
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 16 February 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.838477
This article is part of the Research TopicInsights in Genitourinary Surgery: 2021View all 4 articles
Objectives: There is a recent paradigm shift to extend robot-assisted radical prostatectomy (RARP) to very senior prostate cancer (PCa) patients based on biological fitness, comorbidities, and clinical PCa assessment that approximates the true risk of progression. Thus, we aimed to assess misclassification rates between clinical vs. pathological PCa burden.
Materials and Methods: We compared senior patients with PCa ≥75 y (n = 847), who were propensity score matched with younger patients <75 y (n = 3,388) in a 1:4 ratio. Matching was based on the number of biopsy cores, prostate volume, and preoperative Cancer of the Prostate Risk Assessment (CAPRA) risk groups score. Multivariable logistic regression models (LRMs) predicted surgical CAPRA (CAPRA-S) upgrade, which was defined as a higher risk of the CAPRA-S in the presence of lower-risk preoperative CAPRA score. LRM incorporated the same variables as propensity score matching. Moreover, patients were categorized as low-, intermediate-, and high-risk, preoperative and according to their CAPRA and CAPRA-S scores.
Results: Surgical CAPRA risk strata significantly differed between the groups. Greater proportions of unfavorable intermediate risk (39 vs. 32%) or high risk (30 vs. 28%; p < 0.001) were observed. These proportions are driven by greater proportions of International Society of Urological Pathology (ISUP) Gleason Grade Group 4 or 5 (33 vs. 26%; p = 0.001) and pathological tumor stage (≥T3a 54 vs. 45%; p < 0.001). Increasing age was identified as an independent predictor of CAPRA-S-based upgrade (age odds ratio [OR] 1.028 95% CI 1.02–1.037; p < 0.001).
Conclusion: Approximately every second senior patient has a misclassification in (i.e., any up or downgrade) and each 4.5th senior patient specifically has an upgrade in his final pathology that directly translates to an unfavorable PCa prognosis. It is imperative to take such substantial misclassification rates into account for this sensitive PCa demographic of senior men. Future prospective studies are warranted to further optimize PCa workflow and diagnostics, such as to incorporate modern imaging, molecular profiling and implement these into biopsy strategies to identify true PCa burden.
Prostate cancer (PCa) is the second most common malignant tumor entity in men, especially in industrialized countries (1), and may present in a variety of oncological profiles, varying from insignificant to highly aggressive diseases.
As the diagnostic tools for PCa evolve, there will be an increase of patient numbers needing counseling with regard to possible treatment options in the upcoming years (1, 2). Among these patients, there will be many senior men over the age of 75 years.
Our series was one of the first to demonstrate that senior age is not a contraindication for local treatment, such as robot-assisted radical prostatectomy (RARP) (3, 4). Specifically, our results demonstrated that senior age is not inherently a risk factor for unfavorable prognosis and that sufficient functional outcomes can be achieved that are highly comparable to younger patients, such as urinary continence recovery. Thus, we contributed to a paradigm shift that selects senior patients who can be in fact counseled for local therapies, such as RARP (3, 4).
However, consistent with several studies developing prediction tools for upgrading and upstaging, when biopsy vs. final histopathology was compared, senior age appeared indeed to have more unfavorable pathology and our own observations indicated greater misclassification between clinical vs. pathological stages. This phenomenon of misclassification likely explains findings of previous studies that reported rather higher rates of biochemical recurrence or even metastatic progression in senior men (5–7), since the studies adjusted for clinical parameters. In fact, such contradicting findings in combination with a rather dated concept to derive life expectancy based on demographics instead at the individual level supported the widely adopted notion to refrain from offering surgical local treatment to senior patients with PCa since the beneficiary potential of RARP appeared compromised (3, 8–11). Alternatively, these patients will be offered radiotherapy or administration of an androgen deprivation therapy (12), both options potentially associated with high morbidity and comparably ineffective treatment of possible Pre-existing urinary tract symptoms (13). Moreover, guidelines, such as the United States Preventive Services Taskforce (USPSTF), recommend no PCa screening in men ≥70 years based on clinical findings, which might lead to further aggravation of the aforementioned misclassification problem in senior aged men. It is of note that senior age sample sizes, which were treated with RARP, are accordingly very small and that most series did not rely on adjustment according to pathological characteristics or that methodological implications apply, such as relying on univariable analyses.
