- 1Department of General Surgery, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Emergency Intensive Care Medicine, The Fifth People's Hospital of Shanghai, Fudan University, Shanghai, China
- 3Department of Pathology, Huangshan People's Hospital, Huangshan, China
- 4Department of Chronic Wound Diagnosis and Treatment Center, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 5Department of Vascular Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
Background: Findings of ectopic hepatocellular carcinoma (EHCC) have been rarely documented. Complicated clinical features and unpredictable medical prognosis make diagnosis and treatment difficult.
Case Presentation: We reported a 59-year-old male patient who came to the hospital with epigastric discomfort and regurgitation of gastric acid. An enhanced CT scan revealed a 1.8 cm × 1.4 cm mass in the tail of pancreas without any positive finding in the liver. Postoperative MRI scan was performed but did not reveal any evidence of hepatic tumor. The tumor was resected in toto. Meanwhile, a 1 cm × 1 cm mass in the body of the stomach was found that was removed simultaneously. Histopathology showed that the pancreatic tumor was ectopic hepatocellular carcinoma (EHCC), and that the gastric nodule was gastrointestinal stromal tumor (GIST). The patient had an uneventful postoperative recovery. He has been living without recurrence for over 7 years since surgery. Owing to our knowledge, this is the second-longest disease-free survival time for EHCC in the literature.
Conclusion: Here, we present a rare case of EHCC in the pancreas, and review the current literature on EHCC. Operation was an effective treatment for patients with curable EHCC. EHCC with metastasis still needs more practice to improve the poor prognosis.
Introduction
The finding of ectopic liver accidentally happens in abdominal surgery and autopsy. It develops in various locations such as the gallbladder, intra-abdominal ligaments, omentum, peritoneum, retroperitoneum, and thorax (1–3). Nevertheless, lesions have a tendency to develop into hepatoma without mother liver malignancy. The underlying mechanism is unclear probably because of compromised vascular supply or biliary drainage (4).
The diagnosis of EHCC before a pathological result is complicated because of various clinical features. Blood tests and imaging cannot give a significant clue. At the same time, treatment for EHCC does not have a gold standard, and it still needs more practice. In this study, we report a rare case of a combination of ECHH and GIST, which were both successfully treated with tumor resection and 7 years follow-up without recurrence. After the case presentation section, a detailed literature review of EHCC was performed.
Case Presentation
A 59-year-old man visited our hospital with epigastric discomfort and regurgitation of gastric acid in April 2014. There were no remarkable findings in the past medical history. He denied alcohol and drug abuse history. The abdomen was soft and without palpable mass. Tests for hepatitis B virus and hepatitis C virus were negative. Other laboratory examinations were regular, including low levels of CEA, CA19-9, and AFP (AFP: 3.1 ng/ml, CEA: 5.3 ng/ml, and CA199: 12.7 U/ml). No significant indication was observed in hepatic function and routine blood examination. The contrast-enhanced computed tomography (CT) demonstrated a slight oval mass with irregular enhancement, which measured ~1.8 cm × 1.4 cm in the tail of pancreas. Moreover, impression of pancreatic duct dilation or stenosis was not detected by magnetic resonance imaging (MRI). By careful evaluation with the radiologist, there was no noticeable abnormality in the liver shown on CT. The postoperative contrast-enhanced magnetic resonance imaging (MRI) also confirmed the speculation on the hepatic condition (Figure 1).
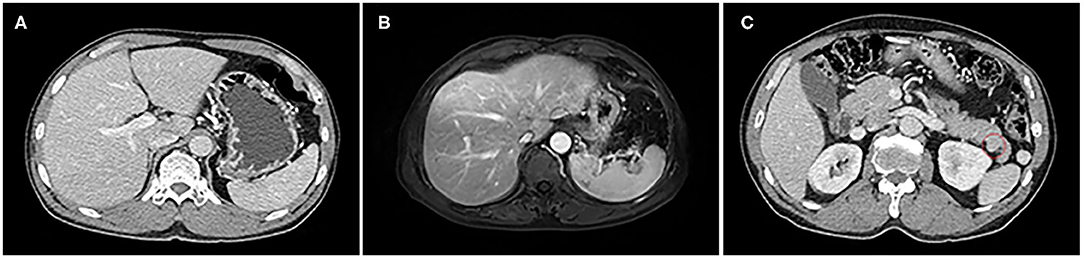
Figure 1. The liver with standard size and shape on CT and MRI; there are no symptoms of liver cirrhosis that can be observed. (A) Abdominal CT scan. (B) MRI scan. A tiny oval and smooth tumor was shown in the enhanced CT scan (C).
