- 1Department of Clinical Laboratory, Fuzhou Second Hospital Affiliated to Xiamen University, Fuzhou, China
- 2Department of Clinical Laboratory Medicine, Fujian Medical University, Fuzhou, China
Background: The Hippo pathway is an essential signaling cascade that regulates cell and organ growth. However, there is no consensus about (i) the expression levels of the Hippo signaling core components yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) in lung cancer, especially in small cell lung cancer (SCLC), or (ii) their association with the prognosis of patients with SCLC.
Methods: We screened relevant articles and identified eligible studies in the PubMed, EMBASE, COCHRANE, and WanFang databases. A combined analysis was performed to investigate (i) the expression levels of the major effectors, YAP and TAZ, in lung cancer and its subsets and (ii) their prognostic role in lung cancer, especially in SCLC.
Results: In total, 6 studies related to TAZ and 13 studies concerning YAP were enrolled in this meta-analysis. We found that high TAZ expression was significantly associated with poor overall survival (OS) of patients with non-small cell lung cancer (NSCLC) in the overall population [Ph < 0.001, crude hazard ratio (HR) = 1.629, 95% CI = 1.199–2.214 for TAZ expression; Ph = 0.029, adjusted HR = 2.127, 95% CI = 1.307–3.460 for TAZ], the Caucasian population (Ph = 0.043, crude HR = 1.233, 95% CI = 1.030–1.477 for TAZ expression), and the Asian population (Ph = 0.551, adjusted HR = 2.676, 95% CI = 1.798–3.982 for TAZ). Moreover, there was a significant negative association between YAP expression and an unsatisfactory survival of patients with lung cancer (Ph = 0.327, crude HR = 1.652, 95% CI = 1.211–2.253 for YAP expression) and patients with NSCLC [disease-free survival (DFS): Ph = 0.693, crude HR = 2.562, 95% CI = 1.876–3.499 for YAP expression; Ph = 0.920, crude HR = 2.617, 95% CI = 1.690–4.052 for YAP-mRNA; OS: Ph = 0.878, crude HR = 1.777, 95% CI = 1.233–2.562 for YAP expression], especially in the Asian population (DFS: Ph = 0.414, crude HR = 2.515, 95% CI = 1.755–3.063; OS: Ph = 0.712, crude HR = 1.772, 95% CI = 1.214–2.587). However, no association was observed in the multivariate combined analysis. High YAP expression was significantly associated with short OS of patients with SCLC in our combined multivariate analysis in the Asian population (Ph = 0.289, crude HR = 4.482, 95% CI = 2.182–9.209), but not with crude data (Ph = 0.033, crude HR = 1.654, 95% CI = 0.434–6.300).
Conclusion: The Hippo pathway is involved in carcinogenesis and progression of NSCLC and SCLC, and high expression levels of YAP and TAZ are independent and novel prognostic factors for lung cancer.
Introduction
Lung cancer is a primary malignancy with one of the highest rates of morbidity and mortality in China (1). According to its histological classification, lung cancer is stratified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), of which the latter subgroup accounts for ~80% of lung cancer cases (2). While emerging molecular-targeted and onco-immunological therapeutics are increasingly used in the clinic (2, 3), the survival rates of patients need to be further improved.
The Hippo pathway is an important signaling cascade related to cell proliferation, apoptosis, and differentiation. Yes-associated protein 1 (YAP1) and transcriptional co-activator with PDZ-binding motif (TAZ) are transcription co-activators and the core downstream effectors of the Hippo signaling pathway (4). When the Hippo pathway is activated, the two molecules are phosphorylated and retained in the cytoplasm in normal cells. On the contrary, YAP/TAZ can translocate into the nucleus to activate the transcription of multiple oncogenes in tumor cells (5). Meanwhile, they are frequently upregulated and biologically function as oncogenes via participation in multiple cancer-related processes, including cancer cell growth, migration, and distal metastasis in various malignancies, such as colorectal, gastric, and lung cancer (6–8).
Several studies have shown that the Hippo pathway is activated upon carcinogenesis and lung cancer progression (9–11). However, different YAP and TAZ expression levels have been reported in lung cancer and its subsets. Moreover, no consensus has been reached with respect to their prognostic values in patients with lung cancer. Yang et al. reported that YAP was not associated with the disease stage and survival of patients with SCLC (12). In another study, higher expression of YAP1 was associated with worse progression-free survival in patients with epidermal growth factor receptor (EGFR)-mutant NSCLC treated with first-line EGFR tyrosine kinase inhibitors (13). Although higher TAZ mRNA and protein levels were associated with shorter survival of patients with NSCLC (14), the nuclear localization of TAZ was increased, which was correlated with poor prognosis in lung squamous cell carcinomas, but not lung adenocarcinomas (15).