We hypothesized that even after multivariable adjustment, there is a substantial misclassification, i.e., unfavorable PCa upgrade at RARP in senior patients compared to younger patients, which might delay treatment or even preclude senior patients from local treatment.
To examine this potentially detrimental misclassification phenomenon in senior men, we applied pre and postoperative Cancer of the Prostate Risk Assessment risk groups (CAPRA and CAPRA-S, respectively) (14), which represent multivariable scores that rely on clinical and pathological variables, respectively. Based on CAPRA vs. CAPRA-S risk scores, we analyzed the rates of an upgrade at RARP between senior patients with PCa aged ≥75 years vs. younger patients with PCa <75 years.
Overall, 13,765 consecutive patients with PCa were identified, who had complete pathological data and were treated with RARP and extended pelvic lymph node dissection at the Prostate Cancer Center Northwest, Gronau, Germany, between May 2006 and December 2019. All patient data were gathered prospectively.
Patients with suspected metastases were excluded and required complete clinico-pathological data to derive both, the University of California, San Francisco (UCSF) preoperative (15) and surgical Cancer of the Prostate Risk Assessment scores (CAPRA and CAPRA-S, respectively) (14).
The preoperative CAPRA incorporates age, clinical tumor stage, Pre-surgical prostate-specific antigen (PSA), biopsy Gleason score, and percentage of positive biopsy cores (15). The final CAPRA score ranges from 0 to 10, with 0–2 representing low risk (LR), 3–5 intermediate risk (IR), and 6–10 high risk (HR). Similarly, the CAPRA-S incorporates pre-surgical PSA, pathological Gleason score, surgical margin status, extracapsular extension, seminal vesicle invasion, and lymph node invasion (16). Patients were stratified according to age groups <75 vs. ≥75 years. The final CAPRA-S score ranges from 0 to 12, with 0–2 representing LR, 3–5 IR, and 6–12 HR. For both, CAPRA and CAPRA-S, each two-point increase in score indicates an approximately 2-fold increase in the risk of PCa recurrence or secondary treatment.
We used propensity score matching at a 4:1 ratio to create similar cohorts between patients with RARP <75 vs. ≥75 years. We ensured that matching improved the balance between the aforementioned age groups by checking the standardized mean differences. Propensity score matching variables consisted of the same variables that were used for the multivariable logistic regression analyses (LRM): age, year of surgery, the number of biopsy cores, and preoperative CAPRA score.
The chi-square test was used for categorical and t-test for continuous variables. Proportions of the respective CAPRA and CAPRA-S risk strata (14), i.e., LR, IR, and HR, were calculated and compared. The CAPRA-S-based upgrade was defined as a lower risk at preoperative CAPRA despite harboring a higher risk CAPRA-S at RARP, for example, preoperative CAPRA LR vs. CAPRA-S IR or even CAPRA-S HR.
Logistic regression model aimed to predict CAPRA-S-based upgrade at RARP and was adjusted for age, year of surgery, the number of biopsy cores sampled, and preoperative CAPRA score. It is important to note that a CAPRA-S-based upgrade cannot be observed in men categorized as preoperative CAPRA HR. Accordingly, LRM was restricted to patients with preoperative CAPRA LR or IR. LRM was additionally performed after propensity score matching.
All tests were two-sided with a statistical significance set at p < 0.05. Analyses were performed with the statistical package for R (R Foundation for Statistical Computing, version 3.2.2).
Clinico-pathological characteristics of 847 senior patients with PCa ≥75 years, who were propensity score matched with 3,388 younger patients with PCa <75 years (4:1 ratio) are presented in Table 1. The respective median age was 76 (interquartile range [IQR] 75–77) vs. 65 (IQR 60–69) years.