Traditional laparotomy was performed, which revealed nothing but a tumor in the pancreatic space that was mentioned in previous CT. The surface of the tumor was yellow and well-demarcated, and had a focal hemorrhagic appearance (Figure 2). The process of separation of the tumor is smooth without adhesion. There was no sign of invasion to the pancreas or other adjacent organs. Hepatic palpation was regular during the surgery, without cirrhosis and other abnormal findings. No lesion was found in the mother liver. Meanwhile, a lump like a GIST was found in the pylorus. Contrary to the hard texture of the pancreatic tumor, the touch of the stomach tumor is between soft and hard. Both tumors were resected with an adequate margin. Microscopy of the pancreatic tumor showed pleomorphic and prominent nuclei and eosinophilic cytoplasm with a section of pancreatic tissue that separated with capsule (Figure 2). The immunohistochemistry of pancreatic tumor was positive for Hep-Par-1, CK, CK8, and CK19, and negative for AFP, S100, Vim, CgA (chromogranin A), and P53 (Figure 2). The final diagnosis was ectopic HCC arising in the tail of pancreas and GIST in the stomach. Postoperative recovery was well without further chemotherapy therapy. Surveillance of AFP, ultrasound, and CT failed to find a sign of recurrence. Up to now, relapse was not observed 7 years after the operation.
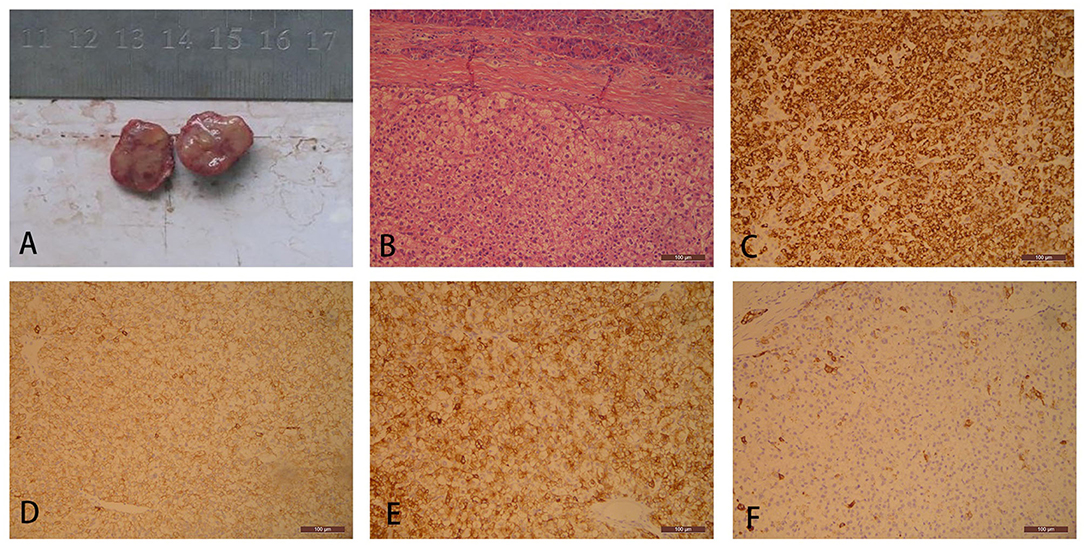
Figure 2. Gross specimen of a pancreatic tumor consists of a well-demarcated and solid firm mass with a yellow appearance (A). Pancreatic tumor cells are separated from normal pancreatic tissue with a prominent fibrous capsule. The tumor cells are polygonal, densely arranged, and abundant eosinophilic granular cytoplasm with partial cellular edema (hematoxylin and eosin, H&E, ×100) (B). Ectopic hepatocellular carcinoma (HCC) demonstrates simultaneous cytoplasm positive for (C) HepPar1, (D) CK, (E) CK8, and (F) CK19.
