- 1Department of Cardiovascular Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Key Laboratory of Ministry of Education for Gastrointestinal Cancer, The School of Basic Medical Sciences, Fujian Medical University, Fuzhou, China
- 3Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University, Fuzhou, China
- 4Department of anesthesiology, Xinyi People’s Hospital, Xuzhou, China
- 5Department of Thoracic Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 6Nursing Department, Fujian Medical University Union Hospital, Fuzhou, China
Background: Corticosteroids can effectively inhibit systemic inflammation induced by cardiopulmonary bypass. Recently clinical trials and meta-analyses and current guidelines for cardiac surgery do not support corticosteroids prophylaxis during cardiac surgery because of an increase in myocardial infarction and no benefit for patients. The aim of this study is to determine whether specific corticosteroids dose ranges might provide clinical benefits without increasing myocardial infarction.
Methods: The PubMed, Web of Science, Embase, Clinical Trials, and Cochrane databases were searched for randomized controlled trials (RCTs) published before August 1, 2021.
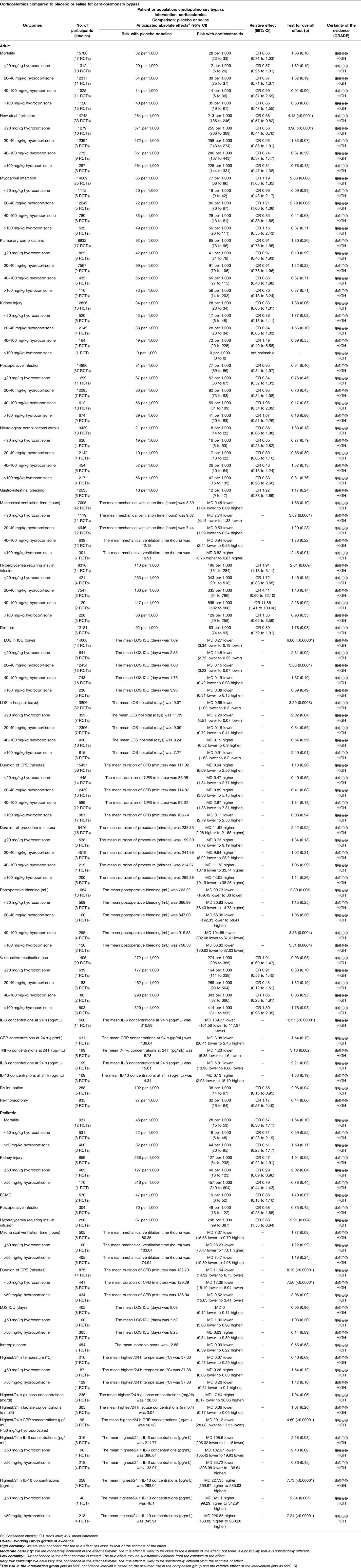
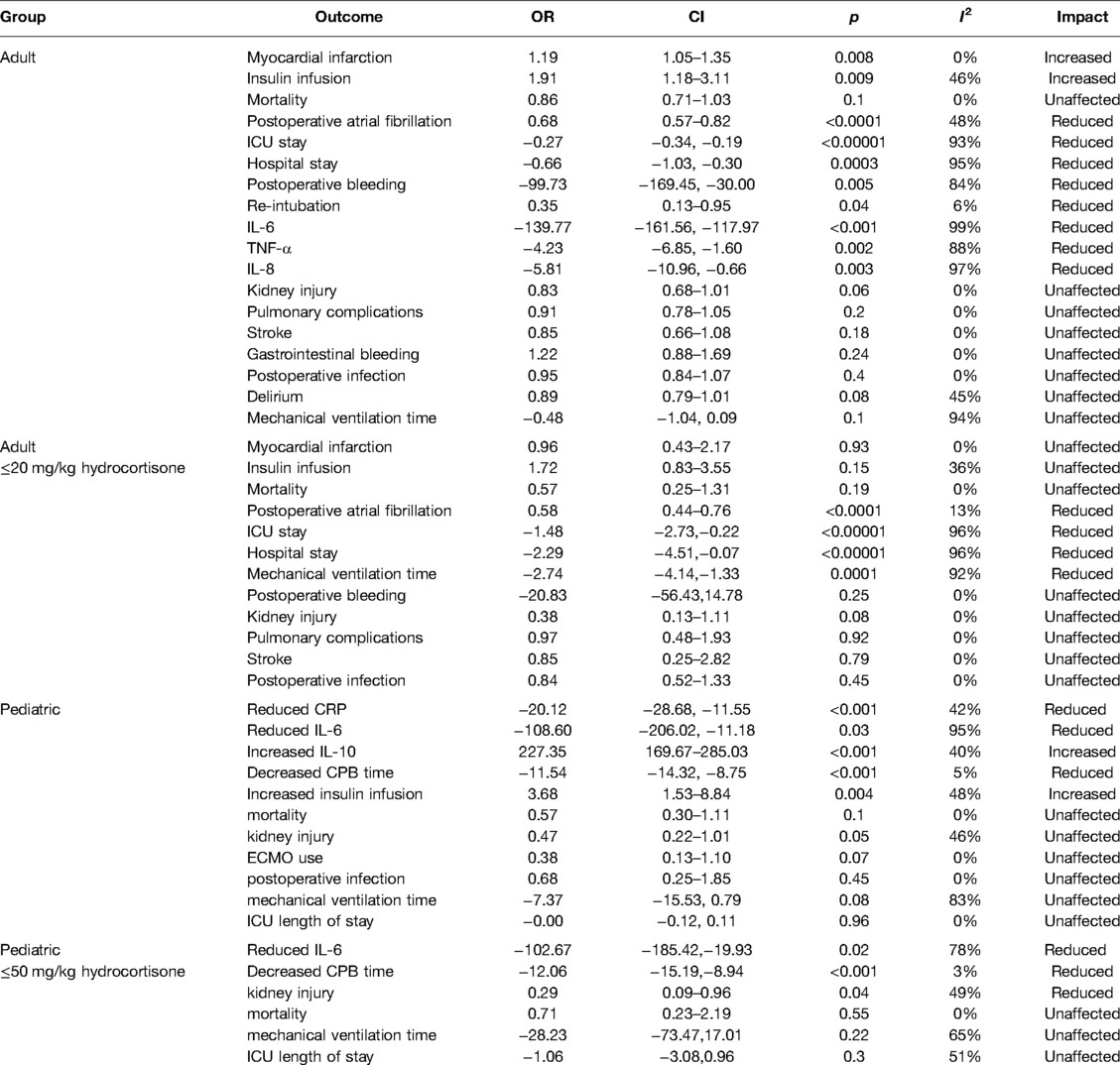
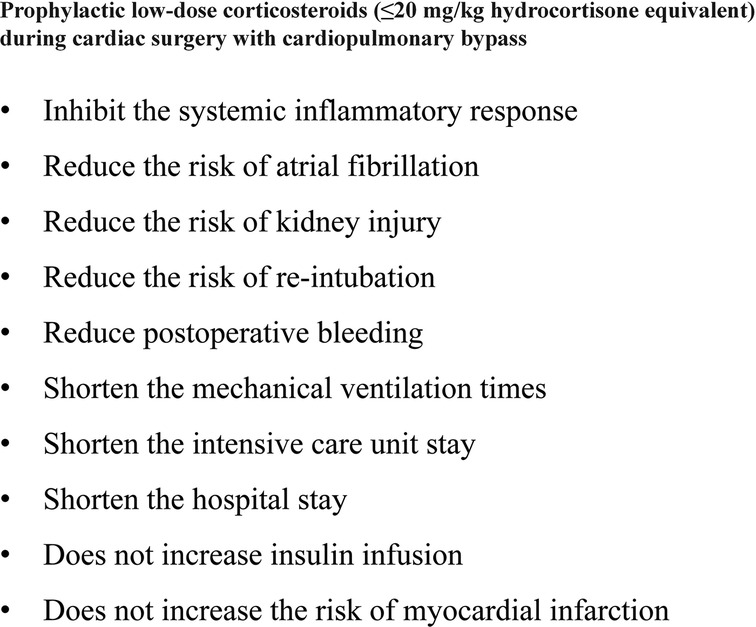
Results: 88 RCTs with 18,416 patients (17,067 adults and 1,349 children) were identified. Relative to placebo and high-dose corticosteroids, low-dose corticosteroids (≤20 mg/kg hydrocortisone) during adult cardiac surgery did not increase the risks of myocardial infarction (odds ratio [OR]: 0.96, 95% confidence interval [CI]: 0.43–2.17; p = 0.93). However, low-dose corticosteroids were associated with lower risks of atrial fibrillation (OR: 0.58, 95% CI: 0.44–0.76; p < 0.0001) and kidney injury (OR: 0.29, 95% CI: 0.09–0.96; p = 0.04). Furthermore, low-dose corticosteroids significantly shortened the mechanical ventilation times (mean difference [MD]: −2.74 h, 95% CI: −4.14, −1.33; p = 0.0001), intensive care unit (ICU) stay (MD: −1.48 days, 95% CI: −2.73, −0.22; p = 0.02), and hospital stay (MD: −2.29 days, 95% CI: −4.51, −0.07; p = 0.04).
Conclusion: Low-dose corticosteroids prophylaxis during cardiac surgery provided significant benefits for adult patients, without increasing the risks of myocardial infarction and other complications.