To better understand the prognostic role of the Hippo pathway in lung cancer, we investigated YAP and TAZ expression in lung cancer and analyzed their associations with the prognosis of patients with lung cancer in this meta-analysis.
Materials and Methods
The Medical Ethics Committee of Fuzhou Second Hospital affiliated with Xiamen University approved this study. To obtain studies for our meta-analysis, we screened and identified the relevant articles according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). First, the following words and phrases were used to identify candidate articles in the PubMed, EMBASE, COCHRANE, and WanFang databases: “lung cancer,” “lung squamous cell carcinomas,” “lung adenocarcinomas,” “non-small cell lung cancer,” “small cell lung cancer,” “YAP1,” “YAP,” “Yes-associated protein,” “Hippo pathway,” “transcriptional co-activator with PDZ-binding motif,” “TAZ,” “WWTR1,” “survival,” “outcome,” “prognosis,” “progression-free survival,” “recurrence-free survival,” “disease-free survival,” and “overall survival.” The retrieval deadline was August 30, 2021. We also screened references of the relevant reports to obtain additional studies. After reading the title and abstract of the candidate articles, we identified the relevant studies. Finally, we identified eligible studies by reading the full texts of the relevant articles. The inclusion criteria were as follows: (1) the study reported the relationship between YAP or TAZ and prognosis of lung cancer and (2) the study provided clinical baseline characteristics and reported the results in hazard ratios (HRs) and 95% CIs. Articles that did not provide detailed survival data and comments were excluded, along with letters, reviews, and meta-analyses.
The following clinical baseline characteristics were extracted from each study: the first author's name, study design, country, race, recruitment time, disease, number of included cases, treatment, outcome, HR, and 95% CI. All data were rechecked.
The HR and 95% CI were selected as parameters to assess the strength of the association between YAP1 or TAZ and the prognosis of the cases with lung cancer. The Q test and estimated I2 were selected to evaluate the heterogeneity of included studies in this meta-analysis, where Ph < 0.1 or I2 > 50% was considered to indicate substantial heterogeneity. The combined analysis of eligible studies was assessed by the Z test based on a fixed (Ph > 0.1) or random (Ph < 0.1) model. Publication bias within the included studies was evaluated by funnel plots (17). The Stata v.11.0 software (Stata Corporation, College Station, TX, USA) was used for all statistical analyses, and p < 0.05 was considered to indicate statistical significance.
Results
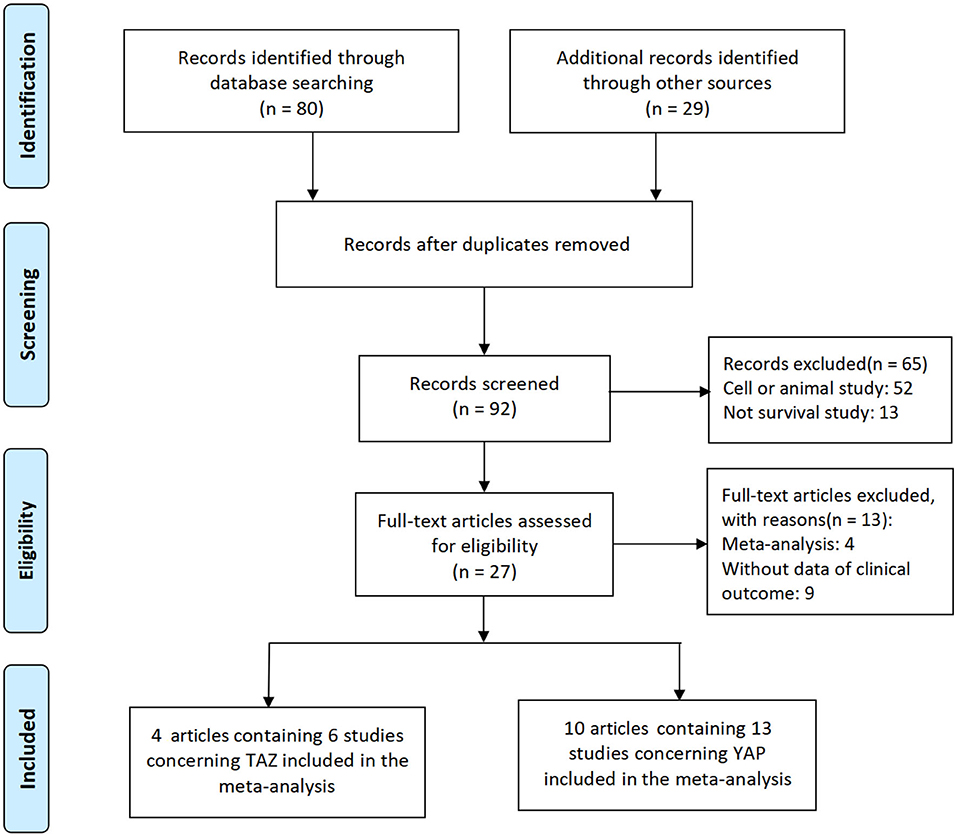
The detailed search and selection procedure of eligible studies is described in Figure 1. Initially, a total of 109 articles were selected. After the exclusion of duplicated articles, unrelated research, and other studies that did not meet the inclusion criteria, 14 articles, including 19 studies, were included in this meta-analysis (13–15, 18–28).
