- 1Department of Anesthesiology, Plastic Surgery Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
- 2Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
Background: Dexmedetomidine (DEX), a highly selective α2-adrenergic receptor agonist, is now widely used in procedural sedation and analgesia. This study was designed to observe and compare the efficacy and safety of DEX administered in two different modes.
Methods: In total, 100 patients were randomly divided into two groups to receive intravenous DEX 1 µg/kg over 15 min followed by 0.4–0.7 µg/kg/h infusion or DEX 1 µg/kg over 30 min followed by 0.4–0.7 µg/kg/h infusion. Heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), bispectral index (BIS), Ramsay Sedation Scores (RSS scores), the lowest respiratory rates (LRR), incidences of respiratory adverse events and frequencies of body movements were recorded. Recovery time, recall of intraoperative events, pain scores in PACU and satisfaction of patients and surgeons were assessed.
Results: The BIS at time points from 5 min after anesthesia to the end of surgery in the intervention group were significantly higher (p < 0.05). The RSS scores at time points from 5 min after anesthesia to immediately after induction with DEX were significantly higher in the intervention group (p < 0.05). The HR at time points from the beginning of surgery to 30 min after local anesthesia, the MAP at time points from 30 min after local anesthesia to the end of surgery, and the RR at time points from 5 min after anesthesia to the end of surgery were significantly higher in the intervention group (p < 0.05). Patients in the intervention group had higher LRR, lower incidences of respiratory adverse events, and shorter recovery time (p < 0.05).
Conclusions: Dexmedetomidine infused with a loading dose over 30 min had less impact on patients’ hemodynamics and respiration and could shorten the recovery time after anesthesia in procedural sedation and analgesia.
Clinical Trial Registration: ClinicalTrials.gov, identifier: ChiCTR1900027958.
Introduction
Dexmedetomidine (DEX) has been used extensively in various superficial surgical procedures and sedation radiographic tests as it induces sedation, analgesia and anxiolysis without significant respiratory depression (1). DEX is currently administered as a loading dose over 10–15 min to rapidly achieve a certain blood concentration, followed by a continuous infusion (2). Due to the limited strength of its sedative and analgesic effects, DEX is often administered to patients with other analgesics or narcotic drugs in sedative and analgesic anesthesia. When used in combination, DEX can improve the anesthetic effect, but may also act to increase the risk of intraoperative respiratory depression (3). Combination of DEX with remifentanil can induce cardiovascular complications such as a lower heart rate (HR) (4).
Previous research reported that omitting the loading dose of DEX avoided hemodynamic side effects without compromising sedation and analgesia (5). No investigation has yet to evaluate whether the prolonged loading dose time of DEX would produce comparable anesthetic effect and decrease the incidences of side effects. With an emphasis on the quality of anesthesia, we designed this study to determine whether the difference in loading dose infusion time would produce different efficacy and safety in procedural sedation and analgesia anesthesia during plastic surgeries.
Materials and Methods
Patients
This randomized controlled trial was approved by Ethic Committee of Plastic Surgery Hospital, Chinese Academy of Medical Science (2016–12) and registered in the Chinese clinical trial registry (ChiCTR1900027958). A total of 100 patients from December of 2019 to December of 2020, American Society of Anesthesiologists (ASA) physical status class I or II, aged 18–60 years, and scheduled for elective plastic surgery under procedural sedation and analgesia were enrolled in this trial. The patients were allocated randomly to one of the two groups (n = 50) using a computer-generated list of random numbers. Exclusion criteria were: bradycardia (HR < 50 beat/min), hypotension (Mean arterial pressure [MAP] <60 mmHg), severe disease (heart, pulmonary, hepatic or renal), obesity (Body mass index [BMI] ≥30), obstructive sleep apnea/hypopnea syndrome, and known allergy to DEX.
Monitoring Indicators
No premedication was given to any of the patients. After being taken to the operating room, standard monitoring included electrocardiogram (ECG), noninvasive blood pressure (BP), HR and oxyhemoglobin saturation (SpO2) (Datex-Ohmeda Division, Instrumentarium Corp., Helsinki, Finland). Respiratory rate (RR) was monitored by a nasal end-tidal CO2 cannula. The bispectral index (BIS) was also continuously monitored (BIS Vista, Covidien, Boulder, CO, USA). A peripheral intravenous (IV) catheter was inserted by nurse.