Introduction
Cardiopulmonary bypass (CPB) is used during most cardiac surgeries, although CPB often induces systemic inflammatory response syndrome (SIRS) (1). The development of SIRS involves activation of complement, platelets, neutrophils, monocytes, macrophages, and cascade reactions, which leads to increased endothelial permeability, blood vessel damage, and parenchymal cell damage (2–4). These events are associated with single and multiple organ dysfunction, myocardial injury and infarction, respiratory failure, and ultimately death (5–7).
Corticosteroids are inexpensive drugs that can effectively inhibit inflammation, limit systemic capillary leak syndrome, and reduce organ damage, which provides a theoretical basis for their use during CPB (8–10). However, corticosteroids can cause side effects, including hyperglycemia, which is associated with immunosuppression and poor wound healing (11, 12). In addition, high-dose corticosteroids are associated with an increased risk of gastrointestinal bleeding and myocardial infarction (11, 12). Thus, the benefits of corticosteroids treatment are controversial for patients undergoing cardiac surgery with CPB (13–15).
Three meta-analyses of small RCTs revealed that prophylactic corticosteroids could reduce the risk of atrial fibrillation after adult cardiac surgery, also caused some side effects (5–7). Two large multi-center RCTs subsequently revealed that corticosteroids therapy provided no benefits and increased the risk of myocardial infarction in adult patients (13, 14). Thus, the adult cardiac surgery guidelines do not recommend routine prophylactic use of corticosteroids during cardiac surgery (16), although there are no specific guidelines regarding corticosteroids use during pediatric cardiac surgery. We hypothesized that the specific corticosteroids dose range might influence the risks and benefits during cardiac surgery with CPB. Therefore, this systematic review and meta-analysis aimed to evaluate the dose-dependent benefits and risks of prophylactic corticosteroids for adults and children undergoing cardiac surgery with CPB.
Methods
Ethical Statement
This study was a meta-analysis of the results of published randomized controlled trials, and ethical approval and informed consent of patients were not required.
Search Strategy and Selection Criteria
Two authors (XJY and MYT) searched the PubMed, Web of Science, Embase, ClinicalTrials, and Cochrane Central Register of Controlled Trials databases for relevant RCTs that were published in any language before August 1, 2021. The reference lists of relevant articles were also manually checked. The study protocol followed the PRISMA-P guidelines (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020193658). The search terms were: (“corticosteroids” OR “dexamethasone” OR “prednisolone” OR “prednisone” OR “methylprednisolone” OR “hydrocortisone”) AND (“cardiopulmonary bypass” OR “cardiac surgery”) AND (“randomized controlled trials”) (Supplementary Table S1).
The meta-analysis only included RCTs that compared corticosteroids with a placebo used before or at the beginning of CPB. Patients undergoing surgery with CPB for heart and/or valvular diseases were included. And the studies were excluded if they used different concomitant medications or evaluated corticosteroids during off-pump heart surgery.
Two authors (TCC and XHZ) independently determined whether the identified articles fulfilled the inclusion criteria. The two authors also independently used pre-designed data extraction forms to record information regarding trial characteristics, clinical outcomes, randomization methods, application of blinding, allocation concealment, inclusion criteria, and exclusion criteria. There were no instances of disagreement regarding the extracted data.
Data Analysis
Study characteristics included first author, publication date, country, study size, study design, randomization, blinding, follow-up duration, patient withdrawals, and study duration. Patient characteristics included age, sex, surgery type, blood pressure, history of diabetes, history of smoking, renal status, and fulfillment of the inclusion criteria. The interventions included the corticosteroids type, dose, timing, and route of administration during CPB.
The primary outcomes included myocardial infarction, insulin use, mortality, new atrial fibrillation, lengths of ICU and hospital stays, acute kidney injury, mechanical ventilation time. The secondary outcomes included postoperative bleeding, re-intubation, duration of CPB and procedure, pulmonary complications (pulmonary edema), postoperative infection, neurological complications (stroke), delirium, gastrointestinal bleeding, extracorporeal membrane oxygenation (ECMO) use, vasoactive medication use, re-thoracotomy, inotropic score, blood transfusion, and and blood concentrations of glucose, lactate, C-reactive protein (CRP), tumor necrosis factor-α(TNF-α), interleukin (IL)-6, IL-8, and IL-10 at 24 h after CPB.
The Cochrane Handbook for Systematic Reviews of Interventions and Jadad score were used to assess the risk of bias for each trial (17, 18). The data were synthesized using Review Manager version 5.3 and Stata software version 16 (StataCorp, College Station, TX, USA). Inter-study heterogeneity was assessed using the chi-squared test and I2 statistic, with the random effects model used for data with high heterogeneity (p < 0.1 or I2 > 50%) and the fixed effects model used for data with less heterogeneity. The Mantel-Haenszel method was used to pool binary data and the results were reported as ORs with 95% CIs. An inverse variance analysis method was used to pool continuous data and the results were reported as MDs with 95% CIs. The GRADEpro GDT (https://www.gradepro.org/) was used to classify the certainty of evidence.
Results
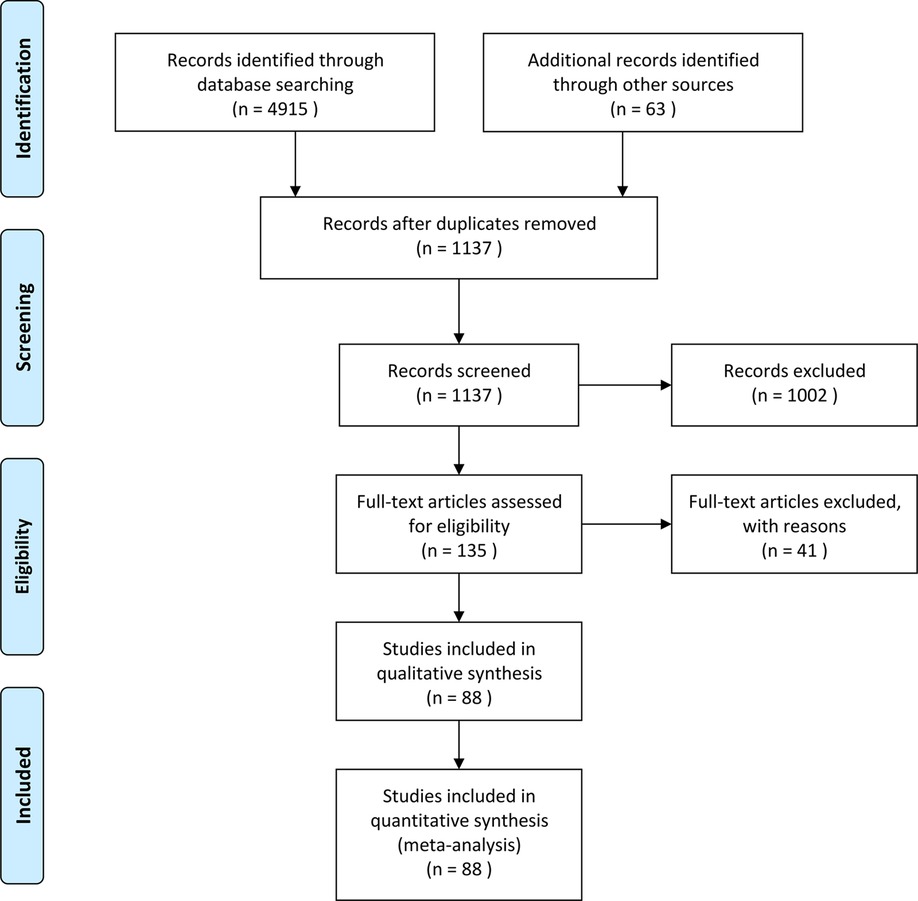
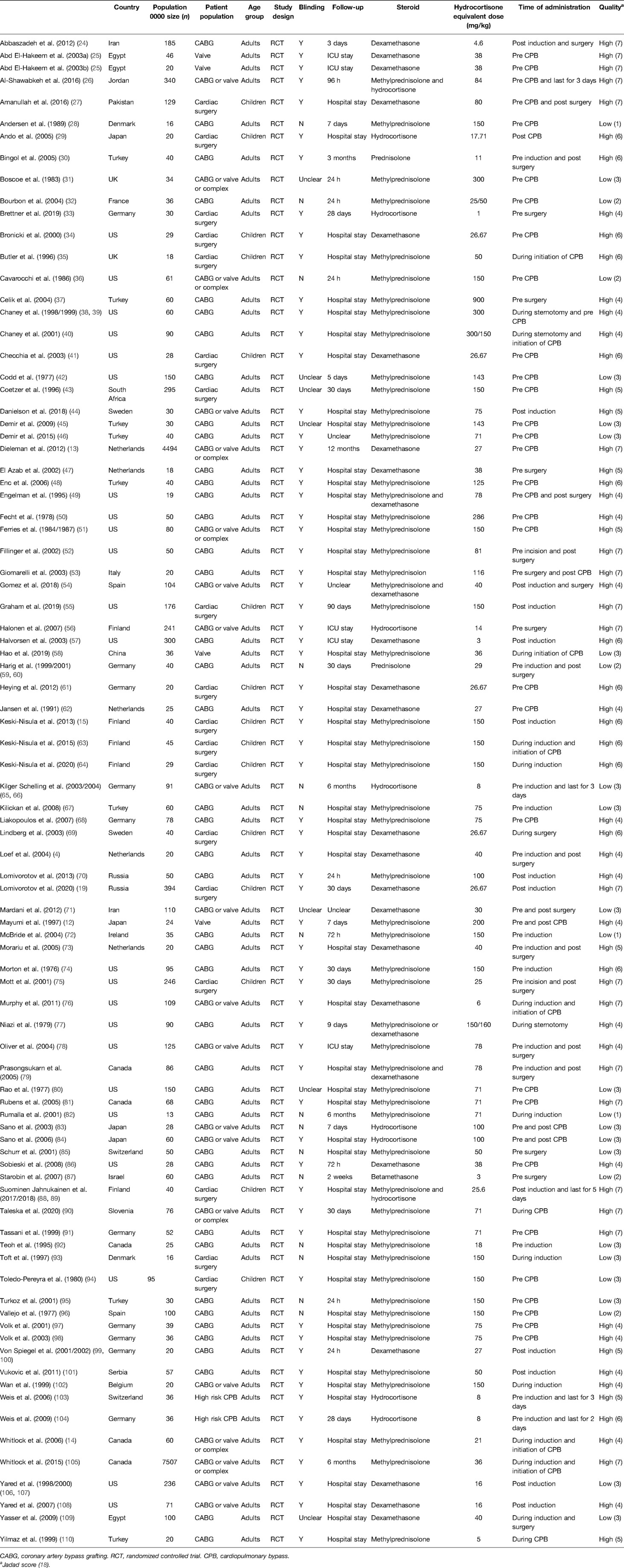
This study identified 88 RCTs from 27 countries that included 18,416 patients (Figure 1). The trials considered adult patients (73 trials and 17,067 patients) or pediatric patients (15 trials and 1,349 patients). The corticosteroids treatments included dexamethasone, betamethasone, methylprednisolone, hydrocortisone, and prednisolone, with a broad range of total doses (1–900 mg/kg hydrocortisone equivalent). Table 1 shows the characteristics of the included studies, Table 2 shows the GRADE summary of the findings and Table 3 summarizes the impact of corticosteroids on adults and pediatric.