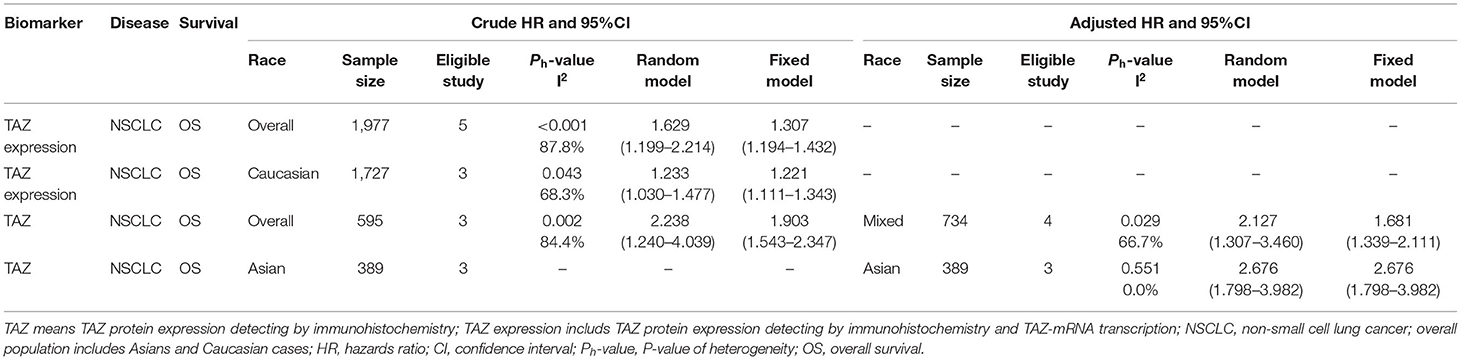
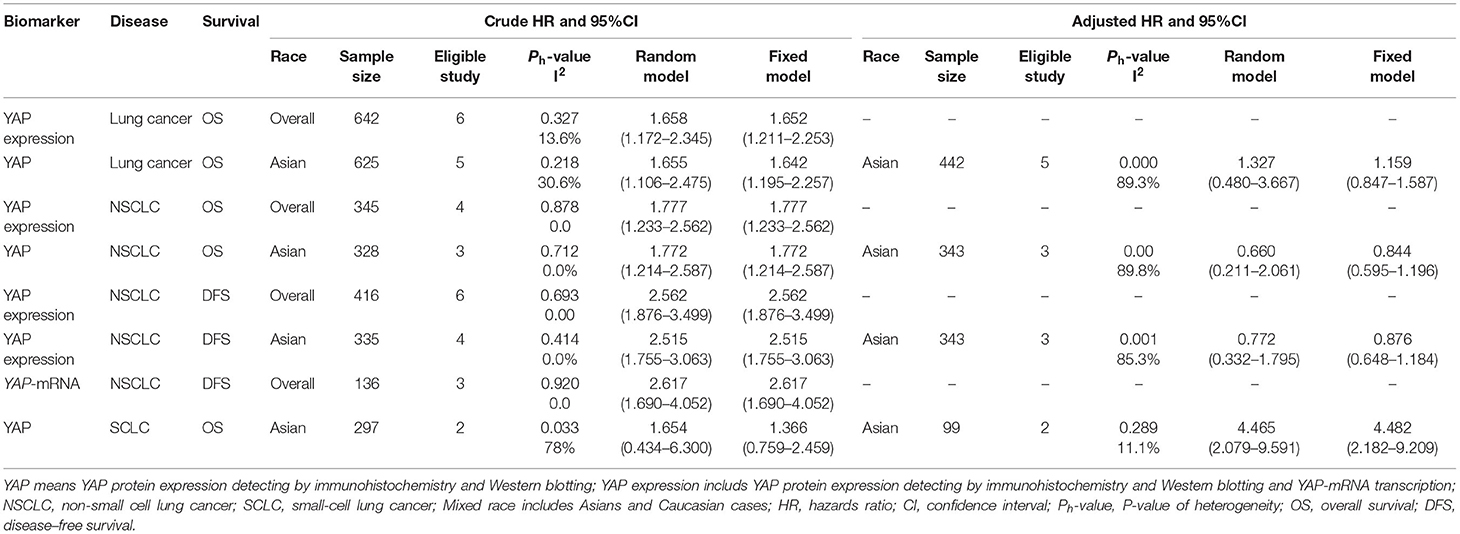
The detailed baseline characteristics of these studies are described in Table 1. Among the 14 included articles, 4 articles including 6 studies (14, 15, 23, 28), and 10 articles (13, 18–22, 24–27) containing 13 studies reported the relationship between TAZ and YAP and the prognosis of patients with lung cancer, respectively. Three studies, including 350 cases, reported these associations concerning SCLC in the Chinese population (24, 26), and the other 16 studies, including 857 cases, reported these relationships concerning NSCLC. Three studies were conducted in the Caucasian population (13, 14, 21), and the others were performed in the East Asian population. Most of the studies reported YAP and TAZ protein expression in lung cancer. However, three studies reported mRNA levels (13, 14, 21).