Consent for publication was signed by our patient and approved by the Ethics Committee of the Fifth People's Hospital of Shanghai.
Literature Review
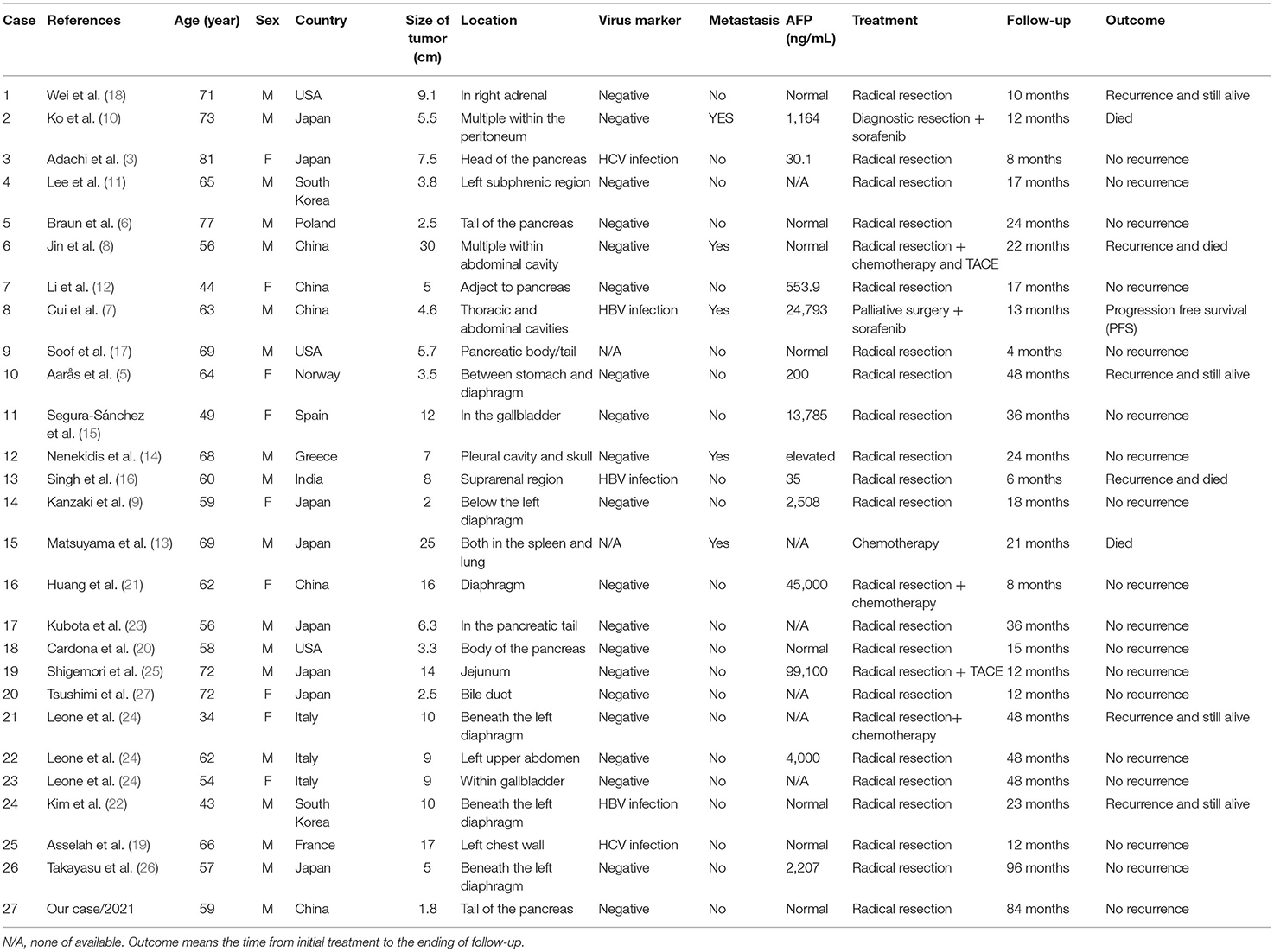
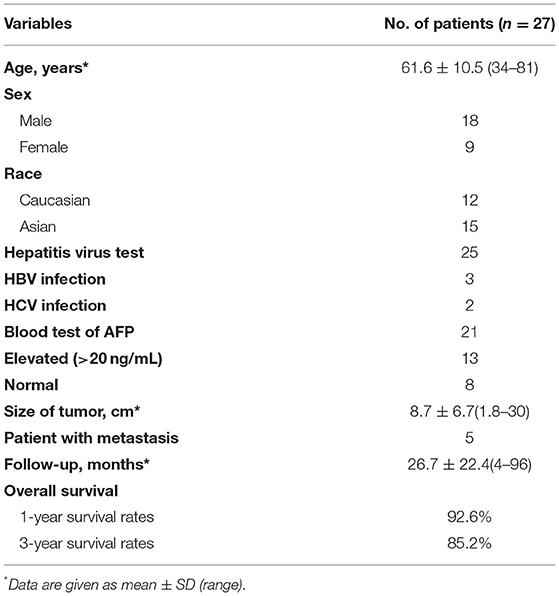
We searched MEDLINE and EMBASE in February 2022 and the search strategy was “ectopic” and “hepatocellular” and “carcinoma” in title, abstract, and key-words. Included cases must provide confirmed pathological images, follow-up examination, and treatment methods. In the end, 27 cases were available for the criteria (including our case) (3, 5–27). Among these cases, male to female ratio was 2:1. Age ranged from 34 to 81 years (median age 61.6 years). Twelve patients were Caucasian and 15 were Asian. Twenty-five patients were detected with hepatitis. Five of these patients had HBV or HCV infection. AFP level was elevated in 13 of 21 patients who had a serological test (AFP > 20 ng/ml). The overall survival rate of 27 patients at 1 and 3 years was 92.6% and 85.2%. Recurrence appeared in 6 of 24 curable patients. Four patients died in our review. The longest follow-up is Takayasu's patient of 8 years, and our case was the second longest follow-up of 7 years (26). The detail of literature review is listed in Tables 1, 2.
Discussion