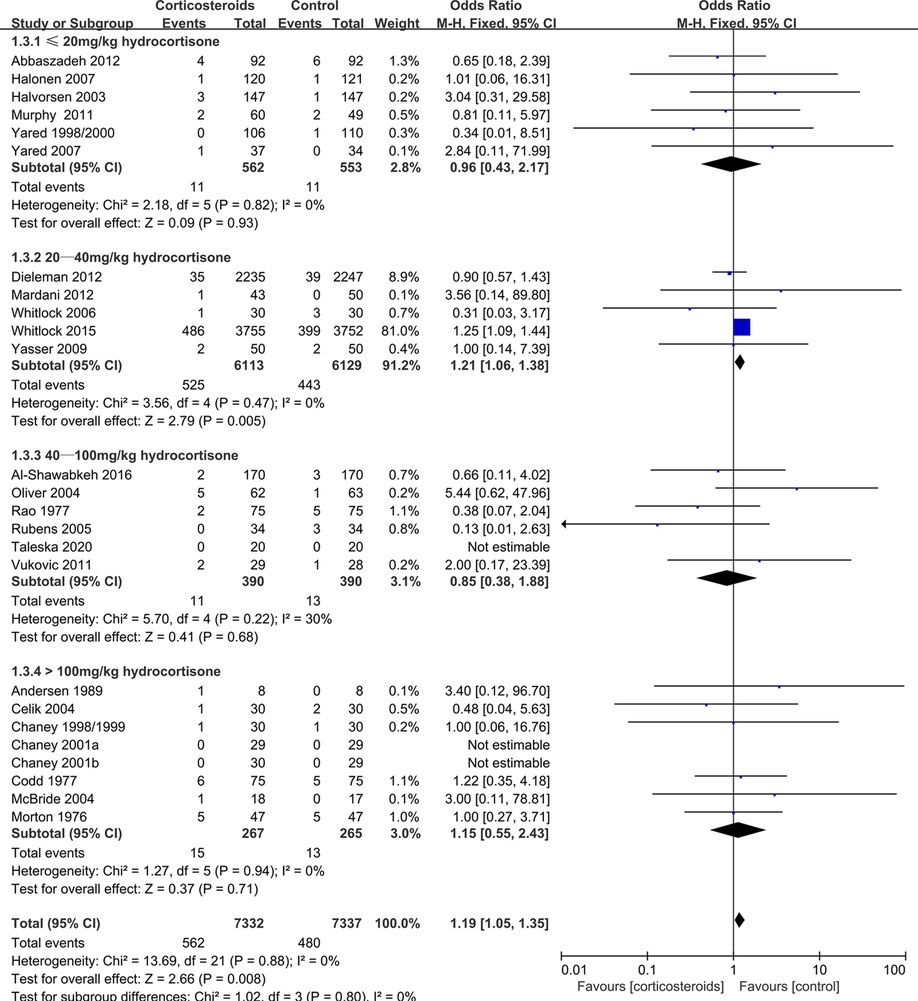
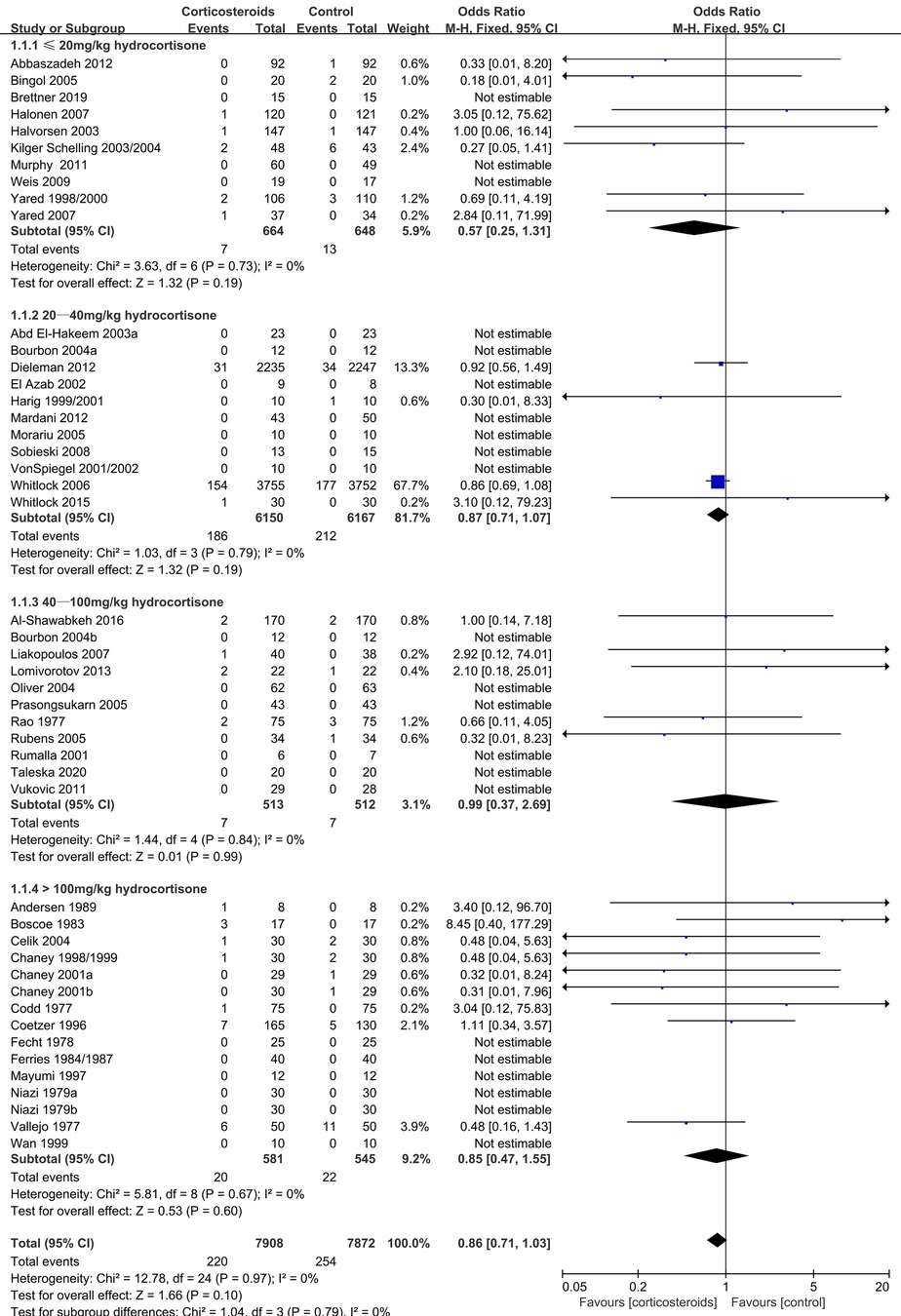
During adult cardiac surgery with CPB, corticosteroids prophylaxis was associated with increased risks of myocardial infarction (OR: 1.19, 95% CI: 1.05–1.35; p = 0.008, I2 = 0%) (Figure 2) and insulin infusion (OR: 1.91, 95% CI: 1.18–3.11; p = 0.009, I2 = 46%) (Supplementary Figure S1), with no obvious improvement in mortality (OR: 0.86, 95% CI: 0.71–1.03; p = 0.10, I2 = 0%) (Figure 3).
