- 1Department of Sports Medicine, Peking University Third Hospital, Beijing, China
- 2Institute of Sports Medicine of Peking University, Beijing Key Laboratory of Sports Injuries, Beijing, China
Purpose: This study aims to evaluate the mid- to long-term outcome of concurrent arthroscopic treatment of osteochondral lesion (OCL) and open anatomical repair of lateral ankle ligaments for severe acute ankle sprain patients and compare them to the outcome of those without OCL.
Methods: A total of 166 patients with grade III acute lateral ankle ligament injuries underwent concurrent ankle arthroscopy and open anatomic ligament repair. Forty-three patients (group A) with OCL underwent arthroscopic treatment followed by open ligament repair. A total of 105 patients (group B) without OCL were followed up as the control. The evaluation parameters included sports recovery, postoperative visual analog scale (VAS) pain score, American Orthopaedic Foot and Ankle Society (AOFAS) score, Tegner score, sprain recurrence, satisfaction, and range of motion. Patients in group A were then subgroup-analyzed according to age, sex, body mass index, injury side, OCL location, and stage (Ferkel and Cheng’s staging system).
Results: The postoperative exercise level of the two groups recovered to more than 90% of the normal level (91.2% ± 11.2% in group A and 90.9% ± 13.3% in group B, n.s.). The average time of group A and group B to return to preinjury sports activity was respectively 4.4 ± 1.0 months and 4.4 ± 1.2 months with no significant difference (p = 0.716). No significant differences were found in the preoperation VAS pain score, AOFAS score, and Tegner score between the two groups. The postoperative VAS pain score in group A was significantly higher than that in group B (0.8 ± 1.7 vs. 0.3 ± 0.8, p = 0.027), but the difference was not clinically important. The postoperative VAS pain score of patients with stage D–F lesions was significantly higher than that of patients with stage B–C lesions (1.3 ± 2.1 vs. 0.3 ± 0.9, p = 0.038).
Conclusions: For the severe acute ankle sprain combined with OCL, the simultaneous arthroscopic treatment and open lateral ankle ligament repair achieved good mid- to long-term outcomes. Except that the pain was more pronounced than in the control group, there were no differences in other outcomes. Postoperative pain was positively correlated with the grade of OCL.
Introduction
Ankle sprains were reported to account for more than 10% of all sports injuries, among which lateral ligament sprain was the most common (1, 2). Rehabilitation with optimal loading in a brace was advocated for most ankle sprains (3). Ankle ligament sprains are usually graded on the basis of the severity of ligament rupture. For patients with grade III ankle ligament (4) and high demand for sports, conservative treatments showed a lower satisfaction rate and surgery is more recommended (5, 6).
The previously reported incidence of osteochondral lesion (OCL) combined with acute ankle sprain was up to 95% (7–10). If not properly treated, the OCL will induce a cascade of events, with the potential of leading to end-stage osteoarthritis, either localized or of the whole joint (11). The surgical treatment for the ankle OCL included abrasion, debridement, microfracture, drilling, and autograft or allograft transplantation, in which bone marrow stimulation (BMS) was the most commonly used. These surgical treatment procedures aim to regenerate tissue similar to natural cartilage, provide symptomatic relief, and return the patient to sports (12, 13). However, excessive intra-articular trauma caused by BMS will cause relatively more bleeding and inflammation, which may lead to complications such as limited joint movement, which has been reported to negatively affect the effect of ligament repair surgery for chronic ankle instability (CAI) (14–16).
Compared to CAI, the OCL with acute ankle sprain contained more osteochondritis dissecans, tangential fractures, and bone marrow edema but fewer subchondral bone cysts and combined joint degeneration (17, 18). These different characteristics of OCL may have different effects on clinical outcomes. So far, there has been no study on the impact of the simultaneous treatment of OCL on the long-term outcome of ligament repair for severe acute ankle sprains.
In the present study, all the acute ankle sprain patients with simultaneous grade III ligament rupture and OCL undergoing concurrent arthroscopy and open ligament repair between 2007 and 2017 in our institute were followed up. Patients with isolated arthroscopic exploration and acute ligament repair during the same period served as controls. The purpose of this study was to evaluate the mid- to long-term outcome of concurrent arthroscopic OCL treatment and open anatomical repair of lateral ankle ligaments of acute ankle sprain and compare them to the outcome of those without OCL. It was hypothesized that simultaneous treatment of OCL does not affect the mid- to long-term outcomes of open ligament repair for acute ankle sprains.