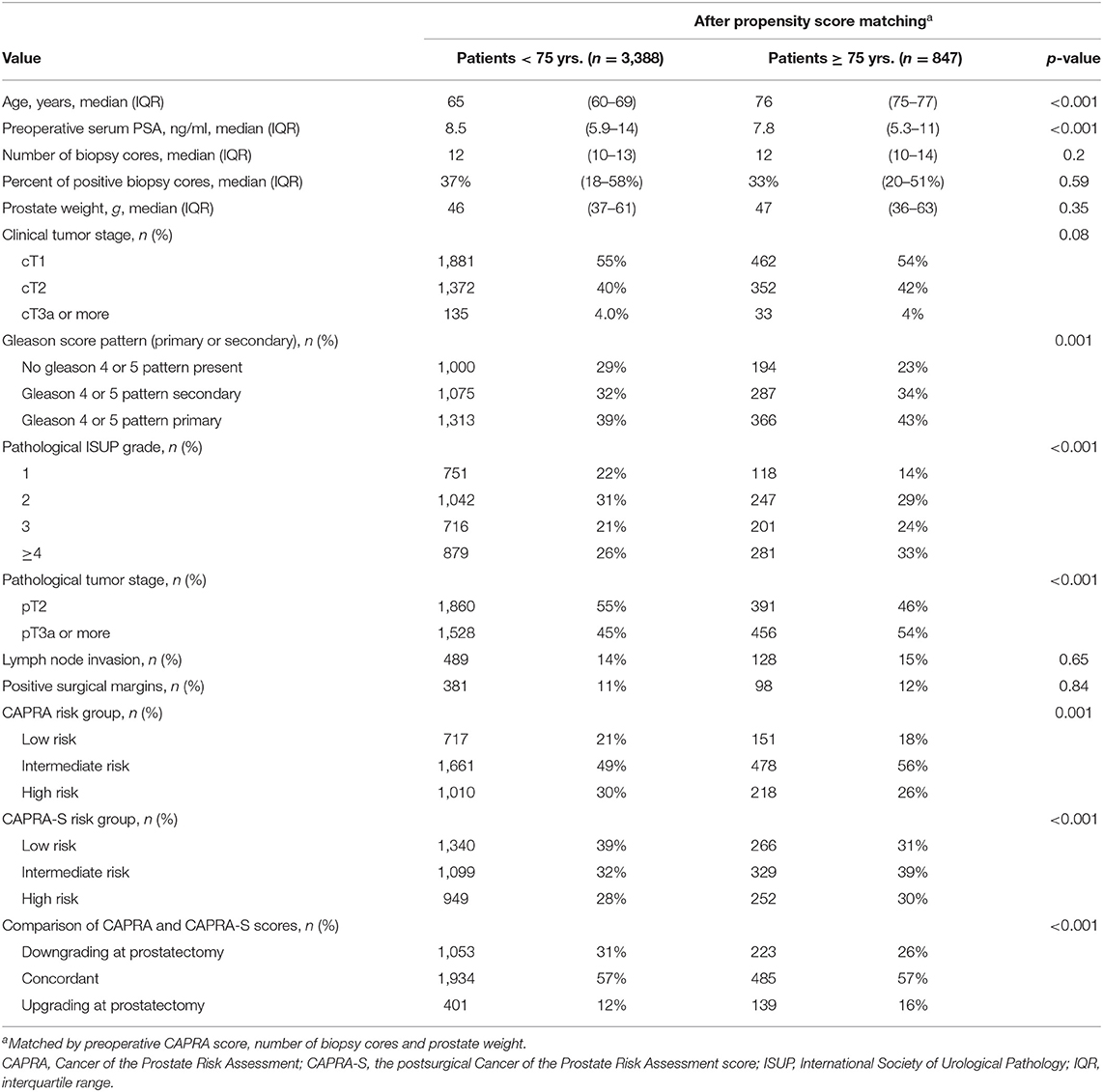
Table 1. Clinicopathological characteristics of 847 senior prostate cancer patients ≥ 75 yrs., who are propensity score matched with 3,388 (4:1 ratio) younger prostate cancer patients <75 yrs., according to preoperative CAPRA score, number of biopsy cores and prostate weight.
Propensity score matching yielded virtually identical proportions of preoperative CAPRA risk strata between age groups, with LR, IR, and HR being 18–21%, 56–49%, and 26–30%, respectively. However, it is of note that minor differences were still observed in underlying clinical characteristics between ≥75 vs. <75 years, such as median PSA (7.8 vs. 8.5; p < 0.001), and primary biopsy Gleason pattern 4 or 5 (43 vs. 39%; p = 0.001).
Despite such virtually identical proportions of preoperative CAPRA risk strata, the RARP-derived CAPRA-S risk strata significantly differed in ≥75 vs. <75 years. Specifically, greater proportions of unfavorable IR (39 vs. 32%) or HR (30 vs. 28%; p < 0.001) were observed in senior patients with PCa. These proportions are driven by greater proportions of ISUP The Gleason Grade Group 4 or 5 (43 vs. 39%; p = 0.001) and pathological tumor stage (≥T3a 54 vs. 45%; p < 0.001) and aforementioned PSA, whereas modestly higher proportions of positive surgical margin (12 vs. 11%; p = 0.84) or nodal metastases (15 vs. 14%; p = 0.65) were observed but not statistically significant.