Anesthesia Management
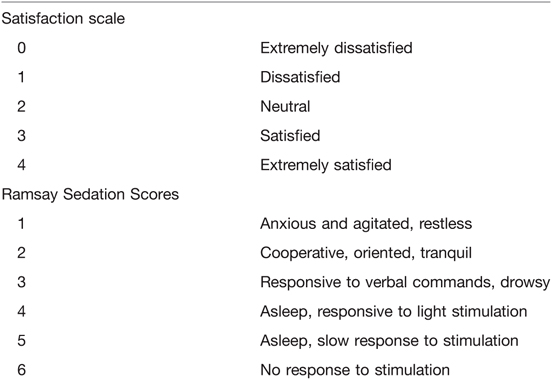
All patients received an IV of midazolam (0.04 mg/kg) and a continuous infusion of remifentanil (0.1 µg/kg/min) at the beginning of anesthesia. Patients in the control group received IV DEX 1 µg/kg over 15 min followed by 0.4–0.7 µg/kg/h infusion and patients in the intervention group received IV DEX 1 µg/kg over 30 min followed by 0.4–0.7 µg/kg/h infusion. During the surgery, the level of sedation status was assessed using the Ramsay Sedation Scores (RSS) (6) (Table 1) and the BIS. The goal of sedation was to achieve an RSS of 3–5 or BIS of 60–80. Therefore, local anesthesia was performed. The patients’ MAP and HR were maintained at a range of baseline ±20%. Atropine (0.3 mg) and ephedrine (6 mg) were intravenously administered for bradycardia (HR < 50 beats/min for >60 s) and hypotension (MAP < 60 mmHg for >60 s), respectively. A facial oxygen mask (6 L/min) was used for hypoxia (SpO2 < 90%). If airway obstruction occurred, the patient was treated by thrusting the jaw. Midazolam (2 mg) was administered as a rescue drug when the infusion rate of DEX was increased to a maximum of 0.7 µg/kg/h and the depth of sedation was not achieved. The infusion of DEX was stopped 30 min before the end of surgery, and the infusion of remifentanil was turned off at the end of the surgery.
Outcome measurements
HR, MAP, RR, BIS, RSS were evaluated and recorded before anesthesia (baseline) (T0), 5 min after anesthesia (T1), 10 min after anesthesia (T2), immediately after induction with DEX (T3), the beginning of local anesthesia (T4), the beginning of surgery (T5), 30 min after local anesthesia (T6), 60 min after local anesthesia (T7), immediately after turning off DEX infusion (T8), and the end of surgery (T9). The lowest respiratory rates (LRR), incidences of respiratory depression (RR < 10 breaths/min), incidences of oxygen supplementation by facial mask and thrusting the jaw, frequencies of body movements, and additional rescue drug administrations were also recorded throughout the procedure. After surgery, recovery time, recall of intraoperative events, and pain scores using a numerical pain scale (0 = no pain to 10 = the worst pain) (7) in PACU were assessed. The satisfaction of patients and surgeons with the anesthesia were assessed using a 5-point numerical rating scale (8) (Table 1).
Statistical Analysis
All statistical analyses were performed using SPSS 23.0 (SPSSFW, SPSS Inc.). The normality of distribution was assessed with the Shapiro-Wilk test. Normally distributed data are presented as mean ± SD, and skewed data were presented as a median with an interquartile range [IQR]). Independent t-test was used to examine the difference of normally distributed continuous variables between groups. Skewed data were analyzed by Mann-Whitney U-test. Categorical data are presented by frequency and analyzed by chi-square test. A p value of <0.05 was considered statistically significant.