The combined HR and 95% CI of TAZ expression and overall survival (OS) of patients with NSCLC are described in Figure 2 and Table 2. TAZ expression and OS of patients with NSCLC were reported for a total of 1,977 patients over five studies. Two studies reported TAZ mRNA levels, and three studies were conducted in the Asian population. The crude and adjusted HR and 95% CI were extracted from five and three studies, respectively. The combined results showed that high TAZ expression was significantly associated with poor OS in the overall population (Ph < 0.001, I2 = 87.8%, crude HR = 1.629, 95% CI = 1.199–2.214 for TAZ and TAZ; Ph = 0.029, I2 = 66.7%, adjusted HR = 2.217, 95% CI = 1.307–3.460 for TAZ), the Caucasian population (Ph = 0.043, I2 = 68.3%, crude HR = 1.233, 95% CI = 1.030–1.477), and the Asian population (Ph = 0.551, I2 = 0.00%, adjusted HR = 2.676, 95% CI = 1.798–3.982).
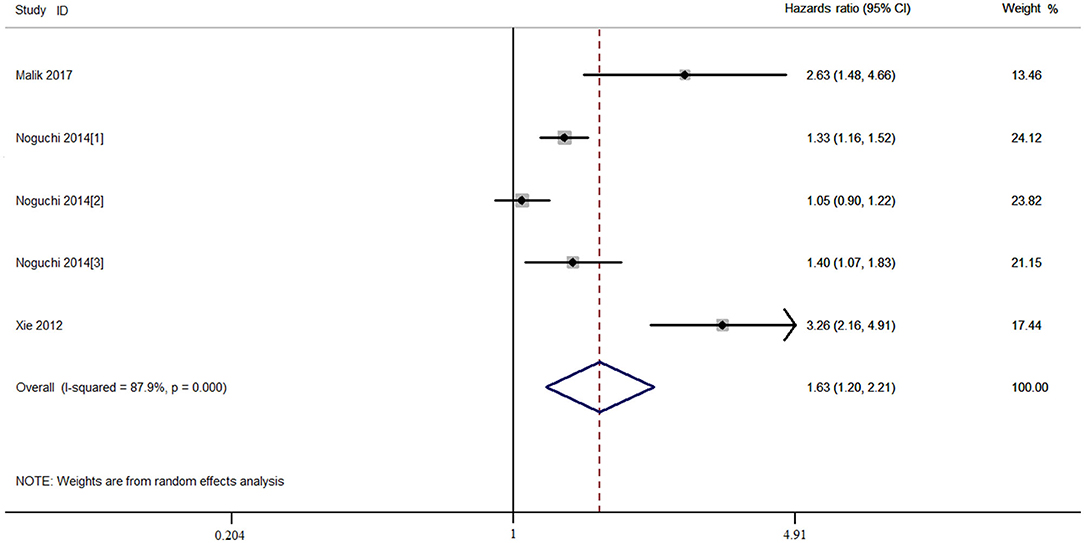
Figure 2. Combined meta-analysis between transcriptional co-activator with PDZ-binding motif (TAZ) expression and overall survival of patients with non-small cell lung cancer (NSCLC).