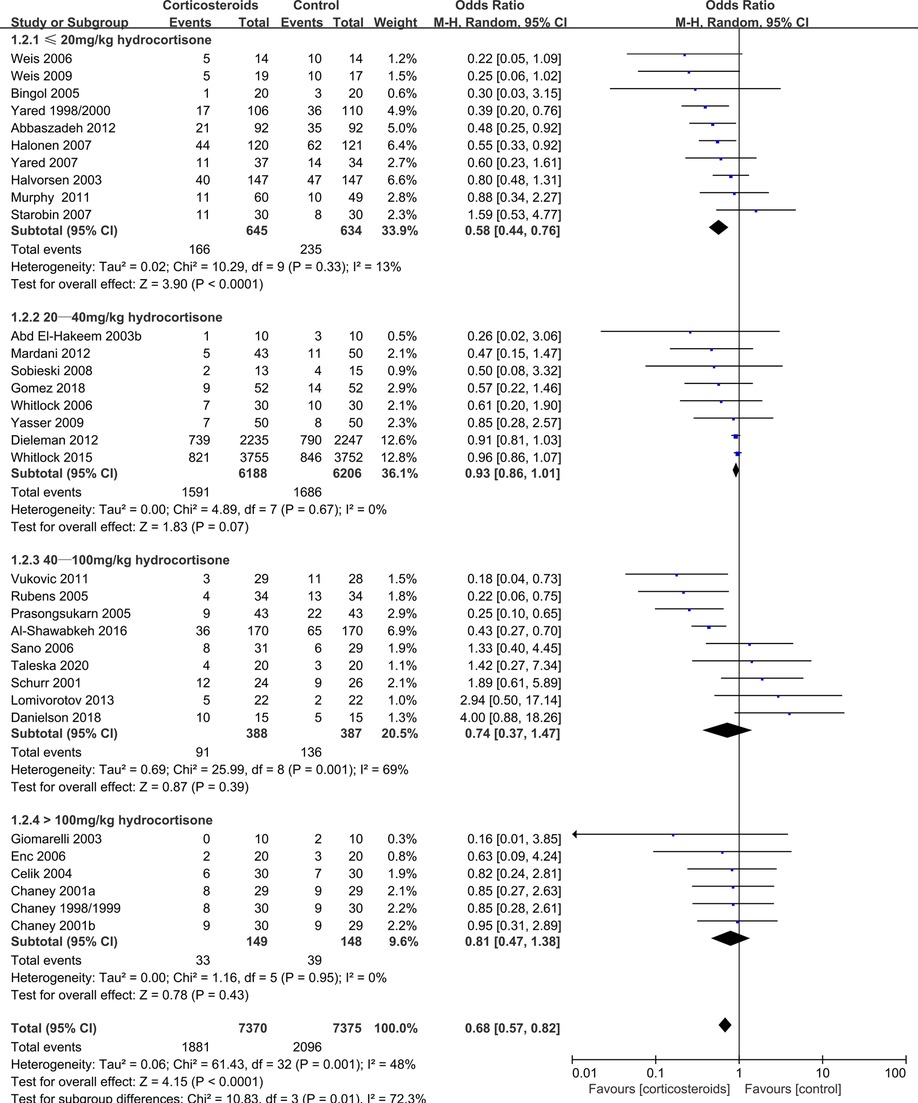
However, corticosteroids prophylaxis reduced the risk of postoperative atrial fibrillation (OR: 0.68, 95% CI: 0.57–0.82; p < 0.0001, I2 = 48%) (Figure 4), shortened the ICU stay (MD: −0.27 days, 95% CI: −0.34, −0.19 days; p < 0.001, I2 = 93%), and shortened the hospital stay (MD: −0.66 days, 95% CI: −1.03, −0.30 days; p = 0.0003, I2 = 95%) (Supplementary Figures S2, S3). In addition, corticosteroids prophylaxis was associated with reduced postoperative bleeding and a reduced risk of re-intubation (Table 3 and Supplementary Figures S4, S5).
Corticosteroids prophylaxis also reduced the blood concentrations of some inflammatory markers in adult patients, which included IL-6, TNF-α, and IL-8 (Table 3 and Supplementary Figures S6–S8). Among children, corticosteroids prophylaxis was associated with a significantly lower peak CRP concentration, a significantly lower IL-6 concentration, and a significantly higher IL-10 concentration (Table 3 and Supplementary Figures S9–S11).
Relative to the placebo group, corticosteroids prophylaxis was not associated with significant improvements in terms of kidney injury, pulmonary complications, stroke, gastrointestinal bleeding, postoperative infection or mechanical ventilation time (Table 3 and Figure 5, Supplementary Figures S12–S17).
Subgroup analysis that the benefits were largely attributable to the prophylactic use of low-dose corticosteroids (≤20 mg/kg hydrocortisone), and these benefits were not observed at higher corticosteroids doses (Table 2). Low-dose corticosteroids prophylaxis was associated with a significantly reduced mechanical ventilation time (MD: −2.74 h, 95% CI: −4.14, −1.33 h; p = 0.0001, I2 = 92%) (Figure 5), without increased risks of myocardial infarction (OR: 0.96, 95% CI: 0.43–2.17; p = 0.93, I2 = 0%) (Figure 2) or insulin infusion (OR: 1.72, 95% CI: 0.83–3.55; p = 0.15, I2 = 36%) (Supplementary Figure S7). Pooled analysis with meta-regression revealed that corticosteroids dose was significantly related to the variation in the mechanical ventilation time (exp: 1.004, 95% CI: 1.002–1.006; p < 0.0001), but not the variation in the other clinical outcomes (Supplementary Figures S18–S26). Funnel plots failed to reveal evidence of publication bias regarding mortality, myocardial infarction, pulmonary complications, kidney injury, postoperative infection, and neurological complications (stroke) (Supplementary Figures S27–S32). However, the funnel plots suggested that there might be some publication bias regarding new atrial fibrillation, mechanical ventilation time, and hyperglycemia requiring insulin infusion (Supplementary Figures S33–S35). Thus, we used the trim and fill method to adjust the analysis, which did not significantly alter the findings.
During pediatric cardiac surgery with CPB, corticosteroids prophylaxis was associated with a decreased CPB time (MD: −11.54 min, 95% CI: −14.32, −8.75 min; p < 0.001, I2 = 5%) and an increased insulin infusion (OR: 3.68, 95% CI: 1.53–8.84; p = 0.004, I2 = 48%), but did not significantly influence mortality, kidney injury, ECMO use, postoperative infection, mechanical ventilation time, and ICU length of stay [LOS] (Tables 2, 3 and Supplementary Figures S36–S43). Relative to placebo and higher dose corticosteroids (>50 mg/kg hydrocortisone), corticosteroids prophylaxis (≤50 mg/kg hydrocortisone) significantly reduced the risk of kidney injury (OR: 0.29, 95% CI: 0.09–0.96; p = 0.04, I2 = 49%) (Table 2 and Supplementary Figure S39). Meta-regression revealed that corticosteroids dose was not related to the variations in mortality (exp: 0.998, 95% CI: 0.981–1.015; p = 0.734) or the duration of CPB (exp: 1.000, 95% CI: 0.993–1.008; p = 0.89) (Supplementary Figures S44, S45). The funnel plots failed to reveal evidence of publication bias regarding mortality, kidney injury, postoperative infection, and ICU LOS (Supplementary Figures S46–S49). However, the funnel plots suggested that there might be some publication bias regarding mechanical ventilation time and CPB duration (Supplementary Figures S50–S51). Thus, we used the trim and fill method to adjust the analysis, which did not significantly alter the findings.
Discussion
This meta-analysis revealed that corticosteroids prophylaxis during cardiac surgery with CPB was associated with significantly decreased blood inflammatory factor concentrations of CRP, TNF-α, IL-6, and IL-8. During adult cardiac surgery, corticosteroids prophylaxis reduced the risks of postoperative atrial fibrillation and re-intubation, shortened the ICU and hospital LOSs, and reduced postoperative bleeding, although it was associated with increased risks of myocardial infarction and hyperglycemia requiring insulin infusion. Interestingly, the benefits among adult patients were largely attributable to low-dose corticosteroids use (≤20 mg/kg hydrocortisone), as the benefits were not observed among patients who received higher corticosteroids doses. In addition, low-dose corticosteroids significantly reduced the mechanical ventilation time without increasing the risks of myocardial infarction and insulin infusion, while high-dose corticosteroids were associated with increased risks of myocardial infarction and prolonged mechanical ventilation. During pediatric cardiac surgery, corticosteroids prophylaxis was associated with a shortened CPB time, an increased risk of insulin infusion, and no substantial changes in terms of mortality, ECMO use, postoperative infection, mechanical ventilation time, and ICU LOS. Moreover, corticosteroids prophylaxis (≤50 mg/kg hydrocortisone) significantly reduced the risk of kidney injury in pediatric patients.
The SIRS plays a vital role in the development of complications after cardiac surgery with CPB (1). Corticosteroids can effectively inhibit SIRS and reduce inflammatory factor concentrations, which provides a theoretical basis for prophylactic administration during cardiac surgery with CPB (2–10). However, several RCTs have indicated that corticosteroids prophylaxis did not provide significant benefits to patients undergoing cardiac surgery with CPB, and was instead associated with an increased risk of myocardial infarction and prolonged mechanical ventilation (10, 13, 14, 19). Thus, the adult cardiac surgery guidelines, as well as routine practice for adult and pediatric cardiac surgery with CPB, involve limited or no prophylactic corticosteroids (16). However, corticosteroids exert dose-dependent anti-inflammatory effects and clinical side effects (3, 4, 7). Thus, we hypothesized that an appropriate dosage range might effectively inhibit SIRS and provide clinical benefits without major side effects, as the optimal corticosteroids dose would protect cardiomyocytes rather than damage them.