Comparison of preoperative CAPRA and RARP CAPRA-S to determine rates of CAPRA-S-based upgrade translated to higher rates of CAPRA-S upgrade in senior patients with PCa (16. vs. 12%; p < 0.001). Conversely, lower downgrade rates were observed in the same senior patients (26 vs. 31%; p < 0.001). Specifically, if analyses were restricted to propensity score-matched senior patients with a preoperative CAPRA LR or IR profile, rates of CAPRA-S upgrade in senior patients with PCa were even more distinct, with 22 vs. 17% (p < 0.001).
Finally, LRM, which were restricted to men with preoperative CAPRA LR or IR and were adjusted for age, number of biopsy cores sampled, prostate weight, and preoperative CAPRA score, revealed increasing age as an independent predictor of CAPRA-S-based upgrade at RARP (age OR 1.028 95% CI 1.02–1.037; p < 0.001, Table 2).
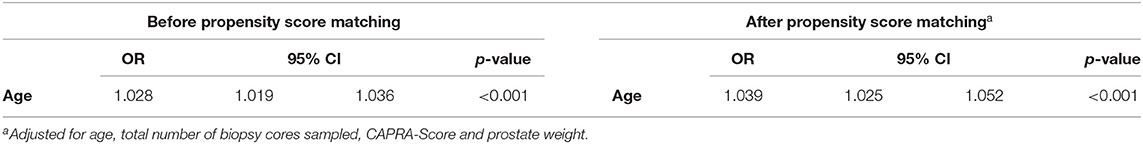
Table 2. Multivariable logistic regression model predicting CAPRA-S based prostate cancer risk upgrade at robot-assisted radical prostatectomy before and after propensity score matching.
To account for the introduction of multiparametric MRI of the prostate and MRI-ultrasound fusion biopsies since around 2015, we additionally performed analyses, which were restricted to recent time intervals 2015–2017 and ≥2018. Even in these most contemporary cohorts, senior age was associated with a higher risk of CAPRA-S-based upgrade at RARP in LRM (data not shown).
The demographic transition in industrialized countries will have a major effect on the burden of the urological departments in the next decades. An increasing number of senior patients will present themselves with newly diagnosed PCa and seek curative treatment (6). However, based on a rather stringent concept of 10 years life expectancy, which is mostly based on national demographic estimates and in compliance with present guidelines, many patients are precluded from local treatment, when such a patient is being consulted for treatment. This is despite recent series, which demonstrated that senior patients, who are treated with RARP, might experience low perioperative morbidity, achieve excellent results of cancer control, quality of life (QoL), and functional results (4, 13, 17, 18). Conversely, it is of utmost importance to consider watchful waiting strategies or active surveillance after carefully weighing risk and benefit and QoL (19).
Thus, the consultation needs to account for several aspects, the individual, his biological fitness, and his clinical PCa characteristics. As a prerequisite, potential misclassification rates must also be considered, particularly, if the treatment is potentially delayed and the window of opportunity of cure might be missed.
Our study yielded several important results:
First, the vast majority of our senior patients with PCa, who were treated with RARP, (82%, Table 1), had preoperative CAPRA IR or HR. This indicates adequate selection criteria for local treatment. However, a comparison of preoperative CAPRA LR with CAPRA-S LR within senior patients revealed substantial differences, i.e., 18 vs. 31%. This indicates misclassification, i.e., downgrading at RARP. This means that these patients might also have benefitted from other solutions than local treatment, such as active surveillance. However, it is of note that CAPRA LR is not as strict as widely adapted active surveillance criteria, such as European Association of Urology (EAU) or Prostate Cancer Research International Active Survaillance study (PRIAS) (20).
Similarly, a comparison of CAPRA HR with CAPRA-S HR within senior patients also reveals Non-negligible differences, 26–30% (Table 1). This indicates misclassification, i.e., upgrading at RARP. Taken together, these combined misclassification rates of 43% at senior age are rather astonishing since CAPRA and CAPRA-S each represent multivariable scores relying on clinical and pathological PCa characteristics, respectively, to denote oncological prognosis. However, the previous series also suggested that CAPRA and CAPRA-S might be further optimized by incorporating better metrics of tumor burden, such as Gleason quantification to optimize the prediction of recurrent disease or metastatic progression (21).