As it is known, ectopic liver tissue and development of cancer from ectopic liver tissue is a rare condition (28). The ectopic liver is regarded as an abnormal tissue, which is similar to liver tissue of morphology and histology, having no linking structure to the mother liver. According to Martinez's report, the incidence of ectopic tissue ranges from 0.24 to 0.47% (29). About 70 patients were detected with an ectopic liver, and 9 of them were diagnosed with EHCC (30). Thus, ectopic liver tissue is considered as having high propensity for hepatocellular carcinoma. This phenomenon is possibly due to pathology difference, which usually appears as abnormality of vascular supply or biliary drainage (4).
What made ectopic liver occur is still unclear. However, the embryology may give us a better understanding of ectopic liver. The original structure of the liver is divided in the caudal part of the foregut during the 4th week of embryonic development. At that time, the primary liver encounters the diaphragm, ventral mesentery, gallbladder, extrahepatic biliary duct, and pancreas (31). According to congenital theory, any variation in hepatic diverticulum development would lead to liver ectopy. Therefore, gallbladder is the most frequent place for finding ectopic liver based on its closest position to the original liver in the embryonic development period. In contrast, transdifferentiation of hepatopancreatic stem cells from the pancreas to the liver is another possible etiology (32). Transformation from pancreatic progenitor cells into hepatocytes has already succeeded in vitro (33, 34). It could explain the abnormal phenomenon with multipotent/stem cell theory.