Our results revealed that corticosteroids prophylaxis reduced the blood concentrations of various inflammatory markers after cardiac surgery, including CRP, TNF-α, IL-6, and IL-8. These findings support the prophylactic administration of corticosteroids to prevent SIRS after cardiac surgery with CPB (8–10). However, we did not detect any significant change in mortality, which is consistent with the results of previous studies (5–7, 13, 14, 19). This may be related to advanced cardiac surgery management and active treatment of complications in the current era.
The SIRS trial and Ho et al.’s meta-analysis of 50 small RCTs revealed that corticosteroids prophylaxis in adults significantly increased the risks of myocardial infarction and hyperglycemia requiring insulin infusion (6, 14). In this context, high doses of corticosteroids can rapidly and significantly induce insulin resistance, reduce cellular utilization of glucose, and cause hyperglycemia (20). Hyperglycemia downregulates glyoxalase 1 and glyoxalase 2, which inhibits the post-injury repair of cardiomyocytes (21). This may be the main mechanism through which high-dose corticosteroids induce myocardial infarction. We found that corticosteroids (>20 mg/kg hydrocortisone), but not low-dose corticosteroids, increased the risk of myocardial infarction and hyperglycemia requiring insulin infusion in adults. This may be because low-dose corticosteroids inhibit SIRS and protect cardiomyocytes, without substantially impairing glucose utilization. We did not observe a substantial change in this relationship when we re-analyzed data from 18 high-quality RCTs (Jadad score of ≥4, 18/25 trials), which all adopted the general definition of myocardial infarction and used cardiac biomarkers to predict its occurrence. In children, corticosteroids increased the use of insulin but did not significantly influence the risk of myocardial infarction, which may be related to neonatal cardiomyocytes having increased glucose uptake and utilization (22).
The DECS trial (13) and the SIRS trial (14) revealed that corticosteroids prophylaxis did not reduce the risk of atrial fibrillation in adult patients after cardiac surgery. However, meta-analyses by Ho et al. (6) and Ng et al. (7) revealed that corticosteroids prophylaxis could significantly reduce the incidence of atrial fibrillation. Ho et al. (6) reported that both low-dose and high-dose corticosteroids could significantly reduce the risk of atrial fibrillation. Ng et al.’s meta-analysis included the DECS and SIRS trials, but did not include a stratified dose analysis (7). Interestingly, we found that only low-dose corticosteroids (≤20 mg/kg hydrocortisone) were effective for reducing the risk of atrial fibrillation, with no positive effects observed at a slightly higher dose (20–40 mg/kg hydrocortisone), a high dose (40–100 mg/kg hydrocortisone), or an ultra-high dose (>100 mg/kg hydrocortisone). These findings were not noticeably different when we re-analyzed 27 high-quality RCTs (27/33 RCTs), with low heterogeneity and no detectable publication bias. Unfortunately, the relevant molecular mechanisms are not clear, although the SIRS can induce atrial fibrillation (23). Thus, we speculate that low-dose corticosteroids might inhibition SIRS without increasing cardiomyocyte damage, which would reduce the incidence of atrial fibrillation. In contrast, high-dose corticosteroids might reduce SIRS but increase cardiomyocyte damage, which would not reduce the risk of atrial fibrillation.
The SIRS can cause systemic multi-organ damage, which often involves kidney damage (5–7). Prophylactic administration of corticosteroids protects the tissues and organs by inhibiting SIRS and thus reduces complications (8–10). We failed to identify significant effects of corticosteroids prophylaxis on the risks of pulmonary complications, neurological complications (stroke), gastrointestinal bleeding, and delirium, which might be related to the low incidences of those outcomes. However, prophylactic corticosteroids (≤50 mg/kg hydrocortisone) significantly reduced the risk of kidney injury in pediatric patients, and low-dose corticosteroids (≤20 mg/kg hydrocortisone) might reduce the risk of kidney injury in adult patients. While corticosteroids suppress the normal immune response and may increase the risk of postoperative infection (7), our results and those from previous studies suggest that corticosteroids prophylaxis did not influence the risk of postoperative infection (6, 13, 14). Ho et al. (6) reported that corticosteroids prophylaxis was closely associated with prolonged mechanical ventilation, and we found that low-dose corticosteroids (≤20 mg/kg hydrocortisone) significantly shortened the mechanical ventilation duration for adult patients, while high doses (>100 mg/kg hydrocortisone) significantly prolonged the mechanical ventilation duration. We also found that low-dose corticosteroids significantly reduced the ICU and hospital LOSs for adult patients, which might be related to accelerated recovery that was caused by suppression of the SIRS and reduced tissue and organ damage. Therefore, corticosteroids may be a cost-effective prophylactic treatment (generally <$5/patient) that can help reduce the burden on patients and hospitals by decreasing the risks of complications and shortening the ICU and hospital LOSs. Furthermore, the lower risk of complications may improve patients’ perioperative quality of life.
Our meta-analysis considered the dose-dependent benefits and risks of prophylactic corticosteroids during adult and pediatric cardiac surgery based on 29 clinical outcomes. Our findings conflict with the lack of support for corticosteroids prophylaxis during cardiac surgery in previous studies (5–7, 13, 14) and the guidelines for adult cardiac surgery (16). Our results suggest that low-dose corticosteroids (≤20 mg/kg hydrocortisone) were not associated with a significant reduction in mortality, but might substantially benefit adult patients by inhibiting SIRS and reducing complications. Therefore, we recommend prophylactic administration of low-dose corticosteroids (≤20 mg/kg hydrocortisone) during adult cardiac surgery. However, the optimal dose range for corticosteroids prophylaxis during pediatric cardiac surgery is unclear, as we only identified a small number of related RCTs. Nevertheless, our results indicate that high-dose glucocorticoids did not provide any benefits and significantly increased insulin use, which may increase the risk of hyperglycemia and related complications.
The evidence from our study was judged to be high based on the GRADE system. The low-dose subgroup for adult cardiac surgery (≤20 mg/kg hydrocortisone) only included 14 small RCTs, although 10 of these RCTs were considered high-quality based on the Jadad scores. Thus, large multi-center RCTs are needed as an additional source of evidence to clarify efficacy and optimal dose range for low-dose prophylactic corticosteroids during adult and pediatric cardiac surgery with CPB.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LWC, TCC, XHZ, and ZHQ designed the study. XJY, ZHQ, and MYT completed the literature search. All authors screened the results, extracted the data, and assessed the risk of bias. LWC, TCC, and XJY performed the statistical analyses. TCC and XHZ wrote the report. All authors participated in evaluating the evidence and critically revising the report. TCC and XHZ contributed equally to this study. All authors have read and approved the final manuscript. LWC and TCC are the study guarantors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [U2005202], the Fujian Province Major Science and Technology Program [2018YZ001-1], the Natural Science Foundation of Fujian Province [2020J01998, 2020J02056], and the Fujian provincial health technology project [2019-ZQN-50].