Moreover, the previous series suggested that senior age was an independent predictor for worse oncological and functional outcomes after radical prostatectomy (6). This point could not be confirmed in a previous study of our center (22), supporting the argument that local surgical treatment of PCa, potentially in the context of a multimodal setting, might confer a survival benefit. Therefore, senior patients should not be excluded from RARP, particularly, when accounting for current demographic trends toward an increasing number of senior PCa patients with unfavorable tumor characteristics (23).
Second, after matching, a comparison of senior patients with PCa ≥75 vs. <75 years, who were either CAPRA LR or IR, revealed higher rates of CAPRA-S-based upgrade at RARP (22 vs. 17%; p = 0.002, data not shown). It is very important to note that baseline characteristics in Table 1 represent comprehensively propensity score-matched data between both age groups, according to the number of biopsy cores sampled, prostate volume, and preoperative CAPRA score. Our findings demonstrate that age is an independent risk factor for CAPRA-S-based upgrade at RARP.
Moreover, it is also of note that a higher CAPRA-S score in senior patients was driven by ISUP Gleason Grade group 4 or 5 and/or extracapsular extension of the PCa. Both, the Gleason Grade group and tumor stage can be assessed with the multiparametric MRI (mpMRI) of the prostate. Unfortunately, many patients with LR PCa at biopsy are unlikely to receive further imaging prior to surgery (mpMRI), as it is not mentioned in the present guidelines and there is no reimbursement covered by health insurance in Germany. This may one of many factors contributing to a systematic underestimation of true PCa aggressiveness in senior men. This argument supports additionally the implementation of prostate MRI as a definite staging tool prior to consultation. Specifically, studies of Nordström et al. and Moore et al. demonstrated that the inclusion of MRI in longitudinal PCa biopsy and diagnostic pathways lead to a clear decrease of unnecessary re-biopsies and a higher yield of the clinical significant PCa (24, 25). Those pathways are likely to further improve if combined with deep learning-based analyses of imaging features, such as apparent diffusion coefficient (ADC) maps (26).
Third, our multivariable analyses confirmed age as an independent risk factor for CAPRA-S-based upgrade at RARP. Before and after propensity score matching, results were virtually identical: increasing age was associated with higher odds of CAPRA-S-based upgrade at RARP (matched OR 1.039, 95% 1.025–1.052; p < 0.001, Table 2). It is of note that several systematic biopsy cores were not statistically significant, further underlining the notion that certain senior patients are insufficiently sampled with current systematic biopsy strategies. Taken together, to mitigate greater susceptibility of misclassification rates in senior men and to account for tumor stage and potential extracapsular extension, mpMRI should be strongly considered at primary diagnosis if the patient has advanced age.
As we are in the middle of the COVID-19 pandemic, the trend of unfavorable characteristics of PCa will continue to develop, leading to a late diagnosis and a delayed treatment with histopathological signs of an advanced stage of cancer (27). We already observed a rise in the proportion of RARP patients with aggressive tumor characteristics in our daily practice. It is mandatory to at least evaluate to extend the indication of RARP to physically and cognitively fit senior patients that might be suitable for surgery (5, 28, 29).
It is important to note that a higher CAPRA-S risk profile, which denotes unfavorable pathology, is associated with a higher chance of adjuvant or salvage treatment. However, recent population-based data revealed that Post-prostatectomy treatment should be carefully evaluated in very senior men due to greater competing other-cause mortality rates compared to men, who were just treated with prostatectomy (30).
Due to our observed misclassification rates, particularly in senior men, it is necessary to not only rely on conventional clinico-pathological parameters but also account for any available preoperative staging information and host characteristics, such as age, cardiovascular, and cognitive fitness. Latter are rarely integrated into widely utilized prediction tools.
Our study has limitations: first of all, all senior patients who received RARP were selected and described as “fit for surgery” after thorough anesthesiological examination with a focus on cardiovascular and mental fitness. However, no dedicated geriatric assessment tools were regularly utilized. Second, all patients were treated in a high-volume center by very experienced surgeons. Therefore, the generalizability of our study findings is limited. Third, our data were derived from a single center.