Hepatoid carcinoma is another histological subtype that mimics the morphology and histology of HCC and demonstrates a trabecular, medullary, ductal, glandular, or endocrine component (35). In contrast, EHCC seems to have pure HCC represented without mixed components. For these two subtypes, IHC results of hepatocyte paraffin (HepPar1/HepPar6) and α-fetoprotein (AFP) usually are both positive (36). Gurzu et al. (37) proved that the cytoplasmic expression of VSIG1/TTF1 is the pivotal biomarker of the development of gastric-type HCCs and hepatoid carcinomas. It may be a potential biomarker that could be used for differential diagnosis of these two subtypes. Furthermore, hepatoid carcinoma, regarded as poorly differentiated adenocarcinoma, is commonly related to unfavorable clinical outcomes, while ectopic HCC always shows better prognosis.
In reviewing, the clinical features of EHCC, the most common clinical manifestations were dizziness, poor appetite, nausea, asymptomatic palpable mass, and abdominal pain (12, 13, 22, 25). The early symptom of EHCC is hard to find, since the presentation is often silent until symptoms such as compression, pain, and bleeding occur. It is usually in the advanced stage when patients come to a hospital (8, 10). Our patient visited the hospital because of epigastric discomfort and regurgitation. It was confirmed that atypical symptoms were usual in EHCC, making them barely able to find early. In an early report, majority of patients with EHCC were of Asian race, and in some reports were Caucasians (20). However, in our literature review, data on Caucasians were 12 of 27 cases (44%). The contradictory data might be due to the greater number of worldwide cases we collected. They made the clinical information seem convincing.
Because of complicated clinical manifestations, only a few clues for diagnosis could be given to doctors. Radiography and laboratory examination rarely help in confirming the diagnosis. Proving EHCC is difficult without biopsy or operation. Only 3 of the 27 patients underwent fine needle aspiration (FNA). Two of three cases were diagnosed with EHCC before treatment or operation (3, 14, 16). Therefore, FNA could be an effective detection tool when a tumor cannot be classified. Immunohistochemistry of EHCC was almost positive for hepatocyte or hepatocyte-paraffin-1 (16 of 23 cases in our review). Thus, it is a vital marker for EHCC diagnosis. On the other hand, in 13 of 21 cases, AFP level was elevated (62%, AFP > 20 ng/ml). The data are similar to those on HCC in Fabio's report (54%, AFP > 20 ng/ml) (38). The traditional testing of AFP, which is always elevated in HCC, can also be evidence for suspected EHCC and a follow-up index.
Twenty-six patients underwent operations in our review, and radical resection was performed on 24 patients. Majority of these patients who underwent a radical operation recovered well. At the same time, six of the 24 patients had recurrence after radical resection (16, 18, 22). Obviously, operation is still the first choice for curable patients. Several patients had a recurrence of HCC in the mother liver after operation (16, 22). Although no lesion was found in the mother liver at the time of initial treatment, TACE was an option to prevent recurrence. Metastatic EHCC was reported on 5 patients (7, 8, 10, 13, 14). Only one of them showed no sign of recurrence after treatment (14). This patient was an old male who had tumors in the chest cavity and skull. Only resection of both tumors was performed. Two-year follow-up did not find any sign of recurrence. Although the remaining four patients received chemotherapy, TACE, or Sorafenib for systemic therapy, three died within 2 years. Based on the above information, metastatic EHCC is still a challenge for doctors. On the other hand, a favorable outcome showed the overall survival rate at 1 and 3 years (92.6 and 85.2%) for all the 27 patients. In contrast to a study on 1,492 patients with HCC, the 1- and 3-year overall survival rate of the patients was 96.6 and 88.8% (39). The concordant result suggested that EHCC without metastasis would have a favorable outcome as HCC expressed. Precise information on the prognosis of EHCC is still unclear, and long-term follow-up is required to evaluate various treatments.
