- 1Department of Orthopedics, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Department of Orthopedic surgery, Changhua Christian Hospital, Changhua, Taiwan
- 4Department of Pathology & Laboratory Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 6Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 7Department of Food Science and Technology, Hung Kuang University, Taichung, Taiwan
We investigated factors associated with postoperative lipiduria and hypoxemia in patients undergoing surgery for orthopedic fractures. We enrolled patients who presented to our emergency department due to traumatic fractures between 2016 and 2017. We collected urine samples within 24 h after the patients had undergone surgery to determine the presence of lipiduria. Hypoxemia was defined as an SpO2 <95% determined with a pulse oximeter during the hospitalization. Patients’ anthropometric data, medical history, and laboratory test results were collected from the electronic medical record. Logistic regression analyses were used to determine the associations of clinical factors with postoperative lipiduria and hypoxemia with multivariate adjustments. A total of 144 patients were analyzed (mean age 51.3 ± 22.9 years, male 50.7%). Diabetes (odd ratio 3.684, 95% CI, 1.256–10.810, p = 0.018) and operation time (odd ratio 1.005, 95% CI, 1.000–1.009, p = 0.029) were independently associated with postoperative lipiduria, while age (odd ratio 1.034, 95% CI, 1.003–1.066, p = 0.029), body mass index (odd ratio 1.100, 95% CI, 1.007–1.203, p = 0.035), and operation time (odd ratio 1.005, 95% CI, 1.000–1.010, p = 0.033) were independently associated with postoperative hypoxemia. We identified several factors independently associated with postoperative lipiduria and hypoxemia in patients with fracture undergoing surgical intervention. Operation time was associated with both postoperative lipiduria and hypoxemia, and we recommend that patients with prolonged operation for fractures should be carefully monitored for clinical signs related to fat embolism syndrome.
Introduction
Fat embolism syndrome (FES) is a clinical condition that arises as a consequence of fat globules in the systemic circulation (1, 2). It usually developed after orthopedic trauma, such as long bone fractures (3, 4). The clinical presentations of FES may vary widely, while the diagnostic criteria remain ill-defined (5). This may result in a wide range of reported incidences of this serious complication (4). The incidence of FES was found to be less than 1% in patients with orthopedic fractures (4, 5). Nevertheless, the rate could be as high as 30% in patients with multiple fractures (6, 7). Moreover, FES is associated with a high mortality rate (8), though its treatment is largely supportive.
The proposed pathophysiology of FES (5) includes endothelial injury followed by release of inflammatory cytokines and acute respiratory distress, hypoxemia, neurological deficit, thrombocytopenia and disseminated intravascular coagulation, all of which may contribute to detrimental outcomes. Currently, there is no established treatment for FES to improve its outcomes, and its care mainly involves supportive measures (5, 9). Early awareness of patients who are at risk for FES might be helpful. Unfortunately, the pathophysiologic mechanisms of FES have not been well-established (5).
Among the proposed diagnostic criteria of FES (5, 10), respiratory involvement (hypoxemia) is an important manifestation, which may result from endothelial injury and subsequent acute respiratory distress syndrome (5). Lipiduria (5) is another criterion for the diagnosis of FES that may be related to renal endothelial injury. Both lipiduria and hypoxemia are not well studied in patients with orthopedic fractures. As postoperative hypoxemia is not uncommon (11), we investigated factors associated with postoperative lipiduria and hypoxemia in patients undergoing surgery for orthopedic fractures in this study.
Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan (approval number: SF17001B). All patients provided written informed consent. We enrolled patients who presented to our emergency department due to traumatic fractures between 2016 and 2017. After initial assessment and preoperative preparation, these patients underwent surgical intervention for their fractures and were admitted. Patients’ anthropometric data, medical history, and laboratory test results were collected from electronic medical records.
We collected urine samples within 24 h after the operation to determine the presence of lipiduria. Approximately 5 mL of urine sample was mixed with 5 mL diethyl ether (E Merck, D-6100 Darmstadt, F.R. Germany), and the mixture was centrifuged at 1,500 rpm for 2 min (Figure 1). We removed the upper layer of transparent fluid, and extracted the opaque layer for determination of lipiduria. Neutral fat stain solution was added to the samples, which were then examined using a microscope. Figure 2 shows a sample with positive neutral fat stain.