Materials and Methods
Patient Selection
Most acute ankle sprain patients were recommended conservative treatments in our institute. For patients with grade III lateral ankle ligament rupture and high demand for sports, the option of surgical treatment was given. After being fully informed of the risk of surgery, patients giving consent were then admitted for surgery. All patients with acute lateral ankle ligament rupture (less than 2 weeks) who underwent arthroscopic exploration and open anatomical ligament repair from June 2007 to May 2017 were enrolled. The ethics license was obtained from the IRB Medical Committee (IRB00006761-2016011).
Patients were excluded if (1) the OCL area was greater than 15 mm2 or the depth of OCL was greater than 8 mm, requiring osteochondral transplantation rather than BMS and (2) they had index ankle surgery.
Surgical Technique
Under general or spinal anesthesia, all patients underwent arthroscopic exploration and necessary treatment of intra-articular lesions before ligament repair. The location of talar cartilage injury is recorded according to the nine-zone method (19). OCL was measured using labeled probes and classified according to Ferkel and Cheng’s classification (20) in group A: stage A: smooth, intact but soft or ballotable; stage B: rough surface; stage C: fibrillation/fissuring; stage D: flap present or bone exposed; stage E: loose, undisplaced fragment; and stage F: displaced fragment. Rough or fibrotic cartilage debridement was performed in patients with stage B–C lesions using a mechanical razor system (Dyonics Power Shaver System; Smith & Nephew, Andover, MA, USA). For D–F stage lesions, in addition to debridement of pterygoid cartilage, BMS was performed, including abrasion, scraping, and microfracture. Other lesions (such as synovial hyperplasia, loose body, etc.) are also treated under arthroscopy. Similarly, for patients in group B, these intra-articular injuries were also explored under arthroscopy before open anatomical ligament repair.
After arthroscopic surgery, a slightly curved longitudinal incision was made 3–4 cm above the distal fibula to expose anterior talofibular ligament (ATFL) and calcaneofibular ligament (CFL). Care was taken to protect the dorsal cutaneous nerve and the sural nerve during the incision. The modified Broström–Gould technique (21) was used for anatomical repair of the lateral ankle ligament, and suture anchors were used for the insertion site rupture. One or two 5-mm-deep holes were drilled at the ruptured insertion position of ATFL or CFL, and 1.8-mm-diameter suture anchors (Mitek Mini GII; Johnson & Johnson, NJ, USA) were placed to fix the ligaments. Then, the extensor retinaculum was sutured to the fibular periosteum. The anterior drawer test and talus tilt were evaluated again to ensure sufficient stability of the ankle.
Postoperative Rehabilitation
The splint was used for all patients 3 weeks after the operation without weight-bearing. Then, the splint was replaced with an ankle brace, and range of motion (ROM) training was performed until week 6. For group A patients, the splint or brace was removed daily from the second week to the sixth week for full-range continuous passive exercise (CPM) training. CPM exercise was performed in a painless range for 30 min a day. Then, reposition the splint to secure the ankle. Varus movement is allowed from week 5. Partial weight-bearing was allowed at 6–8 weeks, and complete weight-bearing was allowed at 8–12 weeks. For patients in group B, ROM training of flexion and extension was started from the third week. Complete weight-bearing was allowed at 4–6 weeks. All the patient resumes exercise according to the patients’ tolerance and recovery.
Clinical Outcome Evaluation
The sports recovery and complications of all patients were recorded and evaluated. During the follow-up, if the sports returned to more than 85% of the preinjury level, it was recorded as returning to the preinjury sports. Assessments at the final follow-up included visual analog scale (VAS) pain score (22), American Orthopaedic Foot and Ankle Society (AOFAS) score (23), Tegner activity score (24), sprain recurrence, ROM, recovery of preinjury sports level, patient satisfaction, skin numbness area, and other complications. For scores that differ between the two groups, a subgroup analysis of group A was performed for the potentially related factors, including age, gender, body mass index (BMI), OCL side, injury zone, and OCL staging.
Statistical Analysis
SPSS statistical software version 23.0 (IBM; Armonk, New York, USA) was used to analyze the data. The chi-square test or Fisher’s exact probability test was used for categorical results, while the paired sample t-test and non-parametric test were used to determine the preoperative and postoperative subjective scores. A non-parametric test was also used to determine the influencing factors of the VAS pain score. The differences between the two groups were analyzed by the t-test and non-parametric test. PASS 11.0 (NCSS, UT, USA) was used to calculate the sample size. The difference was considered significant at p < 0.05.