Results
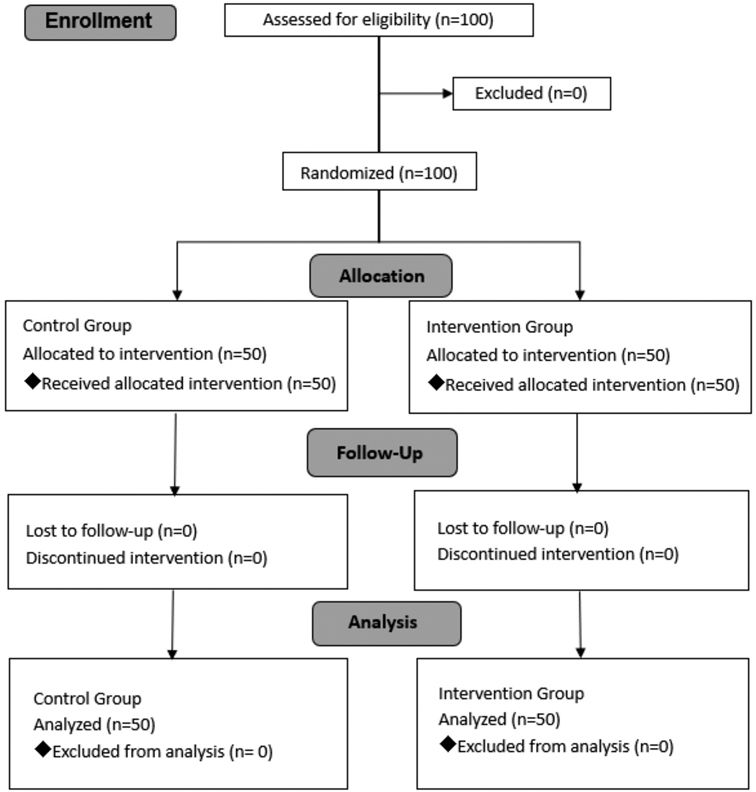
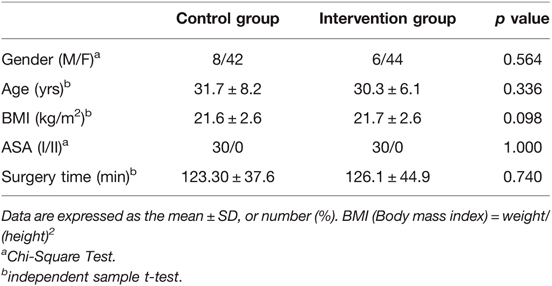
The procedure was successfully performed in each of the 100 patients recruited in the study (Figure 1). No significant differences were observed in patient demographic data including gender, age, ASA classification and BMI (Table 2). The surgery time did not differ between groups.
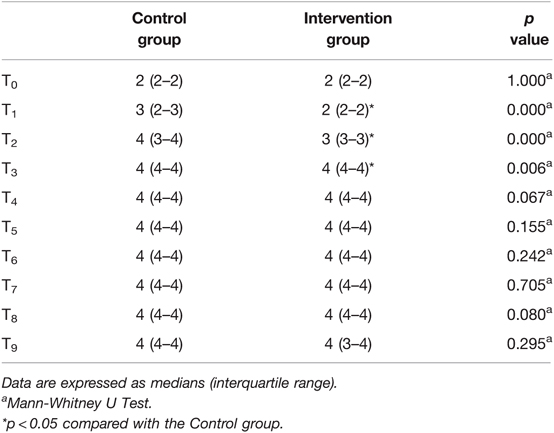
Figure 2A and Table 3 show the changes in sedation level during the study. Patients in the intervention group had higher BIS at time points from 5 min after anesthesia to the end of surgery (p < 0.05). Patients in the intervention group also had higher RSS scores compared with the control group at time points from 5 min after anesthesia to immediately after induction with DEX (p < 0.05).
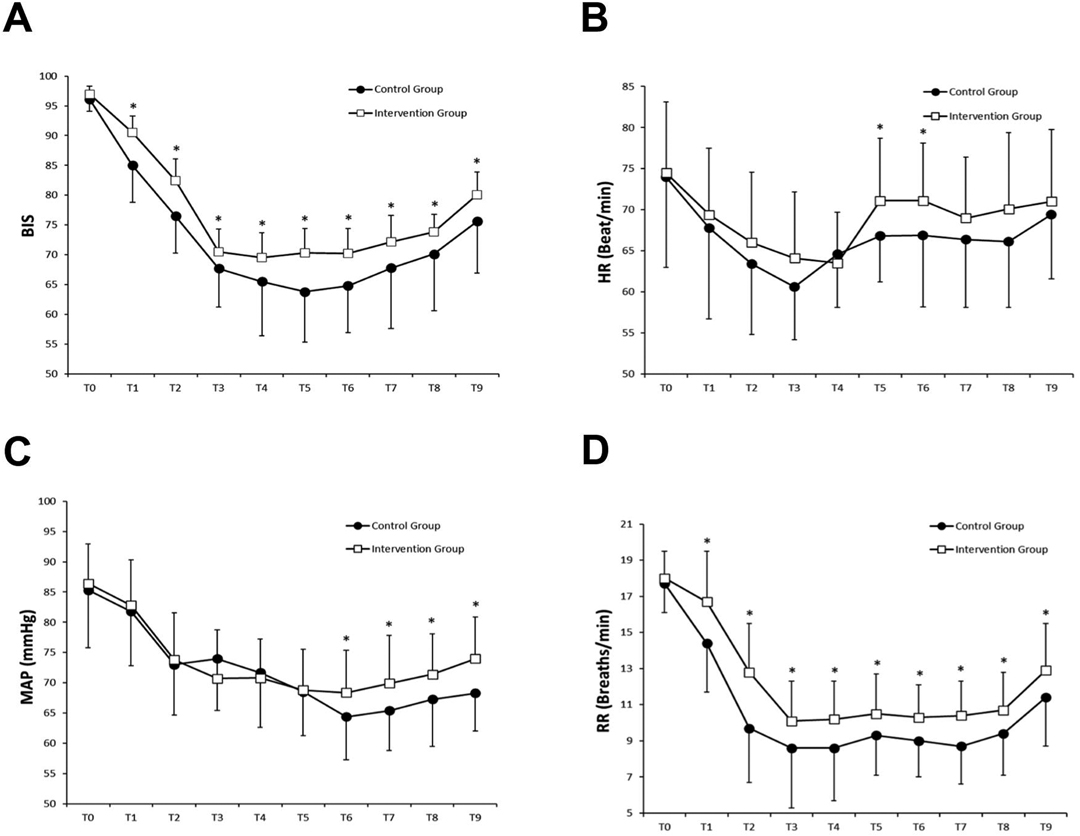
Figure 2. Changes in BIS (A), HR (B), MAP (C) and RR (D) were compared by the independent sample t-test for comparisons between the two groups. *p < 0.05 compared with the Control group.
Figure 2B–D displays the hemodynamic and respiratory parameters changes during the study. Patients in the intervention group had higher HR at time points from the beginning of surgery to 30 min after local anesthesia, higher MAP at time points from 30 min after local anesthesia to the end of surgery, and higher RR at time points from 5 min after anesthesia to the end of surgery (p < 0.05).
Compared with the control group, patients in the intervention group had higher LRR, lower incidences of respiratory depression, lower incidences of oxygen supplementation by facial mask and thrusting the jaw, and a shorter recovery time (p < 0.05) (Table 4).
No significant differences were found in body movements, additional rescue drug administrations, recall of intraoperative events, pain scores and the satisfaction levels of patients and surgeons between groups (p > 0.05) (Table 4).
Discussion
Due to its numerous advantages, DEX is one of the main anesthetics currently used for procedural sedation and analgesia. In this study, we found that although DEX infused with a loading dose over 15 min provided deeper sedation than DEX infused with a loading dose over 30 min, these two different DEX administrations could both be effectively applied in the surgery. In addition, DEX infused with a loading dose over 30 min resulted in more stable hemodynamics, less respiratory depression, and shorted recovery time for patients in procedural sedative and analgesic anesthesia.