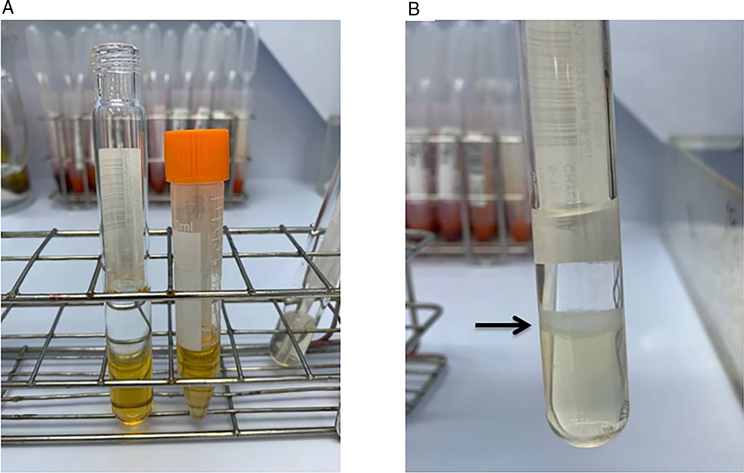
Figure 1. (A) A urine sample before the experiment and (B) after mixing with 5mL diethyl ether (E Merck, D-6100 Darmstadt, F.R. Germany) and centrifugation at 1,500rpm for 2min. The black arrow indicates the opaque layer we extracted for determination of lipiduria.
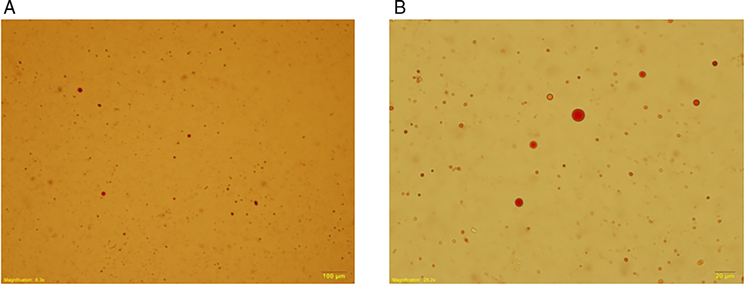
Figure 2. A urine sample stained with neutral fat stain solution observed using a microscope. (A) 100×. (B) 400×.
SpO2 <90% (∼PaO2 <60 mm Hg) (12) has been used to define FES (13). In this study, we defined hypoxemia as an SpO2 <95% (14) determined with a pulse oximeter during the hospitalization. The monitoring of SpO2 was accompanied with vital signs measurements (usually every 6–8 h). More frequent monitoring may be required, depending on the patients’ clinical condition. Patients’ renal function was determined using estimated glomerular filtration rate (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (15). Information about operation time, blood loss, and blood transfusion was recorded according to the operation notes.
The statistical analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS version 22.0; International Business Machines Corp, NY, USA). We divided our patients into two groups according to whether or not they had lipiduria within 24 h after the operation. The statistical differences in continuous and categorical variables between the two groups were examined using the Student's t test and the Chi-square test, respectively. Logistic regression analyses were used to determine the associations of clinical factors with postoperative lipiduria and hypoxemia with multivariate adjustments. In all of the analyses, a two-sided P value of less than 0.05 was considered statistically significant.
Results
A total of 144 patients were analyzed (mean age 51.3 ± 22.9 years, male 50.7%). Table 1 shows the characteristics of the study population according to whether or not they had lipiduria after the surgery. Patients who had lipiduria after operation had a higher proportion of diabetes (25.0% vs. 11.3%, p = 0.030), a longer operation time (214 ± 124 vs. 174 ± 84 min, p = 0.027), and a higher rate of postoperative hypoxemia (SpO2 <95%) (40.6% vs. 20.0%, p = 0.007) compared with those who had no lipiduria. There were no significant between-group differences in the other variables.