Conclusion
The recommendation for diagnosis of EHCC is biopsy before operation. The AFP of blood test and hepatocyte-paraffin-1 of IHC are valuable for a definitive diagnosis of EHCC. Radical operation is necessary for a curable case. TACE, chemotherapy, and targeted therapy (like Sorafenib) are optional for patients with metastasis or multiple nodules. More cases of EHCC still need to be recorded to evaluate the prognosis and option for different treatments, especially long-time surveillance.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of The Fifth People's Hospital of Shanghai. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BG and JL collected the data. QL analyzed the data and wrote the manuscript. BG, XZ, YP, and JL revised the important intellectual content and helped in the final approval of the article. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author would like to thank Dr. Huangyi for the help in conceptualizing this study.
Abbreviations
EHCC, ectopic hepatocellular carcinoma; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; TACE, transhepatic arterial chemotherapy and embolization; IHC, immunohistochemistry; Hep-Par-1, hepatocyte-paraffin-1; AFP, alpha-fetoprotein; FNA, fine needle aspiration; H&E, hematoxylin and eosin stain.
References
2. Martínez-Acitores D, Hernández Ainsa M, Cortés García L, Bengochea Martínez ML, Palacios Fanlo MJ. Ectopic hepatocellular carcinoma arising from the peritoneum. Rev Esp Enferm Dig. (2019) 111:809–11. doi: 10.17235/reed.2019.6408/2019
3. Adachi Y, Hayashi H, Yusa T, Takematsu T, Matsumura K, Higashi T, et al. Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor: a case report. World J Gastroenterol. (2020) 26:2268–75. doi: 10.3748/wjg.v26.i18.2268
4. Caygill CP, Gatenby PA. Ectopic liver and hepatocarcinogenesis. Eur J Gastroenterol Hepatol. (2004) 16:727–9. doi: 10.1097/01.meg.0000131037.92864.df
5. Aarås AM, Reitan-Gjersøe TA, Waage A, Mala T, Edwin B, Løberg EM, et al. Laparoscopic resection of recurrent ectopic hepatocellular carcinoma: a case report with review of the literature and guidelines for follow-up. Int J Surg Case Rep. (2015) 17:92–5. doi: 10.1016/j.ijscr.2015.10.014
6. Braun M, Kuncman W, Teresiński L, Kupnicki P, Jesionek-Kupnicka D, Kordek R. Pure hepatocellular carcinoma originates from an ectopic liver nodule located in the pancreas. Contemp Oncol (Pozn). (2017) 21:311–4. doi: 10.5114/wo.2017.72403
7. Cui T, Diao X, Chen X, Huang S, Sun J. A case report: delayed high fever and maculopapules during Sorafenib treatment of ectopic hepatocellular carcinoma. BMC Cancer. (2016) 16:543. doi: 10.1186/s12885-016-2590-9
8. Jin R, Yu Q, Liang X. Ectopic hepatocellular carcinoma manifesting multiple abdominal masses: a case report. Medicine (Baltimore). (2017) 96:e8968. doi: 10.1097/MD.0000000000008968
9. Kanzaki R, Yamada T, Gotoh K, Takahashi H, Ohigashi H, Ishikawa O. Ectopic hepatocellular carcinoma arising in the left triangular ligament of the liver. Case Rep Gastroenterol. (2010) 4:138–43. doi: 10.1159/000314042
10. Ko YL, Takata K, Tanaka T, Ohishi J, Takeshita M, Yamauchi R, et al. Unresectable ectopic hepatocellular carcinoma treated with sorafenib. Case Rep Gastroenterol. (2020) 14:226–33. doi: 10.1159/000506929
11. Lee JY, Kim KH, Kang MS, Kim KH. Ectopic hepatocellular carcinoma arising from the peritoneum in a patient with a history of oropharyngeal cancer: a case report. Case Rep Oncol. (2015) 8:456–60. doi: 10.1159/000441020
12. Li Z, Wu X, Wen T, Li C, Peng W. Multiple ectopic hepatocellular carcinomas in the pancreas: a case report. Medicine (Baltimore). (2017) 96:e6747. doi: 10.1097/MD.0000000000006747
13. Matsuyama M, Sugiura S, Kakita A, Sato Y, Kuroda M. Hepatocellular carcinoma arising from ectopic liver tissue in the spleen producing insulin-like growth factor II. Pathol Res Pract. (2011) 207:124–6. doi: 10.1016/j.prp.2010.09.003