Results
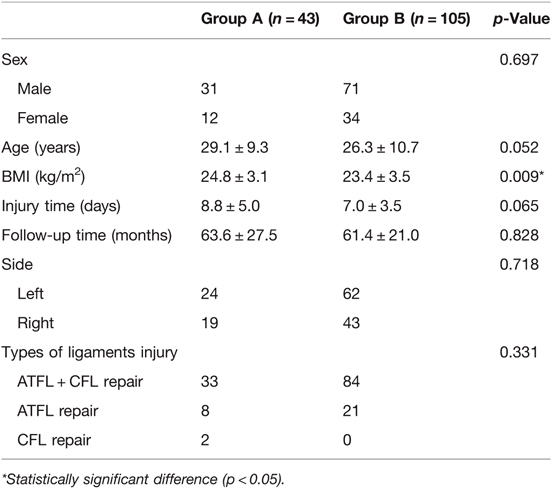
Of the 166 patients who met the inclusion criteria, 148 patients (89.2%) were available for the final follow-up, including 43 patients with OCL (group A) and 105 patients without OCL (group B). Post hoc power analysis showed that the sample size of 90 could achieve 95% power to detect the observed differences in the recovery of sports; thus, the sample of our research cases was enough. Preoperative demographic data and characteristics of two groups are compared in Table 1. The mean follow-up time of group A and group B was 63.6 ± 27.5 and 61.4 ± 21.0 months, respectively (n.s.). There was no significant difference in age, sex, injured side, types of ligament injury, and injured time (n.s.) between the two groups. The BMI of group A was significantly higher than that of group B (p < 0.05) (Table 1).
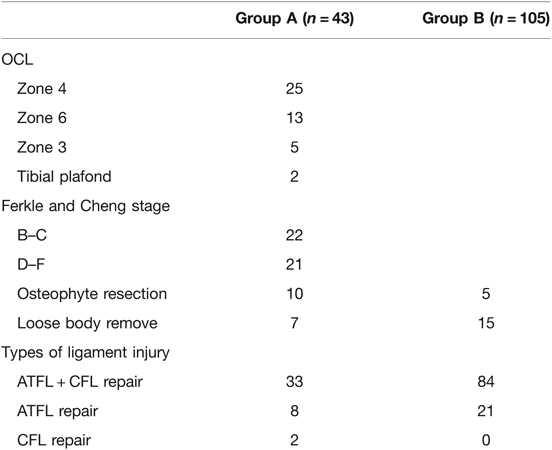
For patients in group A, the OCL was accounted for 25 (58%) in zone 4, 13 (30%) in zone 6, and 5 (12%) in zone 3. According to the Ferkle and Cheng staging system, there were 22 (51%) cases of stage B–C OCL and 21 (49%) cases of stage D–F OCL; osteophyte resection was performed in 10 (7%) and 5 (3%) patients in groups A and B, respectively, with no significant difference. A total of 7 (5%) and 15 (10%) patients underwent loose body removal in groups A and B, respectively. Two patients demonstrated OCLs in both the medial talus and the tibial plafond (Table 2).
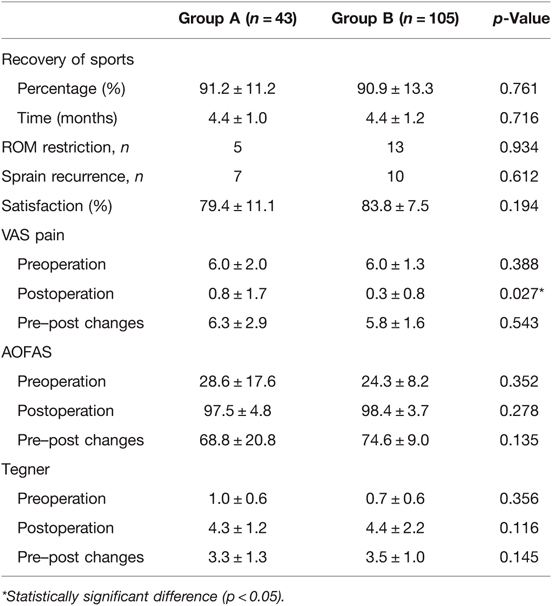
At the final follow-up, a total of 5 (3%) and 13 (9%) patients reported mild ROM restriction (<10°) in groups A and B, respectively; 7 (5%) and 10 (7%) patients experienced sprain recurrence in groups A and B, respectively. The postoperative VAS pain score, AOFAS score, and Tegner score were significantly improved from the preoperative level for group A and group B (p < 0.001). There was no significant difference in the preoperative VAS pain score, AOFAS score, or Tegner scores between the two groups (n.s.) (Table 3). The average postoperative satisfaction of patients in group B was slightly higher than that of patients in group A (83.8 ± 7.5% vs. 79.4 ± 11.1%), but the difference was not statistically significant. The postoperative exercise level of group A and group B recovered to more than 90% of the normal level (91.2% ± 11.2%, 90.9% ± 13.3%, respectively, n.s.). The average time to return to sports of group A and group B was 4.4 ± 1.0 and 4.4 ± 1.2 months after surgery, respectively (n.s.). Significant differences were found in the postoperational VAS pain score between the two groups. The patients with OCL showed higher postoperational VAS pain score (0.8 ± 1.7) than patients without OCL (0.3 ± 0.8) (p < 0.05).