Table 2 shows the associations between clinical factors and postoperative lipiduria. In the univariate analysis, diabetes (odd ratio 2.630, 95% CI, 1.074–6.436, p = 0.034) and operation time (odd ratio 1.001, 95% CI, 1.000–1.007, p = 0.025) were significantly associated with postoperative lipiduria. The associations of diabetes (odd ratio 3.684, 95% CI, 1.256–10.810, p = 0.018) and operation time (odd ratio 1.005, 95% CI, 1.000–1.009, p = 0.029) with postoperative lipiduria remained significant after adjustments for age, sex, and other relevant variables (Table 2).
We examined the associations between clinical factors and postoperative hypoxemia (SpO2 <95%), as shown in Table 3. Significant associations of age, diabetes, hypertension, eGFR, and operation time with postoperative hypoxemia were noted in the univariate analysis. After adjustments for relevant variables, age (odd ratio 1.034, 95% CI, 1.003–1.066, p = 0.029), body mass index (odd ratio 1.100, 95% CI, 1.007–1.203, p = 0.035), and operation time (odd ratio 1.005, 95% CI, 1.000–1.010, p = 0.033) were independently associated with postoperative hypoxemia.
Discussion
In this study, we demonstrated that diabetes and operation time were associated with postoperative lipiduria in patients with fractures undergoing surgical intervention (Table 2). Moreover, operation time was independently associated with postoperative hypoxemia (Table 3). Both postoperative lipiduria and hypoxemia are common presentations of FES (5, 11), which is an uncommon but severe postoperative complication with a poor prognosis (8, 16). Our findings are clinically relevant and might help identify patients with high risk of postoperative FES.
Lipiduria has been associated with nephrotic syndrome (17). The cause of lipiduria in nephrotic syndrome may be secondary to hyperlipidemia (18). Our finding that diabetes was associated with lipiduria (odd ratio 3.684, 95% CI, 1.256–10.810, p = 0.018, Table 2) is perhaps not surprising, as diabetes has been associated with proteinuria and nephrotic syndrome (19). We speculate that an increase in oxidative stress after acute trauma and surgical intervention (20–22) might be related to postoperative lipiduria. Oxidative stress has been reported to contribute to acute kidney injury after orthopedic trauma in an animal model (23). Oxidative stress may result in an increase in glomerular permeability (24), which has been associated with lipiduria (25, 26). This scenario may help explain the finding in a recent study (16) that showed delayed time to operation was associated with FES in patients with acute trauma, and may also account for our result, which indicated operation time (odd ratio 1.005, 95% CI, 1.000–1.009, p = 0.029) was independently associated with postoperative lipiduria.
Moreover, we found that operation time was independently associated with postoperative hypoxemia (odd ratio 1.005, 95% CI, 1.000–1.010, p = 0.033). An increase in oxidative stress associated with surgical intervention could lead to an increase in pulmonary permeability (22), which in turn may result in postoperative hypoxemia. Both age and body mass index were also independently associated with postoperative hypoxemia, and these findings were consistent with previous reports (27–29). Twenty-five of our patients had both lipiduria and hypoxemia after surgery for their fractures. The mean operation time of these patients was 227 ± 130 min, compared with 178 ± 82 min (p = 0.034) for the 64 patients who did not have postoperative lipiduria and hypoxemia. As bone marrow manipulation during orthopedic surgery might increase the risk of FES (30), it is reasonable to postulate that this could explain the association of operation time with postoperative lipiduria and hypoxemia. Based on our findings, we recommend that patients who had prolonged operation for fractures should be carefully monitored for clinical signs related to the FES.
There were several limitations in this study. First, the number of our study patients was relatively small. As the incidence of FES was less than 1% (4, 5), a larger number of patients are needed to investigate predisposing factors of this serious complication. Second, we did not investigate proteinuria and lipiduria before the operation for all study patients. We cannot exclude the possibility that some of our patients had preoperative lipiduria which was not related to the operation. Third, we used SpO2 <95% determined using a pulse oximeter, rather than low PaO2 determined by an arterial blood gas analysis, as the definition of hypoxemia. This must be acknowledged as a potential confounder, although low SpO2 (<90%) was used to define FES in a recent study (13).