In order to achieve satisfactory sedation and analgesia, DEX is often used clinically in combination with other anesthetic drugs such as midazolam and opioids (9–11). Midazolam reduces the incidence of recall of intraoperative events by producing anterograde amnesia (12). Studies have shown that DEX combined with midazolam provided better sedation than midazolam alone in patients undergoing fiberoptic bronchoscopy (13). The analgesic effect of DEX combined with opioids is beneficial in invasive operations. For example, a previous study demonstrated dexmedetomidine combined with remifentanil provided better analgesia and higher satisfaction levels amongst surgeons than midazolam combined with remifentanil in patients undergoing radiofrequency ablation for atrial fibrillation (8). In our study, DEX was administered with midazolam and remifentanil to produce sufficient sedation and analgesia for the surgery.
While enhancing the anesthetic effect, the combination of sedation and analgesic drugs may also increase the incidences of respiratory and circulatory depressions. Clinically, the safety of anesthesia can be improved by reducing the dose of drugs or adjusting the method of drug administration (14, 15). In our study, we extended the infusion time of DEX loading dose from 15 to 30 min to delay the increment of DEX plasma concentration and attenuate the DEX peak plasma concentration. As shown in the present study, patients in the intervention group had a more gradual BIS change after DEX infusion. However, no differences were found between the groups in RSS from the beginning of local anesthesia to the end of surgery, intraoperative body movements, additional rescue drug administrations, recall of intraoperative events, pain scores in PACU, or the satisfaction levels of patients and surgeons. Our results revealed that although the sedation depth in the intervention group was relatively shallow, this mode of DEX administration could achieve the sedation and analgesia required for the surgery. In a study of sedative and analgesic anesthesia, use of dexmedetomidine could significantly prolong postoperative recovery time (16). In this study, the DEX plasma concentration of the intervention group after the loading dose infusion was theoretically lower than that of the control group. We found no differences in the surgery time between groups which lead to a better early postoperative recovery for patients in the intervention group.