In the combined analysis of YAP expression and survival of patients with lung cancer, the OS of the patients with lung cancer with high YAP expression was significantly shorter than in patients with low YAP expression in the overall population (Ph = 0.327, I2 = 13.6%, crude HR = 1.652, 95% CI = 1.211–2.253) (Figure 3; Table 3), and even more so in the Asian population (Ph = 0.218, I2 = 30.6%, crude HR = 1.642, 95% CI = 1.195–2.257). The significant negative association between YAP expression and survival of patients with NSCLC was also observed in the overall population [disease-free survival (DFS): Ph = 0.878, I2 = 0.00%, crude HR = 2.562, 95% CI = 1.876–3.499; OS: Ph = 0.878, I2 = 0.00%, crude HR = 1.777, 95% CI = 1.233–2.562] and the Asian population (DFS: Ph = 0.414, I2 = 0.00%, crude HR = 2.515, 95% CI = 1.755–3.063; OS: Ph = 0.712, I2 = 0.00%, crude HR = 1.772, 95% CI = 1.214–2.587). Moreover, high YAP-mRNA was also significantly associated with the survival of patients with NSCLC (DFS: Ph = 0.920, I2 = 0.00%, crude HR = 2.617, 95% CI = 1.690–4.052). However, no association between them was observed in the multivariate combined analysis. High YAP expression was significantly associated with short OS of patients with SCLC in our combined multivariate analysis in the Asian population (Ph = 0.289, I2 = 11.10%, crude HR = 4.482, 95% CI = 2.182–9.209), but not with the crude data (Ph = 0.033, I2 = 78.00%, crude HR = 1.654, 95% CI = 0.434–6.300) (Figure 3; Table 3).
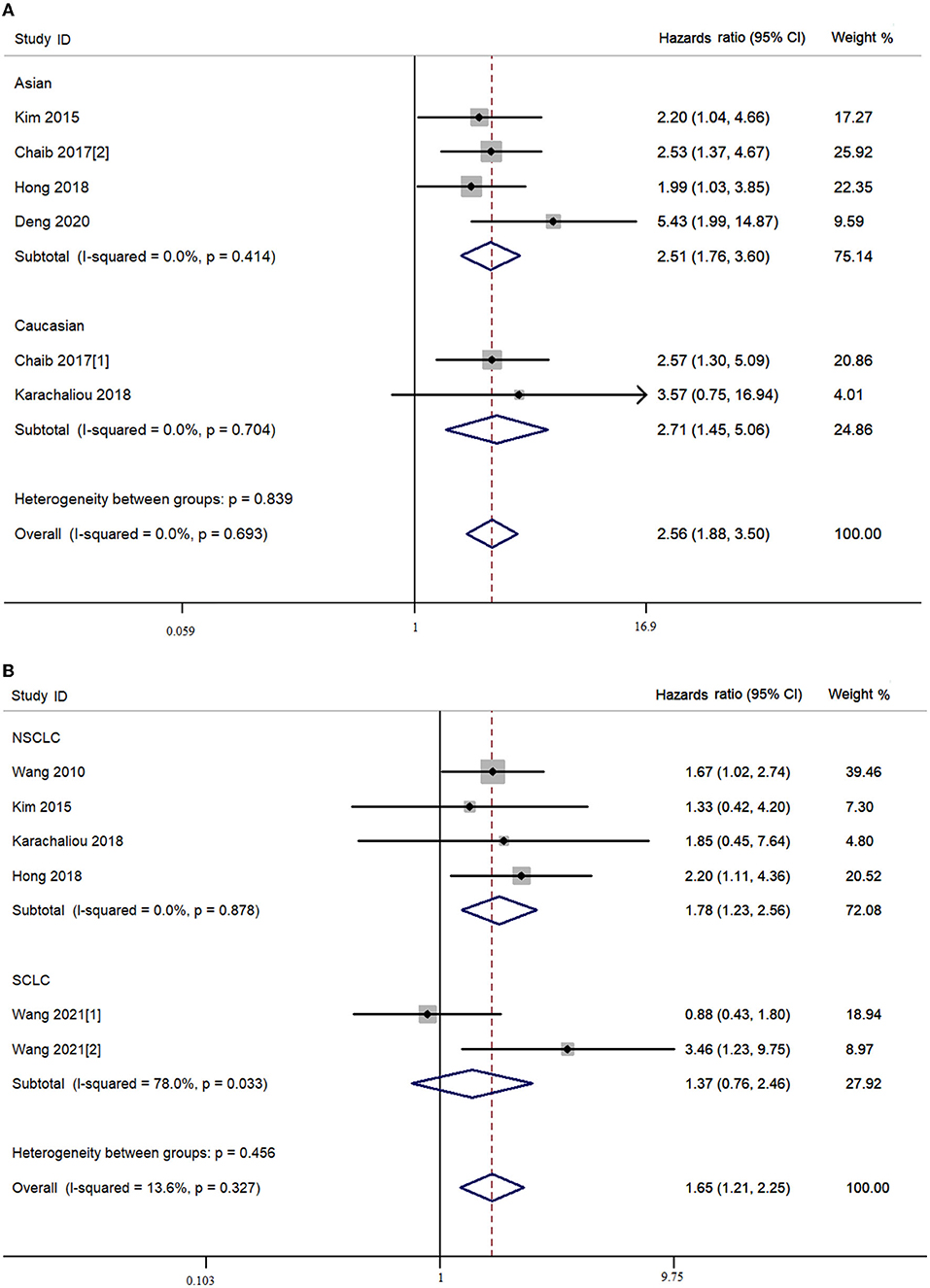