Conclusion
In summary, we identified several factors independently associated with postoperative lipiduria and hypoxemia in patients with fracture undergoing surgical intervention. Operation time was associated with both postoperative lipiduria and hypoxemia, and we recommend that patients with prolonged operation for fractures should be carefully monitored for clinical signs related to FES.
Data Availability Statement
The datasets presented in this article are not readily available because of privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
C-HC, Y-CW, and C-HL designed and conducted the research; Y-CW, Y-CL, F-AT, J-YL, and J-SW contributed acquisition of data, analysis and interpretation of data; J-SW and Y-CW wrote the first draft of the manuscript; and C-HC, Y-CL, F-AT, J-YL, and C-HL revised the manuscript critically for important intellectual content. All authors approved the final draft of the manuscript.
Funding
This work was supported by Taichung Veterans General Hospital, Taichung, Taiwan [grant numbers TCVGH-1093504C, 2020; TCVGH-1103504C, 2021]. The funder was not involved in the study design, data collection, analysis, interpretation of the results, preparation of the article, and the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. (1974) 14:955–62. PMID: 4419160
2. Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J Crit Illn Inj Sci. (2013) 3:64–8. doi: 10.4103/2229-5151.109426
3. Akhtar S. Fat embolism. Anesthesiol Clin. (2009) 27:533–50, table of contents. doi: 10.1016/j.anclin.2009.07.018
4. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. (2008) 336:472–7. doi: 10.1097/MAJ.0b013e318172f5d2
5. Kosova E, Bergmark B, Piazza G. Fat embolism syndrome. Circulation. (2015) 131:317–20. doi: 10.1161/CIRCULATIONAHA.114.010835
6. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. (1996) 19:41–8 doi: 10.3928/0147-7447-19960101-09
7. Georgopoulos D, Bouros D. Fat embolism syndrome: clinical examination is still the preferable diagnostic method. Chest. (2003) 123:982–3. doi: 10.1378/chest.123.4.982
8. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. (1974) 56B:408–16. doi: 10.1302/0301-620X.56B3.408
9. Mellor A, Soni N. Fat embolism. Anaesthesia. (2001) 56:145–54. doi: 10.1046/j.1365-2044.2001.01724.x
10. Koul PA, Ahmad F, Gurcoo SA, Khan UH, Naqash IA, Sidiq S, et al. Fat embolism syndrome in long bone trauma following vehicular accidents: experience from a tertiary care hospital in north India. Lung India. (2013) 30:97–102. doi: 10.4103/0970-2113.110413
11. Sun Z, Sessler DI, Dalton JE, Devereaux PJ, Shahinyan A, Naylor AJ, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. (2015) 121:709–15. doi: 10.1213/ANE.0000000000000836
12. Walsh BK, Smallwood CD. Pediatric oxygen therapy: a review and update. Respir Care. (2017) 62:645–61. doi: 10.4187/respcare.05245
13. Kirk A, Elliott J, Varma D, Kimmel LA. Fat embolism syndrome: experience from an Australian trauma centre. Int J Orthop Trauma Nurs. (2020) 36:100746. doi: 10.1016/j.ijotn.2019.100746
14. Bach J. A quick reference on hypoxemia. Vet Clin North Am Small Anim Pract. (2017) 47:175–9. doi: 10.1016/j.cvsm.2016.10.004
15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
16. Kainoh T, Iriyama H, Komori A, Saitoh D, Naito T, Abe T. Risk factors of fat embolism syndrome after trauma: a nested case-control study with the use of a nationwide trauma registry in Japan. Chest. (2021) 159:1064–71. doi: 10.1016/j.chest.2020.09.268