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fsurg.2022.832205/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. D’Agostino RS, Jacobs JP, Badhwar V, Paone G, Rankin JS, Han JM, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg. (2018) 107(1):24–32. doi: 10.1016/j.athoracsur.2018.10.004
2. Westaby. Complement and the damaging effects of cardiopulmonary bypass. Thorax. (1983) 38(5):321–5. doi: 10.1136/thx.38.5.321
3. McGuinness J, Bouchier-Hayes D, Redmond J. Understanding the inflammatory response to cardiac surgery. Surgeon. (2008) 6(3):162–71. doi: 10.1016/S1479-666X(08)80113-8
4. Loef BG, Henning RH, Epema AH, Rietman GW, van Oeveren W, Navis GJ, et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. Br J Anaesth. (2004) 93(6):793–8. doi: 10.1093/bja/aeh266
5. Whitlock RP, Chan S, Devereaux PJ, Sun J, Rubens FD, Thorlund K, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta-analysis of randomized trials. Eur Heart J. (2008) 29(21):2592–600. doi: 10.1093/eurheartj/ehn333
6. Ho K, Tan J. Benefits and risks of corticosteroids prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. (2009) 119(14):1853–66. doi: 10.1161/CIRCULATIONAHA.108.848218
7. Ng KT, Van Paassen J, Langan C, Sarode DP, Arbous MS, Alston RP, et al. The efficacy and safety of prophylactic corticosteroids for the prevention of adverse outcomes in patients undergoing heart surgery using cardiopulmonary bypass: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. (2020) 57:620–7. doi: 10.1093/ejcts/ezz325
8. Pesonen E, Keski-Nisula J, Andersson S, Palo R, Salminen J, Suominen P. High-dose methylprednisolone and endothelial glycocalyx in paediatric heart surgery. Acta Anaesthesiol Scand. (2016) 60(10):1386–94. doi: 10.1111/aas.12785
9. Heying R, Wehage E, Schumacher K, Tassani P, Haas F, Lange R, et al. Dexamethasone pretreatment provides antiinflammatory and myocardial protection in neonatal arterial switch operation. Ann Thorac Surg. (2012) 93(3):869–7. doi: 10.1016/j.athoracsur.2011.11.059
10. Bronicki RA, Backer CL, Baden HP, Mavroudis C, Crawford SE, Green TP. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. (2000) 69(5):1490–5. doi: 10.1016/S0003-4975(00)01082-1
11. Chaney M. corticosteroids and cardiopulmonary bypass : a review of clinical investigations. Chest. (2002) 121(3):921–31. doi: 10.1378/chest.121.3.921
12. Mayumi H, Zhang QW, Nakashima A, Masuda M, Kohno H, Kawachi Y, et al. Synergistic immunosuppression caused by high-dose methylprednisolone and cardiopulmonary bypass. Ann Thorac Surg. (1997) 63(1):129–37. doi: 10.1016/S0003-4975(96)00682-0
13. Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. (2012) 308(17):1761–7. doi: 10.1001/jama.2012.14144
14. Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. (2015) 386(10000):1243–53. doi: 10.1016/S0140-6736(15)00273-1
15. Keski-Nisula J, Pesonen E, Olkkola K, Peltola K, Neuvonen PJ, Tuominen N, et al. Methylprednisolone in neonatal cardiac surgery: reduced inflammation without improved clinical outcome. Ann Thorac Surg. (2013) 95(6):2126–32. doi: 10.1016/j.athoracsur.2013.02.013
16. Kunst G, Milojevic M, Boer C, De Somer F, Gudbjartsson T, van den Goor J, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. BrJ Anaesth. (2019) 123(6):713–57. doi: 10.1016/j.bja.2019.09.012
17. Cumpston M, Li T, Page M, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142.31643080
18. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17. doi: 10.1016/0197-2456(95)00134-4
19. Lomivorotov V, Kornilov I, Boboshko V, Shmyrev V, Bondarenko I, Soynov I, et al. Effect of Intraoperative Dexamethasone on Major Complications and Mortality Among Infants Undergoing Cardiac Surgery: The DECISION Randomized Clinical Trial. JAMA. (2020) 323(24):2485–92. doi: 10.1001/jama.2020.8133
20. Hartmann K, Koenen M, Schauer S, Wittig-Blaich S, Ahmad M, Baschant U, et al. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol Rev. (2016) 96(2):409–47. doi: 10.1152/physrev.00011.2015
21. Molgat ASD, Tilokee EL, Rafatian G, Vulesevic B, Ruel M, Milne R, et al. Hyperglycemia inhibits cardiac stem cell-mediated cardiac repair and angiogenic capacity. Circulation. (2014) 130:S70–6. doi: 10.1161/CIRCULATIONAHA.113.007908
22. Calmettes G, John S, Weiss J, Ribalet B. Hexokinase-mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J Gen Physiol. (2013) 142(4):425–36. doi: 10.1085/jgp.201310968
23. Hu Y-F, Chen Y-J, Lin Y-J, Chen S-A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
24. Abbaszadeh M, Khan ZH, Mehrani F, Jahanmehr H. Perioperative intravenous corticosteroids reduce incidence of atrial fibrillation following cardiac surgery: a randomized study. Rev Bras Cir Cardiovasc. (2012) 27:18–23. doi: 10.5935/1678-9741.20120005
25. Abd El-Hakeem EE, Ashry MA, El-Minshawy A, Maghraby EA. Influence of dexamethasone on cytokine balance in patients undergoing valve replacement surgery. Egyptian J Anaesth. (2003) 19(3):205–14. doi: 10.13140/RG.2.2.33395.96800
26. Al-Shawabkeh Z, Al-Nawaesah K, Anzeh RA, Al-Odwan H, Al-Rawashdeh WAB, Altaani H. Use of short-term steroids in the prophylaxis of atrial fibrillation after cardiac surgery. J Saudi Heart Assoc. (2017) 29:23–9. doi: 10.1016/j.jsha.2016.03.005
27. Amanullah MM, Hamid M, Hanif HM, Muzaffar M, Siddiqui MT, Adhi F, et al. Effect of steroids on inflammatory markers and clinical parameters in congenital open heart surgery: a randomised controlled trial. Cardiol Young. (2016) 26:506–15. doi: 10.1017/S1047951115000566
28. Andersen LW, Baek L, Thomsen BS, Rasmussen JP. Effect of methylprednisolone on endotoxemia and complement activation during cardiac surgery. J Cardiothorac Anesth. (1989) 3:544–9. doi: 10.1016/0888-6296(89)90150-6
29. Ando M, Park IS, Wada N, Takahashi Y. Steroid supplementation: a legitimate pharmacotherapy after neonatal open heart surgery. Ann Thorac Surg. (2005) 80:1672–8. doi: 10.1016/j.athoracsur.2005.04.035
30. Bingol H, Cingoz F, Balkan A, Kilic S, Bolcal C, Demirkilic U, et al. The effect of oral prednisolone with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. J Card Surg. (2005) 20:252–6. doi: 10.1111/j.1540-8191.2005.200392.x
31. Boscoe MJ, Yewdall VM, Thompson MA, Cameron JS. Complement activation during cardiopulmonary bypass: quantitative study of effects of methylprednisolone and pulsatile flow. Br Med J (Clin Res Ed). (1983) 287:1747–50. doi: 10.1136/bmj.287.6407.1747
32. Bourbon A, Vionnet M, Leprince P, Vaissier E, Copeland J, McDonagh P, et al. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardiothorac Surg. (2004) 26:932–8. doi: 10.1016/j.ejcts.2004.07.044
33. Brettner F, Chappell D, Nebelsiek T, Hauer D, Schelling G, Becker BF, et al. Preinterventional hydrocortisone sustains the endothelial glycocalyx in cardiac surgery. Clin Hemorheol Microcirc. (2019) 71:59–70. doi: 10.3233/CH-180384
34. Bronicki RA, Backer CL, Baden HP, Mavroudis C, Crawford SE, Green TP. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. (2000) 69:1490–5. doi: 10.1016/s0003-4975(00)01082-1
35. Butler J, Pathi VL, Paton RD, Logan RW, MacArthur KJ, Jamieson MP, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg. (1996) 62:538–42. https://www.ncbi.nlm.nih.gov/pubmed/86946198694619
36. Cavarocchi NC, Pluth JR, Schaff HV, Orszulak TA, Homburger HA, Solis E, et al. Complement activation during cardiopulmonary bypass. Comparison of bubble and membrane oxygenators. J Thorac Cardiovasc Surg. (1986) 91:252–8. https://www.ncbi.nlm.nih.gov/pubmed/35113283511328
37. Celik JB, Gormus N, Okesli S, Gormus ZI, Solak H. Methylprednisolone prevents inflammatory reaction occurring during cardiopulmonary bypass: effects on TNF-alpha, IL-6, IL-8, IL-10. Perfusion. (2004) 19:185–91. doi: 10.1191/0267659104pf733oa
38. Chaney MA, Nikolov MP, Blakeman B, Bakhos M, Slogoff S. Pulmonary effects of methylprednisolone in patients undergoing coronary artery bypass grafting and early tracheal extubation. Anesth Analg. (1998) 87:27–33. doi: 10.1097/00000539-199807000-00007
39. Chaney MA, Nikolov MP, Blakeman BP, Bakhos M, Slogoff S. Hemodynamic effects of methylprednisolone in patients undergoing cardiac operation and early extubation. Ann Thorac Surg. (1999) 67:1006–11. doi: 10.1016/s0003-4975(99)00067-3
40. Chaney MA, Durazo-Arvizu RA, Nikolov MP, Blakeman BP, Bakhos M. Methylprednisolone does not benefit patients undergoing coronary artery bypass grafting and early tracheal extubation. J Thorac Cardiovasc Surg. (2001) 121:561–9. doi: 10.1067/mtc.2001.112343
41. Checchia PA, Backer CL, Bronicki RA, Baden HP, Crawford SE, Green TP, et al. Dexamethasone reduces postoperative troponin levels in children undergoing cardiopulmonary bypass. Crit Care Med. (2003) 31:1742–5. doi: 10.1097/01.CCM.0000063443.32874.60
42. Codd JE, Wiens RD, Barner HB, Kaiser GC, Willman VL. Steroids and myocardial preservation. J Thorac Cardiovasc Surg. (1977) 74:418–22. https://www.ncbi.nlm.nih.gov/pubmed/302373302373
43. Coetzer M. The effect of methylprednisolone, given prior to cardiopulmonary bypass, on indices of gas exchange. South African Medical JournalS Afr Med J. (1996) 86(4):C188–92. https://hdl.handle.net/10520/AJA10159657_484
44. Danielson M, Reinsfelt B, Westerlind A, Zetterberg H, Blennow K, Ricksten SE. Effects of methylprednisolone on blood-brain barrier and cerebral inflammation in cardiac surgery-a randomized trial. J Neuroinflammation. (2018) 15:283. doi: 10.1186/s12974-018-1318-y
45. Demir T, Demir H, Tansel T, Kalko Y, Tireli E, Dayioglu E, et al. Influence of methylprednisolone on levels of neuron-specific enolase in cardiac surgery: a corticosteroid derivative to decrease possible neuronal damage. J Card Surg. (2009) 24:397–403. doi: 10.1111/j.1540-8191.2009.00842.x
46. Demir T, Ergenoglu MU, Demir HB, Tanrikulu N, Sahin M, Gok E, et al. Pretreatment with methylprednisolone improves myocardial protection during on-pump coronary artery bypass surgery. Heart Surg Forum. (2015) 18:E171–7. doi: 10.1532/hsf.1367
47. El Azab SR, Rosseel PM, de Lange JJ, Groeneveld AB, van Strik R, van Wijk EM, et al. Dexamethasone decreases the pro- to anti-inflammatory cytokine ratio during cardiac surgery. Br J Anaesth. (2002) 88:496–501. doi: 10.1093/bja/88.4.496
48. Enc Y, Karaca P, Ayoglu U, Camur G, Kurc E, Cicek S. The acute cardioprotective effect of glucocorticoid in myocardial ischemia-reperfusion injury occurring during cardiopulmonary bypass. Heart Vessels. (2006) 21:152–6. doi: 10.1007/s00380-005-0887-8
49. Engelman RM, Rousou JA, Flack 3rd JE, Deaton DW, Kalfin R, Das DK. Influence of steroids on complement and cytokine generation after cardiopulmonary bypass. Ann Thorac Surg. (1995) 60:801–4. doi: 10.1016/0003-4975(95)00211-3
50. Fecht DC, Magovern GJ, Park SB, Merkow LP, Dixon CM, Dosios T, et al. Beneficial effects of methylprednisolone in patients on cardiopulmonary bypass. Circ Shock. (1978) 5:415–22. https://www.ncbi.nlm.nih.gov/pubmed/378452378452
51. Ferries LH, Marx J Jr, Ray JF. The effect of methylprednisolone on complement activation during cardiopulmonary bypass. J Extra Corpor Technol. (1984) 16:83–8.