With respect to hemodynamic stability, Bloor et al. (17) reported that the amount of DEX loading dose and the duration of administration affected the blood pressure, with blunting of the blood pressure by 23% 60 min after infusion of 1 µg/kg DEX over 2 min. The remifentanil used in this study is a non-accumulative, ultra-short-acting opioid which allows faster recovery after anesthesia (18, 19). We know from earlier studies that both remifentanil and DEX reduce HR (20, 21). We observed that the intervention group had higher MAP at time points from 30 min after local anesthesia to the end of surgery and higher HR at time points from the beginning of surgery to 30 min after local anesthesia, which indicated that prolonged DEX loading dose time contributed to more stable hemodynamics.
DEX is seldom administered in clinical practice as a single agent for procedural sedation and analgesia. It is often administered in combination with analgesics. Though DEX provides sedation and analgesia without significant respiratory depression, respiratory changes needed to be measured in the case of drug combinations (22). The depth of sedation can significantly affect patients’ minute ventilation and RR (23). In the present study, we found both groups showed respiratory depression during anesthesia. However, the intervention group had higher RR throughout the study and also had higher LRR, lower incidences of respiratory depression, oxygen supplementation by facial mask and thrusting the jaw. We concluded that prolonged DEX loading dose time reduced adverse respiratory reactions.
Limitations
There are a few limitations to this study. The method of DEX administration in the control group is extensively applied in clinical practice. Though higher respiratory depression was observed in this group, the problem could be solved by oxygen supplementation with facial mask or thrusting the jaw. Before the study, we excluded patients with known obesity or obstructive sleep apnea/hypopnea syndrome. For patients with a predictable risk of respiratory depression, further research is needed to identify the safety of this method of DEX administration.
Conclusions
In conclusion, our study demonstrated that DEX is an excellent drug when combined with other narcotic drugs in sedative and analgesic anesthesia. Prolonging the DEX loading dose infusion time from 15 to 30 min can provide sufficient sedation during the surgery with more stable hemodynamics, as well as less respiratory adverse reactions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethic Committee of Plastic Surgery Hospital, Chinese Academy of Medical Science (2016–12). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WX and XD designed and conducted the study. WX and SW analyzed and interpreted the data and drafted the manuscript. JS, LW and JL were responsible for the collection of data. XD and DY revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the Special Research Fund for Plastic Surgery Hospital, Chinese Academy of Medical Science and Peking Union Medical College (No. Y2018002).
Acknowledgements
The authors thank the anesthetists and surgeons at our hospital who participated in this study. We would also like to acknowledge the professional assistance provided by Gilbert Xavier Gonzalez (Institute for Biomedical Sciences, Georgia State University, Atlanta, GA, USA) in reviewing and editing the English grammar in this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. (2010) 110:47–56. doi: 10.1213/ane.0b013e3181ae0856
2. Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother. (2007) 41:245–54. doi: 10.1345/aph.1H314
3. Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). (2001) 14:13–21. doi: 10.1080/08998280.2001.11927725
4. Kim N, Yoo YC, Lee SK, Kim H, Ju HM, Min KT. Comparison of the efficacy and safety of sedation between dexmedetomidine-remifentanil and propofol-remifentanil during endoscopic submucosal dissection. World J Gastroenterol. (2015) 21:3671–8. doi: 10.3748/wjg.v21.i12.3671
5. Ickeringill M, Shehabi Y, Adamson H, Ruettimann U. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: haemodynamic effects and efficacy. Anaesth Intensive Care. (2004) 32:741–5. doi: 10.1177/0310057X0403200602