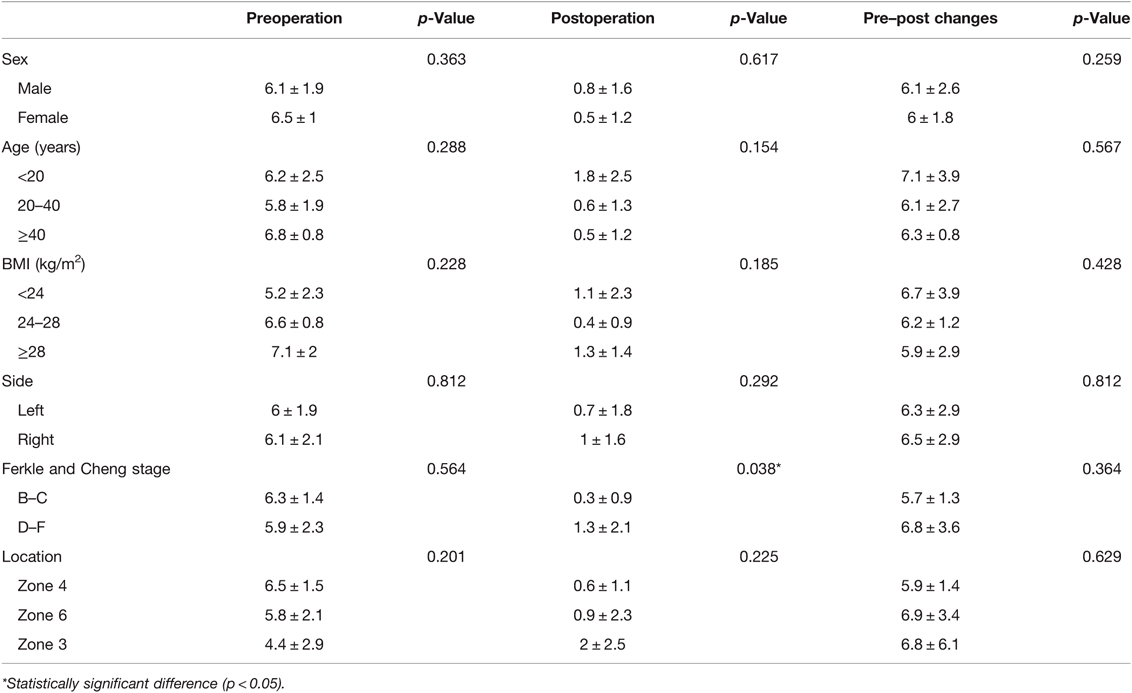
According to the subgroup analysis of the VAS score of group A, the postoperative VAS score of patients with stage D–F OCL was significantly higher than that of patients with stage B–C OCL (1.3 ± 2.1 vs. 0.3 ± 0.9, p = 0.038). No significant difference was found in the other parameters (Table 4).
Discussion
The most important finding of this study was that concurrent arthroscopic treatment of OCL and open anatomic ligament repair achieved good mid- to long-term results and could be a reliable procedure for patients after severe acute ankle sprains. Except that the pain was more pronounced than in the control group, there were no differences in other outcomes. Postoperative pain was positively correlated with the grade of OCL.