52. Fillinger MP, Rassias AJ, Guyre PM, Sanders JH, Beach M, Pahl J, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth. (2002) 16:163–9. doi: 10.1053/jcan.2002.31057
53. Giomarelli P, Scolletta S, Borrelli E, Biagioli B. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Ann Thorac Surg. (2003) 76:117–23. doi: 10.1016/s0003-4975(03)00194-2
54. Gomez Polo JC, Vilacosta I, Gomez-Alvarez Z, Vivas D, Martin-Garcia AG, Fortuny-Frau E, et al. 3275 Short term use of corticosteroids in the prophylaxis of atrial fibrillation after cardiac surgery and impact on the levels of acute phase proteins in this context. Eur Heart J. (2018) 39(suppl_1). doi: 10.1093/eurheartj/ehy563.3275
55. Graham EM, Martin RH, Buckley JR, Zyblewski SC, Kavarana MN, Bradley SM, et al. Corticosteroid therapy in neonates undergoing cardiopulmonary bypass: randomized controlled trial. J Am Coll Cardiol. (2019) 74:659–68. doi: 10.1016/j.jacc.2019.05.060
56. Halonen J, Halonen P, Jarvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. (2003) 297:1562–7. doi: 10.1001/jama.297.14.1562
57. Halvorsen P, Raeder J, White PF, Almdahl SM, Nordstrand K, Saatvedt K, et al. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesth Analg. (2003) 96:1578–83. doi: 10.1213/01.ANE.0000063922.90966.3A
58. Hao X, Han J, Zeng H, Wang H, Li G, Jiang C, et al. The effect of methylprednisolone prophylaxis on inflammatory monocyte subsets and suppressive regulatory T cells of patients undergoing cardiopulmonary bypass. Perfusion. (2019) 34:364–74. doi: 10.1177/0267659118820777
59. Harig F, Feyrer R, Mahmoud FO, Blum U, von der Emde J. Reducing the post-pump syndrome by using heparin-coated circuits, steroids, or aprotinin. Thorac Cardiovasc Surg. (1999) 47:111–8. doi: 10.1055/s-2007-1013121
60. Harig F, Hohenstein B, von der Emde J, Weyand M. Modulating IL-6 and IL-10 levels by pharmacologic strategies and the impact of different extracorporeal circulation parameters during cardiac surgery. Shock. (2001) 16(Suppl 1):33–8. doi: 10.1097/00024382-200116001-00007
61. Heying R, Wehage E, Schumacher K, Tassani P, Haas F, Lange R, et al. Dexamethasone pretreatment provides antiinflammatory and myocardial protection in neonatal arterial switch operation. Ann Thorac Surg. (2012) 93:869–76. doi: 10.1016/j.athoracsur.2011.11.059
62. Jansen NJ, van Oeveren W, van den Broek L, Oudemans-van Straaten HM, Stoutenbeek CP, Joen MC, et al. Inhibition by dexamethasone of the reperfusion phenomena in cardiopulmonary bypass. J Thorac Cardiovasc Surg. (1991) 102:515–25. https://www.ncbi.nlm.nih.gov/pubmed/16561491656149
63. Keski-Nisula J, Suominen PK, Olkkola KT, Peltola K, Neuvonen PJ, Tynkkynen P, et al. Effect of timing and route of methylprednisolone administration during pediatric cardiac surgical procedures. Ann Thorac Surg. (2015) 99:180–5. doi: 10.1016/j.athoracsur.2014.08.042
64. Keski-Nisula J, Arvola O, Jahnukainen T, Andersson S, Pesonen E. Reduction of inflammation by high-dose methylprednisolone does not attenuate oxidative stress in children undergoing bidirectional glenn procedure with or without aortic arch or pulmonary arterial repair. J Cardiothorac Vasc Anesth. (2020) 34:1542–7. doi: 10.1053/j.jvca.2019.10.015
65. Kilger E, Weis F, Briegel J, Frey L, Goetz AE, Reuter D, et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit Care Med. (2003) 31:1068–74. doi: 10.1097/01.CCM.0000059646.89546.98
66. Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. (2004) 55:627–33. doi: 10.1016/j.biopsych.2003.09.014
67. Kilickan L, Yumuk Z, Bayindir O. The effect of combined preinduction thoracic epidural anaesthesia and glucocorticoid administration on perioperative interleukin-10 levels and hyperglycemia. A randomized controlled trial. J Cardiovasc Surg (Torino). (2008) 49:87–93. https://www.ncbi.nlm.nih.gov/pubmed/1821269318212693
68. Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. (2007) 84:110–8. doi: 10.1016/j.athoracsur.2007.01.003
69. Lindberg L, Forsell C, Jogi P, Olsson AK. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. Br J Anaesth. (2003) 90:728–32. doi: 10.1093/bja/aeg125
70. Lomivorotov VV, Efremov SM, Kalinichenko AP, Kornilov IA, Knazkova LG, Chernyavskiy AM, et al. Methylprednisolone use is associated with endothelial cell activation following cardiac surgery. Heart Lung Circ. (2013) 22:25–30. doi: 10.1016/j.hlc.2012.08.001
71. Mardani D, Bigdelian H. The effect of dexamethasone prophylaxis on postoperative delirium after cardiac surgery: a randomized trial. J Res Med Sci. (2012) 17(1 SPL.1):S113–9. PMID: 23914217; PMCID: PMC3724375
72. McBride WT, Allen S, Gormley SM, Young IS, McClean E, MacGowan SW, et al. Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery. Cytokine. (2004) 27:81–9. doi: 10.1016/j.cyto.2004.03.018
73. Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, et al. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. (2005) 128:2677–87. doi: 10.1378/chest.128.4.2677
74. Morton JR, Hiebert CA, Lutes CA, White RL. Effect of methylprednisolone on myocardial preservation during coronary artery surgery. Am J Surg. (1976) 131:419–22. doi: 10.1016/0002-9610(76)90150-1
75. Mott AR, Fraser CD Jr, Kusnoor AV, Giesecke NM, Reul GJ Jr, Drescher KL, et al. The effect of short-term prophylactic methylprednisolone on the incidence and severity of postpericardiotomy syndrome in children undergoing cardiac surgery with cardiopulmonary bypass. J Am Coll Cardiol. (2001) 37:1700–6. doi: 10.1016/s0735-1097(01)01223-2
76. Murphy GS, Sherwani SS, Szokol JW, Avram MJ, Greenberg SB, Patel KM, et al. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. (2011) 25:950–60. doi: 10.1053/j.jvca.2011.03.002
77. Niazi Z, Flodin P, Joyce L, Smith J, Mauer H, Lillehei RC. Effects of glucocorticosteroids in patients undergoing coronary artery bypass surgery. Chest. (1979) 76:262–8. doi: 10.1378/chest.76.3.262
78. Oliver WC Jr, Nuttall GA, Orszulak TA, Bamlet WR, Abel MD, Ereth MH, et al. Hemofiltration but not steroids results in earlier tracheal extubation following cardiopulmonary bypass: a prospective, randomized double-blind trial. Anesthesiology. (2004) 101:327–39. doi: 10.1097/00000542-200408000-00013
79. Prasongsukarn K, Abel JG, Jamieson WR, Cheung A, Russell JA, Walley KR, et al. The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: a prospective randomized trial. J Thorac Cardiovasc Surg. (2005) 130:93–8. doi: 10.1016/j.jtcvs.2004.09.014
80. Rao G, King J, Ford W, King G. The effects of methylprednisolone on the complications of coronary artery surgery. Vasc Surg. (1977) 11:1–7. doi: 10.1177/153857447701100101
81. Rubens FD, Nathan H, Labow R, Williams KS, Wozny D, Karsh J, et al. Effects of methylprednisolone and a biocompatible copolymer circuit on blood activation during cardiopulmonary bypass. Ann Thorac Surg. (2005) 79:655–65. doi: 10.1016/j.athoracsur.2004.07.044
82. Rumalla V, Calvano SE, Spotnitz AJ, Krause TJ, Lin E, Lowry SF. The effects of glucocorticoid therapy on inflammatory responses to coronary artery bypass graft surgery. Arch Surg. (2001) 136:1039–44. doi: 10.1001/archsurg.136.9.1039
83. Sano T, Morita S, Masuda M, Tomita Y, Nishida T, Tatewaki H, et al. Cardiopulmonary bypass, steroid administration, and surgical injury synergistically impair memory T cell function and antigen presentation. Interact Cardiovasc Thorac Surg. (2003) 2:598–602. doi: 10.1016/S1569-9293(03)00168-3
84. Sano T, Morita S, Masuda M, Yasui H. Minor infection encouraged by steroid administration during cardiac surgery. Asian Cardiovasc Thorac Ann. (2006) 14:505–10. doi: 10.1177/021849230601400613
85. Schurr UP, Zund G, Hoerstrup SP, Grunenfelder J, Maly FE, Vogt PR, et al. Preoperative administration of steroids: influence on adhesion molecules and cytokines after cardiopulmonary bypass. Ann Thorac Surg. (2001) 72:1316–20. doi: 10.1016/s0003-4975(01)03062-4