14. Nenekidis I, Anagnostakou V, Paralikas I, Kokkori A, Dedeilias P, Zisis C. Ectopic hepatocellular carcinomas developed in the chest wall and skull. Asian Cardiovasc Thorac Ann. (2011) 19:360–2. doi: 10.1177/0218492311419460
15. Segura-Sánchez J, Torres-Domínguez Y, Ruiz-García E. Ectopic hepatocellular carcinoma in the gallbladder. Rev Esp Enferm Dig. (2014) 106:149–50. doi: 10.4321/S1130-01082014000200015
16. Singh V, Sinha RJ, Sankhwar SN, Kumar S, Mehrotra B, Puri M, et al. Primary hepatocellular carcinoma in ectopic liver masquerading as left adrenal carcinoma: a rare occurrence. Rare Tumors. (2010) 2:e35. doi: 10.4081/rt.2010.e35
17. Soofi Y, Kanehira K, Abbas A, Aranez J, Bain A, Ylagan L. Pancreatic hepatoid carcinoma: a rare form of pancreatic neoplasm. Diagn Cytopathol. (2015) 43:251–6. doi: 10.1002/dc.23195
18. Wei N, Wong V, Matz A, Vemulakonda LA, Wang X, Phillips J. Ectopic hepatocellular carcinoma presenting as a right adrenal mass with IVC thrombus: case report and review of the literature. Urol Case Rep. (2022) 40:101900. doi: 10.1016/j.eucr.2021.101900
19. Asselah T, Condat B, Cazals-Hatem D, Hassani Z, Bernuau J, Groussard O, et al. Ectopic hepatocellular carcinoma arising in the left chest wall: a long-term follow-up. Eur J Gastroenterol Hepatol. (2001) 13:873–5. doi: 10.1097/00042737-200107000-00018
20. Cardona D, Grobmyer S, Crawford JM, Liu C. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. (2007) 450:225–9. doi: 10.1007/s00428-006-0353-8
21. Huang TW, Chan DC, Lee HS, Yao NS, Lee SC, Cheng YL. Ectopic hepatocellular carcinoma of the diaphragm. Dig Dis Sci. (2007) 52:1118–20. doi: 10.1007/s10620-006-9329-4
22. Kim KA, Park CM, Kim CH, Choi SY, Park SW, Hong SJ, et al. Hepatocellular carcinoma in an ectopic liver: CT findings. Eur Radiol. (2003) 13(Suppl. 4):L45–L7. doi: 10.1007/s00330-003-1908-6
23. Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, et al. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. (2007) 13:4270–3. doi: 10.3748/wjg.v13.i31.4270
24. Leone N, De Paolis P, Carrera M, Carucci P, Musso A, David E, et al. Ectopic liver and hepatocarcinogenesis: report of three cases with four years' follow-up. Eur J Gastroenterol Hepatol. (2004) 16:731–5. doi: 10.1097/01.meg.0000131044.05434.f7
25. Shigemori M, Kondo M, Azechi H, Inoue F, Tamura J, Kobayashi H, et al. A case of ectopic hepatocellular carcinoma in the jejunum. J Gastroenterol. (2006) 41:913–8. doi: 10.1007/s00535-006-1872-4
26. Takayasu K, Itabashi M, Moriyama N. Case report: ectopic hepatocellular carcinoma arising from the left diaphragm. Clin Radiol. (1994) 49:579–81. doi: 10.1016/S0009-9260(05)82944-7
27. Tsushimi T, Enoki T, Harada E, Orita M, Noshima S, Masuda M, et al. Ectopic hepatocellular carcinoma arising in the bile duct. J Hepatobiliary Pancreat Surg. (2005) 12:266–8. doi: 10.1007/s00534-004-0963-y
28. Akbulut S, Demyati K, Ciftci F, Koc C, Tuncer A, Sahin E, et al. Ectopic liver tissue (choristoma) on the gallbladder: a comprehensive literature review. World J Gastrointest Surg. (2020) 12:534–48. doi: 10.4240/wjgs.v12.i12.534
29. Martinez CA, de Resende HC Jr., Rodrigues MR, Sato DT, Brunialti CV, et al. Gallbladder-associated ectopic liver: a rare finding during a laparoscopic cholecystectomy. Int J Surg Case Rep. (2013) 4:312–5. doi: 10.1016/j.ijscr.2013.01.006
30. Arakawa M, Kimura Y, Sakata K, Kubo Y, Fukushima T, Okuda K. Propensity of ectopic liver to hepatocarcinogenesis: case reports and a review of the literature. Hepatology (Baltimore, Md). (1999) 29:57–61. doi: 10.1002/hep.510290144
31. Moore KL, Persaud TVN. The Developing Human: Clinically Oriented Embryology, 5th Edn. Philadelphia: Saunders Press (1993). 243 p.