Figure 3. Combined meta-analysis between Yes-associated protein (YAP) expression and survival of lung cancer. (A) Disease-free survival. (B) Overall survival.
In our study, relatively symmetric funnel plots were observed in our prognostic comparisons of patients with high- and low-TAZ NSCLC. Moreover, symmetric funnel plots were also found in the combined survival analysis of the patients with high- and low-YAP lung cancer, NSCLC, and SCLC (Figure 4).
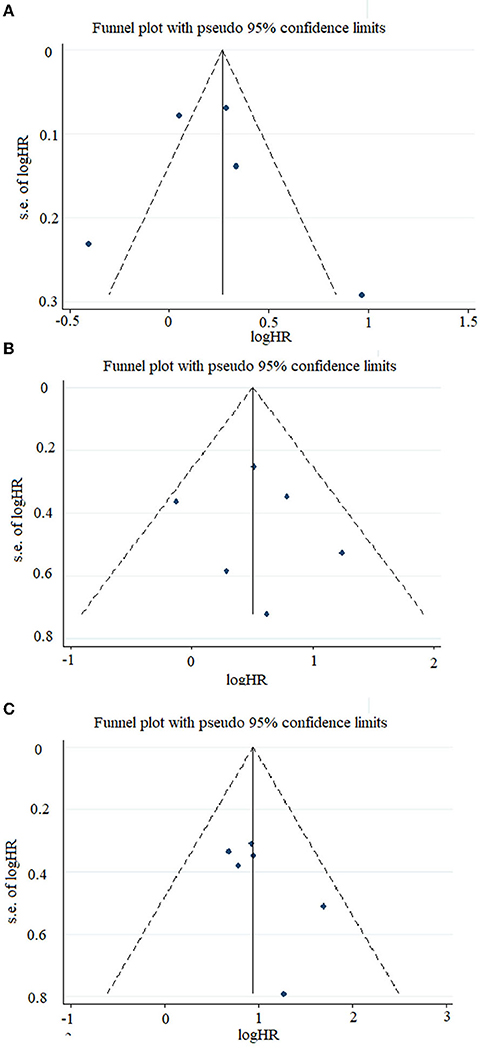
Figure 4. Funnel plots of the studies included in this meta-analysis. (A) Included studies related to TAZ expression and overall survival in the overall population. (B) Included studies related to YAP expression and disease-free survival in the overall population. (C) Included studies related to YAP expression and overall survival in the overall population.
Discussion
The association of YAP and TAZ expression and lung cancer survival, particularly in patients with SCLC, remains unclear. This meta-analysis revealed that TAZ is significantly associated with poor OS of patients with NSCLC in the overall population and the Asian population. Moreover, a significant association was observed between high YAP expression and unsatisfactory survival of patients with lung cancer and patients with NSCLC, especially in the Asian population. However, no association between them was observed in the multivariate combined analysis. YAP expression was significantly associated with short OS in our combined analysis in the Asian SCLC population based on multivariate data, but not crude data.