86. Sobieski 2nd MA, Graham JD, Pappas PS, Tatooles AJ, Slaughter MS. Reducing the effects of the systemic inflammatory response to cardiopulmonary bypass: can single dose steroids blunt systemic inflammatory response syndrome? ASAIO J. (2008) 54:203–6. doi: 10.1097/MAT.0b013e3181640331
87. Starobin D, Kramer MR, Garty M, Shitirt D. Morbidity associated with systemic corticosteroid preparation for coronary artery bypass grafting in patients with chronic obstructive pulmonary disease: a case control study. J Cardiothorac Surg. (2007) 2:25. doi: 10.1186/1749-8090-2-25
88. Suominen PK, Keski-Nisula J, Ojala T, Rautiainen P, Jahnukainen T, Hastbacka J, et al. Stress-dose corticosteroid versus placebo in neonatal cardiac operations: a randomized controlled trial. Ann Thorac Surg. (2017) 104:1378–85. doi: 10.1016/j.athoracsur.2017.01.111
89. Jahnukainen T, Keski-Nisula J, Tainio J, Valkonen H, Patila T, Jalanko H, et al. Efficacy of corticosteroids in prevention of acute kidney injury in neonates undergoing cardiac surgery-A randomized controlled trial. Acta Anaesthesiol Scand. (2018). doi: 10.1111/aas.13134
90. Taleska Stupica G, Sostaric M, Bozhinovska M, Rupert L, Bosnic Z, Jerin A, et al. Extracorporeal hemadsorption versus glucocorticoids during cardiopulmonary bypass: a prospective, randomized, controlled trial. Cardiovasc Ther. (2020) 2020:7834173. doi: 10.1155/2020/7834173
91. Tassani P, Richter JA, Barankay A, Braun SL, Haehnel C, Spaeth P, et al. Does high-dose methylprednisolone in aprotinin-treated patients attenuate the systemic inflammatory response during coronary artery bypass grafting procedures? J Cardiothorac Vasc Anesth. (1999) 13:165–72. doi: 10.1016/s1053-0770(99)90081-2
92. Teoh KH, Bradley CA, Gauldie J, Burrows H. Steroid inhibition of cytokine-mediated vasodilation after warm heart surgery. Circulation. (1995) 92:II347–53. doi: 10.1161/01.cir.92.9.347
93. Toft P, Christiansen K, Tonnesen E, Nielsen CH, Lillevang S. Effect of methylprednisolone on the oxidative burst activity, adhesion molecules and clinical outcome following open heart surgery. Scand Cardiovasc J. (1997) 31:283–8. doi: 10.3109/14017439709069549
94. Toledo-Pereyra LH, Lin CY, Kundler H, Replogle RL. Steroids in heart surgery: a clinical double-blind and randomized study. Am Surg. (1980) 46:155–60. https://www.ncbi.nlm.nih.gov/pubmed/73776597377659
95. Turkoz A, Cigli A, But K, Sezgin N, Turkoz R, Gulcan O, et al. The effects of aprotinin and steroids on generation of cytokines during coronary artery surgery. J Cardiothorac Vasc Anesth. (2001) 15:603–10. doi: 10.1053/jcan.2001.26539
96. Vallejo JL, Gimenez-Fernandez R, Mainer JL, Rivera R. Clinical analysis of the protective effect of methylprednisolone on the heart in anoxic arrest (random study). Rev Esp Cardiol. (1977) 30:705–9. https://www.ncbi.nlm.nih.gov/pubmed/609839609839
97. Volk T, Schmutzler M, Engelhardt L, Docke WD, Volk HD, Konertz W, et al. Influence of aminosteroid and glucocorticoid treatment on inflammation and immune function during cardiopulmonary bypass. Crit Care Med. (2001) 29:2137–42. doi: 10.1097/00003246-200111000-00015
98. Volk T, Schmutzler M, Engelhardt L, Pantke U, Laule M, Stangl K, et al. Effects of different steroid treatment on reperfusion-associated production of reactive oxygen species and arrhythmias during coronary surgery. Acta Anaesthesiol Scand. (2003) 47:667–74. doi: 10.1034/j.1399-6576.2003.00145.x
99. von Spiegel T, Giannaris S, Wrigge H, Schorn B, Hoeft A. Effects of dexamethasone on extravascular lung water and pulmonary haemodynamics in patients undergoing coronary artery bypass surgery. Anasthesiol Intensivmed Notfallmed Schmerzther. (2001) 36:545–51. doi: 10.1055/s-2001-17260
100. von Spiegel T, Giannaris S, Wietasch GJ, Schroeder S, Buhre W, Schorn B, et al. Effects of dexamethasone on intravascular and extravascular fluid balance in patients undergoing coronary bypass surgery with cardiopulmonary bypass. Anesthesiology. (2002) 96:827–34. doi: 10.1097/00000542-200204000-00008
101. Vukovic PM, Maravic-Stojkovic VR, Peric MS, Jovic M, Cirkovic MV, Gradinac S, et al. Steroids and statins: an old and a new anti-inflammatory strategy compared. Perfusion. (2011) 26:31–37. doi: 10.1177/0267659110385607
102. Wan S, LeClerc JL, Huynh CH, Schmartz D, DeSmet JM, Yim AP, et al. Does steroid pretreatment increase endotoxin release during clinical cardiopulmonary bypass? J Thorac Cardiovasc Surg. (1999) 117:1004–8. doi: 10.1016/S0022-5223(99)70382-X
103. Weis F, Kilger E, Roozendaal B, de Quervain DJ, Lamm P, Schmidt M, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. (2006) 131:277–82. doi: 10.1016/j.jtcvs.2005.07.063
104. Weis F, Beiras-Fernandez A, Schelling G, Briegel J, Lang P, Hauer D, et al. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: effects on interleukin-6 to interleukin-10 ratio and early outcome. Crit Care Med. (2009) 37:1685–90. doi: 10.1097/CCM.0b013e31819fca77
105. Whitlock RP, Young E, Noora J, Farrokhyar F, Blackall M, Teoh KH. Pulse low dose steroids attenuate post-cardiopulmonary bypass SIRS; SIRS I. J Surg Res. (2006) 132:188–94. doi: 10.1016/j.jss.2006.02.013
106. Yared JP, Starr NJ, Hoffmann-Hogg L, Bashour CA, Insler SR, O’Connor 3rd M, et al. Dexamethasone decreases the incidence of shivering after cardiac surgery: a randomized, double-blind, placebo-controlled study. Anesth Analg. (1998) 87:795–9. doi: 10.1097/00000539-199810000-00010
107. Yared JP, Starr NJ, Torres FK, Bashour CA, Bourdakos G, Piedmonte M, et al. Effects of single dose, postinduction dexamethasone on recovery after cardiac surgery. Ann Thorac Surg. (2000) 69:1420–4. doi: 10.1016/s0003-4975(00)01180-2
108. Yared JP, Bakri MH, Erzurum SC, Moravec CS, Laskowski DM, Van Wagoner DR, et al. Effect of dexamethasone on atrial fibrillation after cardiac surgery: prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. (2007) 21:68–75. doi: 10.1053/j.jvca.2005.10.014
109. Yasser Mohamed A, Elmistekawy E, El-Serogy H. Effects of dexamethasone on pulmonary and renal functions in patients undergoing CABG with 38 cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. (2009) 13:231–7. doi: 10.1177/1089253209351598
Keywords: corticosteroids prophylaxis, cardiac surgery, cardiopulmonary bypass, myocardial infarction, randomized controlled trial
Citation: Chai T, Zhuang X, Tian M, Yang X, Qiu Z, Xu S, Cai M, Lin Y and Chen L (2022) Meta-Analysis: Shouldn’t Prophylactic Corticosteroids be Administered During Cardiac Surgery with Cardiopulmonary Bypass?. Front. Surg. 9:832205. doi: 10.3389/fsurg.2022.832205
Received: 9 December 2021; Accepted: 2 May 2022;
Published: 1 June 2022.
Edited by:
Laura Pasin University Hospital of Padua, ItalyReviewed by:
Savvas Lampridis, Guy’s and St Thomas’ NHS Foundation Trust, United KingdomIGiuseppe Filiberto Serraino, Department of Clinical and Experimental Medicine, Magna Græcia University of Catanzaro, Italy
Copyright © 2022 Chai, Zhuang, Tian, Yang, Qiu, Xu, Cai, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangwan Chen Y2hlbmxpYW5nd2FuQGZqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Specialty section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
Abbreviations: CPB, cardiopulmonary bypass; RCT, randomized controlled trial; OR, odds ratio; CI, confidence interval; MD, mean difference; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome; ECMO, extracorporeal membrane oxygenation; LOS, length of stay; CRP, C-reactive protein.
 Tianci Chai1,2,3,4†
Tianci Chai1,2,3,4† Xinghui Zhuang
Xinghui Zhuang Xiaojie Yang
Xiaojie Yang Meiling Cai
Meiling Cai Liangwan Chen
Liangwan Chen