Despite the matching, there are still important caveats. CAPRA and CAPRA-S do not account for highly variable differences of tumor burden and Gleason quantification that might occur within the same risk-strata. For the same reason, it is of note that CAPRA relies on the same clinical variables (PSA, biopsy Gleason grade, and clinical tumor stage) as the widely utilized D'Amico classification, and additionally includes age at diagnosis and percent of biopsy cores involved with cancer as a metric of cancer burden. These variables represent essential variables for multivariable predictions of misclassification (31). Thus, we relied on CAPRA to allow for a more precise risk stratification than the D'Amico classification system.
Moreover, as some series strongly indicate, there are potentially very distinct molecular patterns underlying PCa that result in an upgrading of the tumor, such as de novo development of high-grade tumors after biopsy, spatial heterogeneity from the same tumor focus, or clonal progression from low- to high-grade cancer (32–35). Moreover, a certain lead time is highly conceivable in senior PCa patients with regard to the untreated natural history of PCa. Finally, ultrasound quality might deteriorate due to a greater probability of hyperechogenic artifacts, such as calcifications, subsequently decreasing systematic biopsy performance.
After a thorough multivariable adjustment, we observed that each 4.5th senior patient has an upgrade in his pathology and virtually every second senior patient has a misclassification in (i.e., any up or downgrade) that directly translates to PCa prognosis. Bearing in mind that select senior patients might greatly benefit from an adequate therapy, such as RARP, it is imperative to take such substantial misclassification rates into account for this PCa demographic, which will be continuously increasing and more relevant in the years to come. Future prospective studies are warranted to further optimize PCa workflow and diagnostics, utilizing tools, such as modern imaging, molecular profiling, and implementing them into new biopsy strategies to identify true PCa burden.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board at the St. Antonius-Hospital, Gronau, approved the retrospective study design and access to the patients' medical records. All methods were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from individual participants in the study. The patients/participants provided their written informed consent to participate in this study.
S-RL-B and NL had full access to all the data in the study, takes responsibility for the integrity of the data, the accuracy of the data analysis, participated in the study concept, and design. NL, S-RL-B, PR, and JW performed the acquisition of data. S-RL-B performed analysis, interpretation of data, and statistical analysis. NL participated in the drafting of the manuscript. S-RL-B and JW participated in the critical revision of the manuscript for important intellectual content, and done supervision. Administrative, technical, or material support was given by PR. All authors contributed to the article and approved the submitted version.
JW is a paid proctor and consultant for Intuitive Surgical and Board member of the German Society of Robot-Assisted Urology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CAPRA, Cancer of the Prostate Risk Assessment score; CAPRA-S, postsurgical Cancer of the Prostate Risk Assessment score; COVID-19, Coronavirus Disease 2019; HR, high risk; IR, intermediate risk; ISUP, International Society of Urological Pathology; IQR, interquartile range; LR, low risk; LRM, logistic regression model; MRI, magnetic resonance imaging; PCa, prostate cancer; PSA, prostate-specific antigen; QoL, quality of life; RARP, robot-assisted radical prostatectomy; yrs., years.
1. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. (2017) 389:1323–35. doi: 10.1016/S0140-6736(16)32381-9
2. Leyh-Bannurah SR, Karakiewicz PI, Pompe RS, Preisser F, Zaffuto E, Dell'Oglio P, et al. Inverse stage migration patterns in North American patients undergoing local prostate cancer treatment: a contemporary population-based update in light of the 2012 USPSTF recommendations. World J Urol. (2019) 37:469–79. doi: 10.1007/s00345-018-2396-2