17. Klahr S, Tripathy K, Bolanos O. Qualitative and quantitative analysis of urinary lipids in the nephrotic syndrome. J Clin Invest. (1967) 46:1475–81. doi: 10.1172/JCI105639
18. Macé C, Chugh SS. Nephrotic syndrome: components, connections, and angiopoietin-like 4-related therapeutics. J Am Soc Nephrol. (2014) 25:2393–8. doi: 10.1681/ASN.2014030267
19. Lee YH, Kim KP, Kim YG, Moon JY, Jung SW, Park E, et al. Clinicopathological features of diabetic and nondiabetic renal diseases in type 2 diabetic patients with nephrotic-range proteinuria. Medicine (Baltimore). (2017) 96:e8047. doi: 10.1097/MD.0000000000008047
20. Tsuchiya M, Sato EF, Inoue M, Asada A. Open abdominal surgery increases intraoperative oxidative stress: can it be prevented? Anesth Analg. (2008) 107:1946–52. doi: 10.1213/ane.0b013e318187c96b
21. O’Leary DP, Wang JH, Cotter TG, Redmond HP. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut. (2013) 62:461–70. doi: 10.1136/gutjnl-2011-300948
22. Matejovic M, Krouzecky A, Rokyta R Jr, Treska V, Spidlen V, Novak I. Effects of intestinal surgery on pulmonary, glomerular, and intestinal permeability, and its relation to the hemodynamics and oxidative stress. Surg Today. (2004) 34:24–31. doi: 10.1007/s00595-003-2642-z
23. Mittwede PN, Xiang L, Lu S, Clemmer JS, Hester RL. Oxidative stress contributes to orthopedic trauma-induced acute kidney injury in obese rats. Am J Physiol Renal Physiol. (2015) 308:F157–63. doi: 10.1152/ajprenal.00537.2014
24. Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. (2010) 53:2056–65. doi: 10.1007/s00125-010-1810-0
25. Zimmer JG, Dewey R, Waterhouse C, Terry R. The origin and nature of anisotropic urinary lipids in the nephrotic syndrome. Ann Intern Med. (1961) 54:205–14. doi: 10.7326/0003-4819-54-2-205
26. Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. (2011) 17:117–22. doi: 10.1038/nm.2261
27. Kaushal A, Goyal P, Dhiraaj S, Agarwal A, Singh PK. Identification of various perioperative risk factors responsible for development of postoperative hypoxaemia. Turk J Anaesthesiol Reanim. (2018) 46:416–23. doi: 10.1038/nm.2261
28. Gao F, Guo W, Sun W, Li Z, Wang W, Wang B, et al. [Mechanisms and influencing factors of hypoxemia after joint arthroplasty]. Zhonghua Yi Xue Za Zhi. (2014) 94:655–9. PMID: 24842202
29. Wu Z, Wang Z, Wu H, Hu R, Ren W, Hu Z, et al. Obesity is a risk factor for preoperative hypoxemia in Stanford A acute aortic dissection. Medicine (Baltimore). (2020) 99:e19186. doi: 10.1097/MD.0000000000019186
Keywords: fracture, hypoxemia, lipiduria, orthopedics, surgery
Citation: Chen C, Wu Y, Li Y, Tsai F, Li J, Wang J and Lee C (2022) Factors Associated with Postoperative Lipiduria and Hypoxemia in Patients Undergoing Surgery for Orthopedic Fractures. Front. Surg. 9:814229. doi: 10.3389/fsurg.2022.814229
Received: 12 November 2021; Accepted: 11 April 2022;
Published: 28 April 2022.
Edited by:
Philip York, Panorama Orthopedic and Spine Center, United StatesReviewed by:
I-Ming Jou, Eda Hospital, Kaohsiung, TaiwanKonstantinos Markatos, Salamina Medical Center, Greece
Copyright © 2022 Chen, Wu, Li, Tsai, Li, Wang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Sing Wang anN3YW5nQHZnaHRjLmdvdi50dw== Cheng-Hung Lee bGVlY2hlbmdodW5nMDExNUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship.
Speciality section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Chih-Hui Chen
Chih-Hui Chen Yun-Che Wu1†
Yun-Che Wu1† Jun-Sing Wang
Jun-Sing Wang