6. Reschreiter H, Maiden M, Kapila A. Sedation practice in the intensive care unit: a UK national survey. Crit Care. (2008) 12:R152. doi: 10.1186/cc7141
7. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. (2001) 94:149–58. doi: 10.1016/S0304-3959(01)00349-9
8. Cho JS, Shim JK, Na S, Park I, Kwak YL. Improved sedation with dexmedetomidine–remifentanil compared with midazolam–remifentanil during catheter ablation of atrial fibrillation: a randomized, controlled trial. Europace. (2013) 16:1000–6. doi: 10.1093/europace/eut365
9. Heard C, Burrows F, Johnson K, Joshi P, Houck J, Lerman J. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. (2008) 107:1832–9. doi: 10.1213/ane.0b013e31818874ee
10. Nie Y, Liu Y, Luo Q, Huang S. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. (2014) 31:197–203. doi: 10.1097/EJA.0000000000000011
11. Hinkelbein J, Lamperti M, Akeson J, Santos J, Costa J, De Robertis E , et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. (2018) 35:6–24. doi: 10.1097/EJA.0000000000000683
12. Boku A, Inoue M, Hanamoto H, Oyamaguchi A, Kudo C, Sugimura M, et al. Effective dosage of midazolam to erase the memory of vascular pain during propofol administration. Anesth Prog. (2016) 63:147–55. doi: 10.2344/15-00034.1
13. Bergese SD, Bender SP, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. (2010) 22:35–40. doi: 10.1016/j.jclinane.2009.02.016
14. Ramaswamy SS, Parimala B. Comparative evaluation of two different loading doses of dexmedetomidine with midazolam-fentanyl for sedation in vitreoretinal surgery under peribulbar anaesthesia. Indian J Anaesth. (2016) 60:89–93. doi: 10.4103/0019-5049.176277
15. Jiang W, Wang Q, Xu M, Li Y, Yang R, Song X, et al. Assessment of different loading doses of dexmedetomidine hydrochloride in preventing adverse reaction after combined spinal-epidural anesthesia. Exp Ther Med. (2017) 13:2946–50. doi: 10.3892/etm.2017.4335
16. Tosun Z, Akin A, Guler G, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. (2006) 20:515–9. doi: 10.1053/j.jvca.2005.07.018
17. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. (1992) 77:1134–42. doi: 10.1097/00000542-199212000-00014
18. Won YJ, Yoo JY, Chae YJ, Kim DH, Park SK, Cho HB, et al. The incidence of postoperative nausea and vomiting after thyroidectomy using three anaesthetic techniques. J Int Med Res. (2011) 39:1834–42. doi: 10.1177/147323001103900526
19. Beecroft CL, Enright SM, O'beirne HA. Remifentanil in the management of severe tetanus. Br J Anaesth. (2004) 94:46–8. doi: 10.1093/bja/aeh288
20. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. (2000) 59:263–8. doi: 10.2165/00003495-200059020-00012
21. Reid JE, Mirakhur RK. Bradycardia after administration of remifentanil. Br J Anaesth. (2000) 84:422–3. doi: 10.1093/oxfordjournals.bja.a013460
22. Palanisamy A, Klickovich RJ, Ramsay M, Ouyang DW, Tsen LC. Intravenous dexmedetomidine as an adjunct for labor analgesia and cesarean delivery anesthesia in a parturient with a tethered spinal cord. Int J Obstet Anesth. (2009) 18:258–61. doi: 10.1016/j.ijoa.2008.10.002
23. Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. (2010) 55:258–64. doi: 10.1016/j.annemergmed.2009.07.030
Keywords: dexmedetomidine, procedural sedation and analgesia, loading dose, hemodynamics, respiration
Citation: Xia W, Wang S, Wei L, Deng X, Yang D, Sui J and Liu J (2022) Comparison of the Efficacy and Safety of Dexmedetomidine Administered in Two Different Modes Under Procedural Sedation and Analgesia in Plastic Surgery. Front. Surg. 9:836398. doi: 10.3389/fsurg.2022.836398
Received: 15 December 2021; Accepted: 12 April 2022;
Published: 2 May 2022.
Edited by:
Sergio Olate, University of La Frontera, ChileReviewed by:
Alessandro Vittori, Intensive Care and Operating Sections, Bambino Gesù Children's Hospital (IRCCS), ItalyZeming Liu, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, China
Copyright © 2022 Xia, Wang, Wei, Deng, Yang, Sui and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Deng ZGVuZ3hpYW9taW5nMjAwM0BzaW5hLmNvbQ==
Speciality section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
 Weipeng Xia
Weipeng Xia Shanshan Wang
Shanshan Wang Lingxin Wei1
Lingxin Wei1