3. Traboulsi SL, Nguyen DD, Zakaria AS, Law KW, Shahine H, Meskawi M, et al. Functional and perioperative outcomes in elderly men after robotic-assisted radical prostatectomy for prostate cancer. World J Urol. (2020) 38:2791–8. doi: 10.1007/s00345-020-03096-0
4. Aas K, Dorothea Fosså S, Åge Myklebust T, Møller B, Kvåle R, Vlatkovic L, et al. Increased curative treatment is associated with decreased prostate cancer-specific and overall mortality in senior adults with high-risk prostate cancer; results from a national registry-based cohort study. Cancer Med. (2020) 9:6646–57. doi: 10.1002/cam4.3297
5. Lu-Yao GL, Albertsen PC, Moore DF, Lin Y, Dipaola RS, Yao SL. Fifteen-year outcomes following conservative management among men aged 65 years or older with localized prostate cancer. Eur Urol. (2015) 68:805–11. doi: 10.1016/j.eururo.2015.03.021
6. Mandel P, Graefen M, Michl U, Huland H, Tilki D. The effect of age on functional outcomes after radical prostatectomy. Urol Oncol. (2015) 33:203.e11–18. doi: 10.1016/j.urolonc.2015.01.015
7. Zhou X, Qiu S, Jin K, Yuan Q, Jin D, Zhang Z, et al. Predicting cancer-specific survival among patients with prostate cancer after radical prostatectomy based on the competing risk model: population-based study. Front Surg. (2021) 8:770169. doi: 10.3389/fsurg.2021.770169
8. Hu JC, Gu X, Lipsitz SR, Barry MJ, D'Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive vs. open radical prostatectomy. JAMA. (2009) 302:1557–64. doi: 10.1001/jama.2009.1451
9. Yamada Y, Teshima T, Fujimura T, Sato Y, Nakamura M, Niimi A, et al. Comparison of perioperative outcomes in elderly (age ≥ 75 years) vs. younger men undergoing robot-assisted radical prostatectomy. PLoS One. (2020) 15: e0234113. doi: 10.1371/journal.pone.0234113
10. Graefen M, Schlomm T. Is radical prostatectomy a useful therapeutic option for high-risk prostate cancer in older men? Oncologist. (2012) 17 (Suppl 1):4–8. doi: 10.1634/theoncologist.2012-S1-04
11. Sanchez-Salas R, Prapotnich D, Rozet F, Mombet A, Cathala N, Barret E, et al. Laparoscopic radical prostatectomy is feasible and effective in “fit” senior men with localized prostate cancer. BJU Int. (2010) 106:1530–6. doi: 10.1111/j.1464-410X.2010.09295.x
12. Bratt O, Folkvaljon Y, Eriksson MH, Akre O, Carlsson S, Drevin L, et al. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur Urol. (2015) 68:53–8. doi: 10.1016/j.eururo.2014.12.026
13. Guo XX, Xia HR, Hou HM, Liu M, Wang JY. Comparison of oncological outcomes between radical prostatectomy and radiotherapy by type of radiotherapy in elderly prostate cancer patients. Front Oncol. (2021) 11: 708373. doi: 10.3389/fonc.2021.708373
14. Droz JP, Albrand G, Gillessen S, Hughes S, Mottet N, Oudard S, et al. Management of prostate cancer in elderly patients: recommendations of a task force of the international society of geriatric oncology. Eur Urol. (2017) 72:521–31. doi: 10.1016/j.eururo.2016.12.025
15. Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, Duchane J, et al. The UCSF cancer of the prostate risk assessment (CAPRA) score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. (2005) 173:1938. doi: 10.1097/01.ju.0000158155.33890.e7
16. Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. (2011) 117:5039. doi: 10.1002/cncr.26169
17. Carbin DD, Tamhankar AS, Ahluwalia P, Gautam G. Robot-assisted radical prostatectomy in Indian men of age 75 years and above: a propensity score-matched analysis. J Robot Surg. (2021) doi: 10.1007/s11701-021-01301-9
18. Ryu JH, Kim SJ, Kim YB, Jung TY, Ko WJ, Kim S Il, et al. Radical prostatectomy for clinically localized prostate cancer in patients aged 75 years or older: comparison with primary androgen deprivation therapy. Aging Male. (2018) 21:17–23. doi: 10.1080/13685538.2017.1365122
19. Leyh-Bannurah S-R, Wagner C, Schuette A, Addali M, Liakos N, Urbanova K, et al. The impact of age on pathological insignificant prostate cancer rates in contemporary robot-assisted prostatectomy patients despite active surveillance eligibility. Minerva Urol Nephrol. (2021) doi: 10.23736/S2724-6051.21.04174-4. [Epub ahead of print].