Lung cancer is a complex and challenging disease that interacts with environmental and genetic factors (29). Many signaling pathways, including the Hippo pathway, are involved in lung cancer tumorigenesis and progression (30–34). YAP and TAZ are transcriptional co-factors. They are considered oncogenes in both NSCLC and SCLC (35, 36). YAP was found to mainly regulate lung cancer cells' growth and proliferation. In contrast, TAZ mainly promotes the migration of cancer cells (11). A recent meta-analysis performed by Feng et al. revealed that overexpression of TAZ is a predictive factor of poor prognosis and is associated with advanced TNM stage, poor tumor differentiation, and lymph node metastasis in various cancers (37). However, no association between TAZ expression and survival of patients with NSCLC was observed in a combined analysis of two studies (37). In our meta-analysis, TAZ was highly expressed in patients with NSCLC in most studies. The combined results showed that high TAZ expression is associated with a worse prognosis of patients with NSCLC in the overall and Asian populations. These findings suggest that TAZ is involved in NSCLC carcinogenesis and progression and that it is an independent prognostic factor of NSCLC.
Three meta-analyses reported the predictive role of YAP in clinical outcomes in various cancers (38–40). They found that both overall and nuclear YAP overexpression are intimately associated with worse OS and DFS in patients with malignancies (38, 40). Wu et al. reported no association between YAP expression and survival of lung cancer cases in their combined meta-analysis (40). However, a meta-analysis including six eligible studies showed that high nuclear expression of YAP1 was associated with shorter survival outcomes in patients with NSCLC (39). In the present study, we found an association between YAP expression and unsatisfactory survival of patients with lung cancer in the overall Asian population in our combined analysis with crude HR and 95% CI. Moreover, YAP expression at the protein and mRNA levels was significantly associated with the survival of patients with NSCLC using unadjusted HR and 95% CI. However, due to the small sample size and different confounding factors in each included study, we did not observe associations between them in combination with multivariate data. These findings demonstrate that YAP is an essential factor promoting the progression of lung cancer and NSCLC and that it is a novel prognostic factor for the disease. Due to the low number of studies in the Asian population in our combined analysis, we found no relationship between YAP expression and OS of patients with SCLC in our univariate analysis, whereas YAP expression was significantly associated with short OS of patients with SCLC in our combined analysis with multivariate data in the Asian population, indicating that YAP can also be considered a predictor of poor survival of patients with SCLC.
To the best of our knowledge, this meta-analysis used the largest sample size to date to analyze the prognostic roles of TAZ and YAP in lung cancer and patients with NSCLC. In addition, this is the first study to report the role of YAP in the prognosis of patients with SCLC. However, there are several limitations to this study. First, no studies on the association between TAZ expression and survival of patients with SCLC were included, so the prognostic role of TAZ in SCLC remains to be elucidated. Second, the sample size of included studies related to YAP expression in patients with SCLC was small. Our findings should be validated by multi-center clinical trials with larger sample sizes. It is also important to emphasize that most studies were conducted in the Asian population. Third, patients with different TNM stages and various treatment options were enrolled in each study. These factors might influence the combined results and hence, the results should be validated by prospective studies with patients with NSCLC or SCLC in specific TNM stages. Fourth, different expression models of YAP or TAZ, different detection methods, and different genetic backgrounds of the patients enrolled in the included studies might lead to inconsistent results.
In summary, YAP and TAZ function as oncogenes in both NSCLC and SCLC and aberrant protein expression levels of YAP and TAZ are independent and novel prognostic factors for these diseases. Further studies are warranted to validate the findings in the Asian population and explore effective biomarkers to predict the prognosis of patients.
Author Contributions
YJ is responsible for the concept or design of the work. W-JX and R-WC are responsible for data collection. W-LY and HC are responsible for drafting the article. W-XC and J-PX are responsible for making important revisions to the article. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Fujian Provincial Natural Science Foundation (2018J01360).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
1. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
2. Ying HQ, Liao YC, Luo YR, Xiong G, Huang Y, Nie RW, et al. Cancer-elicited inflammation attenuates response and outcome in tyrosine kinase inhibitor naive patients with advanced NSCLC. Pharmacol Res. (2021) 170:105734. doi: 10.1016/j.phrs.2021.105734
3. Cui P, Huang D, Wu Z, Tao H, Zhang S, Ma J, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol. (2020) 12:1758835920922033. doi: 10.1177/1758835920922033
4. Morciano G, Vezzani B, Missiroli S, Boncompagni C, Pinton P, Giorgi C. An updated understanding of the role of YAP in driving oncogenic responses. Cancers. (2021) 13:3100. doi: 10.3390/cancers13123100