The present study indicated that no significant difference was found in most outcomes and returned to sports between the two groups. Simultaneous treatment of OCL does not affect the outcomes of open ligament repair for acute ankle sprains. However, the literature on CAI showed controversial results. Gregush et al. concluded that isolated lateral ligament repair had higher ankle and hindfoot scores compared to repair with concomitant treatment of an OCL (25), while other studies have shown that the association of OCL treatment did not affect the efficacy in the treatment of CAI up to 6 years after operation (26, 27). The result of the present research might be due to better regeneration potential and special characteristics of OCL in acute ankle sprain compared to those of CAI. In the present study, stage B–C OCL was found in 22 (51%) patients and stage D–F OCL was found in the other 21 (49%) patients, but no subchondral cyst was found. On the other hand, subchondral bone cystic degeneration and combined joint degeneration were commonly associated with CAI and negatively affected osteochondral regeneration (17, 18). In addition, during acute injury, the organism responded rapidly to activate repair capability within a few hours, and the inflammatory process was stimulated immediately to promote the regeneration of injured tissue (28).
The results also showed that the postoperative VAS pain score of OCL with arthroscopic treatment was higher than patients without OCL. The reason for the higher VAS pain score incidence in group A might be mostly due to the debridement or microfracture during the arthroscopic OCL treatment, which led to inflammation or the stimulation of subchondral bone tissue. The soft tissue and bone were reported as the major source of pain during OCL (29–31). In addition, bone marrow edema of subchondral bone often occurs caused by OCL, and BMS in the early stage may increase the risk of postoperative pain (32, 33). However, it should be noted that although there were differences in VAS scores between the two groups, they were both at a low level. A difference of less than 1.4 points might not have much clinical significance (34). In addition, patients with acute ankle sprain combined with OCL recovered 91.2 ± 11.2% of preinjury sports level, and the time to return to sport was 4.4 ± 1.0 months, which achieved similar clinical effects compared with previous studies on open ankle lateral ligament repair without OCL (35, 36). The results of the present study indicated that simultaneous arthroscopic OCL treatment and open ligament repair could be a reliable operation with good mid- to long-term results.
The subgroup analysis showed that stage D–F OCL (involving subchondral bone) caused more long-term pain than stage B–C OCL (not involving subchondral bone), which was similar to previous studies on CAI (37, 38). The results further indicated that the subchondral bone irritation was highly correlated with pain. Other reasons might be due to the lack of formation of hyaline cartilage. Fibrocartilage could be obtained after BMS treatment instead of hyaline cartilage, but the former does not have sufficient mechanical properties during exercise, thereby resulting in postoperative pain (13–15). However, radiography and MRI were not performed at the follow-up and should be further accessed in future studies.
The results of this study indicated that the medial (zone 4) and lateral (zones 3 and 6) OCL accounted for 58% and 30%, respectively, which was consistent with previous research (19). Clinical outcomes in this study were not affected by the location of OCL. In CAI, medial lesions were demonstrated to be more common as well as larger in depth and surface area, so the recovery of lateral OCL was reported to be better than medial OCL (39–41). However, according to the present study, there was no significant difference in clinical results between the acute ankle sprains with medial and lateral OCL. The reason for similar clinical outcomes between different locations might be mostly that the severity of the lesion did not vary with the location of OCL in the present research. In addition, the articular surface injury of the corresponding tibial plafond was also reported to negatively affect the postoperative recovery (42), but only two cases were complicated with tibial OCL in the current study and had little effect on the outcome. A study with more cases was needed in the future.
This study also found that the BMI of patients with OCL was significantly higher than that of patients without OCL. Higher BMI was associated with higher absolute tibiofemoral compression (43, 44). In addition, a motion analysis study showed that in obese patients, the disproportionate stress is borne by force scattered in a small part of the whole articular surface (45). Biomechanical gait studies of the knee also showed that obese patients spent more time standing and knee weight-bearing (46). In addition, BMI was also found to be higher in patients with OCL than in patients without OCL in chronic lateral ankle instability (47). Therefore, patients with higher BMI were considered to have a greater possibility of OCL, but more in-depth research was needed in the future.
It should be noted that the patients of the two groups underwent different postoperative rehabilitation procedures, which might affect the outcome. In fact, a well-recognized rehabilitation program was used after ankle arthroscopy with or without OCL, rather than deliberately using different rehabilitation programs for the two groups of patients. Patients with OCL had CPM exercise 2 weeks after BMS to promote fibrocartilage regeneration by mechanical stimulus. Although the patients in group B did not have such training, they also underwent passive ankle ROM exercises daily from week 2 to avoid joint adhesion and resume the normal ROM. During the 2–3 weeks after the operation, except during the CPM activity, the ankle joint was still in a fixed state, so the immobilization time of the two groups was basically the same and the two groups were comparable. In terms of the weight-bearing time, the postoperative weight-bearing of patients undergoing OCL in group A was delayed by about 4 weeks compared with patients without OCL. Although some studies proposed immediate weight-bearing after BMS (6, 48), the delayed weight-bearing for postoperative cartilage procedure was more widely accepted to reduce postoperative pain and promote cartilage regeneration (49). In addition, our research focused on the mid- to long-term clinical effect and have a relatively long time from postoperative rehabilitation, so we proposed that the difference in conclusion mainly comes from OCL treatment itself but not the rehabilitation program.