20. Leyh-Bannurah SR, Karakiewicz PI, Dell'Oglio P, Briganti A, Schiffmann J, Pompe RS, et al. Comparison of 11 active surveillance protocols in contemporary european men treated with radical prostatectomy. Clin Genitourin Cancer. (2017) 16:e141–9. doi: 10.1016/j.clgc.2017.08.005
21. Leyh-Bannurah S-R, Dell'oglio P, Zaffuto E, Briganti A, Schiffmann J, Pompe RS, et al. Assessment of oncological outcomes after radical prostatectomy according to preoperative and postoperative cancer of the prostate risk assessment scores: results from a large, two-center experience. (2019) 5:568–576. doi: 10.1016/j.euf.2017.10.015
22. Leyh-Bannurah S-R, Wagner C, Schütte A, Liakos N, Karagiotis T, Mendrek M, et al. Feasibility of robot-assisted radial prostatectomy in men at senior age ≥75 years: perioperative, functional and oncological outcomes of a high-volume center. Aging Male. (2021) 25:8–16 doi: 10.1080/13685538.2021.2018417
23. Reisz PA, Laviana AA, Zhao Z, Huang LC, Koyama T, Conwill R, et al. Assessing the quality of surgical care for clinically localized prostate cancer: results from the CEASAR study. J Urol. (2020) 204:1236–41. doi: 10.1097/JU.0000000000001198
24. Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. (2021) 22:1240–9. doi: 10.1016/S1470-2045(21)00348-X
25. Moore CM. An important step towards smarter screening for prostate cancer. Lancet Oncol. (2021) 22:1201–2. doi: 10.1016/S1470-2045(21)00449-6
26. Xie J, Li B, Min X, Zhang P, Fan C, Li Q, et al. Prediction of pathological upgrading at radical prostatectomy in prostate cancer eligible for active surveillance: a texture features and machine learning-based analysis of apparent diffusion coefficient maps. Front Oncol. (2021) 10:604266. doi: 10.3389/fonc.2020.604266
27. Fallara G, Sandin F, Styrke J, Carlsson S, Lissbrant IF, Ahlgren J, et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand J Urol. (2021) 55:184–91. doi: 10.1080/21681805.2021.1910341
28. Hoeh B, Preisser F, Mandel P, Wenzel M, Humke C, Welte MN, et al. Inverse stage migration in radical prostatectomy-a sustaining phenomenon. Front Surg. (2021) 8:612813. doi: 10.3389/fsurg.2021.612813
29. Rider JR, Sandin F, Andrén O, Wiklund P, Hugosson J, Stattin P. Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study. Eur Urol. (2013) 63:88–96. doi: 10.1016/j.eururo.2012.08.001
30. Wenzel M, Würnschimmel C, Chierigo F, Tian Z, Shariat SF, Terrone C, et al. Non-cancer mortality in elderly prostate cancer patients treated with combination of radical prostatectomy and external beam radiation therapy. Prostate. (2021) 81:728–35. doi: 10.1002/pros.24169
31. Wenzel M, Würnschimmel C, Chierigo F, Flammia RS, Tian Z, Shariat SF, et al. Nomogram predicting downgrading in national comprehensive cancer network high-risk prostate cancer patients treated with radical prostatectomy. Eur Urol Focus. (2021). doi: 10.1016/j.euf.2021.07.008. [Epub ahead of print].
32. Salami SS, Tosoian JJ, Nallandhighal S, Jones TA, Brockman S, Elkhoury F, et al. Platinum priority-prostate cancer serial molecular profiling of low-grade prostate cancer to assess tumor upgrading: a longitudinal cohort study. Eur Urol. (2021) 79:456–65. doi: 10.1016/j.eururo.2020.06.041
33. Epstein JI, Walsh PC, Carter HB. Dedifferentiation of prostate cancer grade with time in men followed expectantly for stage T1c disease. J Urol. (2001) 166:1688–91. doi: 10.1016/S0022-5347(05)65654-6
34. Sowalsky AG, Ye H, Bubley GJ, Balk SP. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. (2013) 73:1050–5. doi: 10.1158/0008-5472.CAN-12-2799
Keywords: frailty, elderly patients, age factors, prostate cancer, upgrading, RARP, propensity score matching
Citation: Liakos N, Witt JH, Rachubinski P and Leyh-Bannurah S-R (2022) The Dilemma of Misclassification Rates in Senior Patients With Prostate Cancer, Who Were Treated With Robot-Assisted Radical Prostatectomy: Implications for Patient Counseling and Diagnostics. Front. Surg. 9:838477. doi: 10.3389/fsurg.2022.838477
Received: 17 December 2021; Accepted: 17 January 2022;
Published: 16 February 2022.
Edited by:
Clemens Mathias Rosenbaum, Asklepios Klinik Barmbek, GermanyReviewed by:
Giovanni Lughezzani, Humanitas Research Hospital, ItalyCopyright © 2022 Liakos, Witt, Rachubinski and Leyh-Bannurah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaos Liakos, bmxpYWtvc0BtZS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.