32. von Mach MA, Hengstler JG, Brulport M, Eberhardt M, Schormann W, Hermes M, et al. In vitro cultured islet-derived progenitor cells of human origin express human albumin in severe combined immunodeficiency mouse liver in vivo. Stem cells (Dayton, Ohio). (2004) 22:1134–41. doi: 10.1634/stemcells.2004-0061
33. Gratte FD, Pasic S, Olynyk JK, Yeoh GCT. Transdifferentiation of pancreatic progenitor cells to hepatocyte-like cells is not serum-dependent when facilitated by extracellular matrix proteins. Sci Rep. (2018) 8:4385. doi: 10.1038/s41598-018-22596-z
34. Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. (2000) 2:879–87. doi: 10.1038/35046522
35. Paner GP, Thompson KS, Reyes CV. Hepatoid carcinoma of the pancreas. Cancer. (2000) 88:1582–9. doi: 10.1002/(SICI)1097-0142(20000401)88:7<1582::AID-CNCR12>3.0.CO;2-A
36. Pasricha S, Grover S, Kamboj M, Bansal D, Batra U, Gupta G, et al. Hepatoid adenocarcinoma of lung: a diagnostic challenge - Series of six cases with histopathological, predictive molecular and PD.L1 assessment. Indian J Pathol Microbiol. (2021) 64:128–31. doi: 10.4103/IJPM.IJPM_334_20
37. Gurzu S, Sugimura H, Szederjesi J, Szodorai R, Braicu C, Kobori L, et al. Interaction between cadherins, vimentin, and V-set and immunoglobulin domain containing 1 in gastric-type hepatocellular carcinoma. Histochem Cell Biol. (2021) 156:377–90. doi: 10.1007/s00418-021-02006-8
38. Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. (2006) 101:524–32. doi: 10.1111/j.1572-0241.2006.00443.x
39. Lin E, Zou B, Zeng G, Cai C, Li P, Chen J, et al. The impact of liver fibrosis on microvascular invasion and prognosis of hepatocellular carcinoma with a solitary nodule: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Ann Transl Med. (2021) 9:1310. doi: 10.21037/atm-21-3731
Keywords: ectopic hepatocellular carcinoma, ectopic liver, pancreatic tumor, case report, literature review
Citation: Liu Q, Li J, Pan Y, Zheng X and Gao B (2022) Challenge in Diagnosis and Treatment of Ectopic Hepatocellular Carcinoma: A Case Report and Literature Review. Front. Surg. 9:827006. doi: 10.3389/fsurg.2022.827006
Received: 01 December 2021; Accepted: 28 February 2022;
Published: 31 March 2022.
Edited by:
Simona Gurzu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Kenan Yusif-zade, Independent Researcher, Baku, AzerbaijanSami Akbulut, İnönü University, Turkey
Copyright © 2022 Liu, Li, Pan, Zheng and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Zheng, ZG9jdG9yemhlbmd4aWFuZyYjeDAwMDQwOzE2My5jb20=; Bin Gao, ZG9jdG9yZ2FvYmluJiN4MDAwNDA7MTYzLmNvbQ==
 Qicen Liu
Qicen Liu Jingyi Li2
Jingyi Li2