To our knowledge, this is the first study to compare the long-term outcome of the acute ligament repair with or without OCL treatment. The follow-up evaluation included comprehensive parameters including recovery of sports, subjective score, ROM, and recurrence of sprain. The results offered insight into the effect of the OCL treatment on the acute ankle sprain outcomes and indicated a possible increased risk of VAS pain score in patients with stage D–F OCL, which should be informed to the patients before operations.
There are still some limitations to this research. First, it was retrospective rather than prospective research, and selection bias might affect the results. In addition, there was no stress x-ray or MRI examination to evaluate the objective ankle stability, OCL fate, and joint degeneration, which could be further accessed in future studies.
Conclusion
Concurrent arthroscopic treatment of OCL and open anatomic ligament repair achieved good mid- to long-term results and could be a reliable procedure for patients after a severe acute ankle sprain. Except that the pain was more pronounced than in the control group, there were no differences in other outcomes. Postoperative pain was positively correlated with the grade of OCL.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the IRB Medical Committee of Peking University Third Hospital (IRB00006761-2016011). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conception and design of the study: DJ. Acquisition of data: M-ZD. Analysis or interpretation of data: TS. Drafting the work: M-ZD. Revising the manuscript critically for important intellectual content: Y-FJ, CJ, Q-WG, Y-LH, and DJ. Final approval of the version to be published: M-ZD, TS, Y-FJ, CJ, Q-WG, Y-LH, and DJ. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: M-ZD, TS, Y-FJ, CJ, Q-WG, Y-LH, and DJ. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (82072428), Beijing Natural Science Foundation (7212132), Beijing Municipal Commission of Science and Technology, National Key Research and Development Program (Grant No. 2019YFB1706905).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fong DT-P, Man C-Y, Yung PS-H, Cheung S-Y, Chan K-M. Sport-related ankle injuries attending an accident and emergency department. Injury. (2008) 39:1222–7. doi: 10.1016/j.injury.2008.02.032
2. Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. (1988) 7:29–36. doi: 10.1016/s0278-5919(20)30956-x
3. Gerber JP, Williams GN, Scoville CR, Arciero RA, Taylor DC. Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot Ankle Int. (1998) 19:653–60. doi: 10.1177/107110079801901002
4. Balduini FC, Tetzlaff J. Historical perspectives on injuries of the ligaments of the ankle. Clin Sports Med. (1982) 1:3–12. doi: 10.1016/s0278-5919(20)31504-0
5. Fong DT-P, Hong Y, Chan L-K, Yung PS-H, Chan K-M. A systematic review on ankle injury and ankle sprain in sports. Sports Med. (2007) 37:73–94. doi: 10.2165/00007256-200737010-00006
6. Attenborough AS, Hiller CE, Smith RM, Stuelcken M, Greene A, Sinclair PJ. Chronic ankle instability in sporting populations. Sports Med. (2014) 44:1545–56. doi: 10.1007/s40279-014-0218-2
7. Soboroff SH, Pappius EM, Komaroff AL. Benefits, risks, and costs of alternative approaches to the evaluation and treatment of severe ankle sprain. Clin Orthop Relat Res. (1984):160–8. doi: 10.1097/00003086-198403000-00026
8. Waterman BR, Belmont PJ, Cameron KL, Deberardino TM, Owens BD. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. (2010) 38:797–803. doi: 10.1177/0363546509350757
9. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. (2013) 95:1045–54. doi: 10.2106/JBJS.L.00773
10. Gianakos AL, Yasui Y, Hannon CP, Kennedy JG. Current management of talar osteochondral lesions. World J Orthop. (2017) 8:12–20. doi: 10.5312/wjo.v8.i1.12
11. Dahmen J, Karlsson J, Stufkens SAS, Kerkhoffs GMMJ. The ankle cartilage cascade: incremental cartilage damage in the ankle joint. Knee Surg Sports Traumatol Arthrosc. (2021) 29:3503–7. doi: 10.1007/s00167-021-06755-w
12. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. (2010) 92:279–86. doi: 10.2106/JBJS.H.01635
13. Delco ML, Kennedy JG, Bonassar LJ, Fortier LA. Post-traumatic osteoarthritis of the ankle: A distinct clinical entity requiring new research approaches. J Orthop Res. (2017) 35:440–53. doi: 10.1002/jor.23462
14. Murawski CD, Foo LF, Kennedy JG. A review of arthroscopic bone marrow stimulation techniques of the talus: the good, the bad, and the causes for concern. Cartilage. (2010) 1:137–44. doi: 10.1177/1947603510364403
15. Cottom JM, Maker JM. Cartilage allograft techniques and materials. Clin Podiatr Med Surg. (2015) 32:93–8. doi: 10.1016/j.cpm.2014.09.012
16. Johnson B, Lever C, Roberts S, Richardson J, McCarthy H, Harrison P, et al. Cell cultured chondrocyte implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin. (2013) 18:135–50. doi: 10.1016/j.fcl.2012.12.008
17. Josten C, Rose T. Acute and chronic osteochondral lesions of the talus. Orthopade. (1999) 28:500–8. doi: 10.1007/pl00003635
18. Steinhagen J, Niggemeyer O, Bruns J. Etiology and pathogenesis of osteochondrosis dissecans tali. Orthopade. (2001) 30:20–7. doi: 10.1007/s001320050569
19. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. (2007) 28:154–61. doi: 10.3113/FAI.2007.0154
20. Ferkel E, Nguyen S, Kwong C. Chronic lateral ankle instability: surgical management. Clin Sports Med. (2020) 39:829–43. doi: 10.1016/j.csm.2020.07.004
21. Maffulli N, Del Buono A, Maffulli GD, Oliva F, Testa V, Capasso G, et al. Isolated anterior talofibular ligament Broström repair for chronic lateral ankle instability: 9-year follow-up. Am J Sports Med. (2013) 41:858–64. doi: 10.1177/0363546512474967
22. Hunt KJ, Hurwit D. Use of patient-reported outcome measures in foot and ankle research. J Bone Joint Surg Am. (2013) 95(1–9):e118. doi: 10.2106/JBJS.L.01476
23. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. (1994) 15:349–53. doi: 10.1177/107110079401500701
24. Ventura A, Terzaghi C, Legnani C, Borgo E. Lateral ligament reconstruction with allograft in patients with severe chronic ankle instability. Arch Orthop Trauma Surg. (2014) 134:263–8. doi: 10.1007/s00402-013-1911-6
25. Gregush RV, Ferkel RD. Treatment of the unstable ankle with an osteochondral lesion: results and long-term follow-up. Am J Sports Med. (2010) 38:782–90. doi: 10.1177/0363546509351556
26. Legnani C, Borgo E, Macchi V, Ventura A. Does the association of microfractures for the treatment of osteochondral lesions of the talus affect the outcome following arthroscopic treatment for chronic ankle instability? J Am Podiatr Med Assoc. (2021) 111:Article_3. doi: 10.7547/19-101
27. Lee M, Kwon JW, Choi WJ, Lee JW. Comparison of outcomes for osteochondral lesions of the talus with and without chronic lateral ankle instability. Foot Ankle Int. (2015) 36:1050–7. doi: 10.1177/1071100715581477
28. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. (2012) 49:35–43. doi: 10.1159/000339613
29. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. (1998) 26:773–7. doi: 10.1177/03635465980260060601
30. Wiewiorski M, Pagenstert G, Rasch H, Jacob AL, Valderrabano V. Pain in osteochondral lesions. Foot Ankle Spec. (2011) 4:92–9. doi: 10.1177/1938640010395749
31. Dye SF, Chew MH. The use of scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. (1994) 43:453–69. doi: 10.2106/00004623-199309000-00015
32. D’Ambrosi R, Maccario C, Ursino C, Serra N, Usuelli FG. The role of bone marrow edema on osteochondral lesions of the talus. Foot Ankle Surg. (2018) 24:229–35. doi: 10.1016/j.fas.2017.02.010
33. Shimozono Y, Hurley ET, Yasui Y, Deyer TW, Kennedy JG. The presence and degree of bone marrow edema influence midterm clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. (2018) 46:2503–8. doi: 10.1177/0363546518782701
34. Sutton RM, McDonald EL, Shakked RJ, Fuchs D, Raikin SM. Determination of minimum clinically important difference (MCID) in visual analog scale (VAS) Pain and foot and ankle ability measure (FAAM) scores after hallux valgus surgery. Foot Ankle Int. (2019) 40:687–93. doi: 10.1177/1071100719834539
