022) Impact of Preoperative Type 2 Diabetes Mellitus on the Outcomes of Gastric Cancer Patients Following Gastrectomy: A Propensity Score Matching Analysis. Front. Surg. 9:850265. doi: 10.3389/fsurg.2022.850265
Received: 07 January 2022; Accepted: 08 February 2022;
Published: 08 March 2022.
Edited by:
Sungsoo Park, Korea University, South KoreaReviewed by:
Yeongkeun Kwon, Korea University, South KoreaJong-Han Kim, Korea University, South Korea
Copyright © 2022 Cheng, Tao, Kang, Liu, Yuan, Zhang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Peng, Y2FycnlfZG9uZ0AxMjYuY29t
†These authors have contributed equally to this work
 Yu-Xi Cheng
Yu-Xi Cheng Wei Tao
Wei Tao Bing Kang
Bing Kang Xiao-Yu Liu
Xiao-Yu Liu Chao Yuan
Chao Yuan Bin Zhang
Bin Zhang Dong Peng
Dong Peng