35. White WJ, McCollum GA, Calder JDF. Return to sport following acute lateral ligament repair of the ankle in professional athletes. Knee Surg Sports Traumatol Arthrosc. (2016) 24:1124–9. doi: 10.1007/s00167-015-3815-1
36. Lynch SA, Renström PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. (1999) 27:61–71. doi: 10.2165/00007256-199927010-00005
37. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. (1999) 81:1229–35. doi: 10.2106/00004623-199909000-00004
38. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. (2008) 36:1750–62. doi: 10.1177/0363546508316773
39. Shea MP, Manoli A. Osteochondral lesions of the talar dome. Foot Ankle. (1993) 14:48–55. doi: 10.1177/107110079301400110
40. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. (1985) 5:165–85. doi: 10.1177/107110078500500403
41. Orr JD, Dutton JR, Fowler JT. Anatomic location and morphology of symptomatic, operatively treated osteochondral lesions of the talus. Foot Ankle Int. (2012) 33:1051–7. doi: 10.3113/FAI.2012.1051
42. van Bergen CJA, van Eekeren ICM, Reilingh ML, Sierevelt IN, van Dijk CN. Treatment of osteochondral defects of the talus with a metal resurfacing inlay implant after failed previous surgery: a prospective study. Bone Joint J. (2013) 95-B:1650–5. doi: 10.1302/0301-620X.95B12.32455
43. Rogers DL, Klyce W, Kajstura TJ, Lee RJ. Association of body mass index with severity and lesion location in adolescents with osteochondritis dissecans of the knee. Orthop J Sports Med. (2021) 9:23259671211045384. doi: 10.1177/23259671211045382
44. Harding GT, Dunbar MJ, Hubley-Kozey CL, Stanish WD, Astephen Wilson JL. Obesity is associated with higher absolute tibiofemoral contact and muscle forces during gait with and without knee osteoarthritis. Clin Biomech (Bristol, Avon). (2016) 31:79–86. doi: 10.1016/j.clinbiomech.2015.09.017
45. McMillan AG, Pulver AME, Collier DN, Williams DSB. Sagittal and frontal plane joint mechanics throughout the stance phase of walking in adolescents who are obese. Gait Posture. (2010) 32:263–8. doi: 10.1016/j.gaitpost.2010.05.008
46. Strutzenberger G, Alexander N, Bamboschek D, Claas E, Langhof H, Schwameder H. Uphill walking: biomechanical demand on the lower extremities of obese adolescents. Gait Posture. (2017) 54:20–6. doi: 10.1016/j.gaitpost.2017.02.013
47. Jiang D, Ao Y-F, Jiao C, Xie X, Chen L-X, Guo Q-W, et al. Concurrent arthroscopic osteochondral lesion treatment and lateral ankle ligament repair has no substantial effect on the outcome of chronic lateral ankle instability. Knee Surg Sports Traumatol Arthrosc. (2018) 26:3129–34. doi: 10.1007/s00167-017-4774-5
48. Petrera M, Dwyer T, Theodoropoulos JS, Ogilvie-Harris DJ. Short- to medium-term outcomes after a modified Broström repair for lateral ankle instability with immediate postoperative weightbearing. Am J Sports Med. (2014) 42:1542–8. doi: 10.1177/0363546514530668
Keywords: acute ankle sprain, osteochondral lesion, arthroscopy, return to play, lateral ankle ligament
Citation: Du M, Su T, Jiang Y, Jiao C, Guo Q, Hu Y and Jiang D (2022) Simultaneous Treatment of Osteochondral Lesion Does Not Affect the Mid- to Long-Term Outcomes of Ligament Repair for Acute Ankle Sprain: A Retrospective Comparative Study with a 3–11-Year Follow-up. Front. Surg. 9:816669. doi: 10.3389/fsurg.2022.816669
Received: 17 November 2021; Accepted: 8 April 2022;
Published: 9 May 2022.
Edited by:
Fahad Mujtaba Iqbal, Imperial College London, United KingdomReviewed by:
Feng Wei, Michigan State University, United StatesBabar Shafiq, The Johns Hopkins Hospital, Johns Hopkins Medicine, United States
Copyright © 2022 Du, Su, Jiang, Jiao, Guo, Hu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Jiang YnlzeWppYW5nZG9uZ0AxMjYuY29t
Specialty section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Ming-Ze Du
Ming-Ze Du Tong Su1,2
Tong Su1,2 Dong Jiang
Dong Jiang