5. Yin F, Dong J, Kang LI, Liu X. Hippo-YAP signaling in digestive system tumors. Am J Cancer Res. (2021) 11:2495–507.
6. Ou C, Sun Z, Li S, Li G, Li X, Ma J. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget. (2017) 8:75727–41. doi: 10.18632/oncotarget.20155
7. Wei J, Wang L, Zhu J, Sun A, Yu G, Chen M, et al. The Hippo signaling effector WWTR1 is a metastatic biomarker of gastric cardia adenocarcinoma. Cancer Cell Int. (2019) 19:74. doi: 10.1186/s12935-019-0796-z
8. Lee TF, Liu YP, Lin YF, Hsu CF, Lin H, Chang WC, et al. TAZ negatively regulates the novel tumor suppressor ANKRD52 and promotes PAK1 dephosphorylation in lung adenocarcinomas. Biochim Biophys Acta Mol Cell Res. (2021) 1868:118891. doi: 10.1016/j.bbamcr.2020.118891
9. Gu C, Chen J, Dang X, Chen C, Huang Z, Shen W, et al. Hippo pathway core genes based prognostic signature and immune infiltration patterns in lung squamous cell carcinoma. Front Oncol. (2021) 11:680918. doi: 10.3389/fonc.2021.680918
10. Lo Sardo F, Pulito C, Sacconi A, Korita E, Sudol M, Strano S, et al. YAP/TAZ and EZH2 synergize to impair tumor suppressor activity of TGFBR2 in non-small cell lung cancer. Cancer Lett. (2021) 500:51–63. doi: 10.1016/j.canlet.2020.11.037
11. Shreberk-Shaked M, Dassa B, Sinha S, Di Agostino S, Azuri I, Mukherjee S, et al. A division of labor between YAP and TAZ in non-small cell lung cancer. Cancer Res. (2020) 80:4145–57. doi: 10.1158/0008-5472.CAN-20-0125
12. Yang K, Zhao Y, Du Y, Tang R. Evaluation of hippo pathway and CD133 in radiation resistance in small-cell lung cancer. J Oncol. (2021) 2021:8842554. doi: 10.1155/2021/8842554
13. Chaib I, Karachaliou N, Pilotto S, Codony Servat J, Cai X, Li X, et al. Co-activation of STAT3 and YES-associated protein 1 (YAP1) pathway in EGFR-mutant NSCLC. J Natl Cancer Inst. (2017) 109. doi: 10.1093/jnci/djx014
14. Noguchi S, Saito A, Horie M, Mikami Y, Suzuki HI, Morishita Y, et al. An integrative analysis of the tumorigenic role of TAZ in human non-small cell lung cancer. Clin Cancer Res. (2014) 20:4660–72. doi: 10.1158/1078-0432.CCR-13-3328
15. Wang Y, Han Y, Guo Z, Yang Y, Ren T. Nuclear TAZ activity distinctly associates with subtypes of non-small cell lung cancer. Biochem Biophys Res Commun. (2019) 509:828–32. doi: 10.1016/j.bbrc.2019.01.012
16. You XH, Jiang YH, Fang Z, Sun F, Li Y, Wang W, et al. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open. (2020) 4:e000605. doi: 10.1136/esmoopen-2019-000605
17. Yimam Y, Woreta A, Mohebali M. Intestinal parasites among food handlers of food service establishments in Ethiopia: a systematic review and meta-analysis. BMC Public Health. (2020) 20:73. doi: 10.1186/s12889-020-8167-1
18. Chen MJ, Wang YC, Wu DW, Chen CY, Lee H. Association of nuclear localization of SHP2 and YAP1 with unfavorable prognosis in non-small cell lung cancer. Pathol Res Pract. (2019) 215:801–6. doi: 10.1016/j.prp.2019.01.027
19. Deng KL, Li YP, Liu GX, Peng QZ. Expression and clinical significance of YAP, Last 1 and Mast 1 proteins in lung cancer tissues. J Intern Intens Med. (2020) 26:418–22+32.
20. Hong SA, Jang SH, Oh MH, Kim SJ, Kang JH, Hong SH. Overexpression of YAP1 in EGFR mutant lung adenocarcinoma prior to tyrosine kinase inhibitor therapy is associated with poor survival. Pathol Res Pract. (2018) 214:335–42. doi: 10.1016/j.prp.2018.01.010
21. Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol. (2018) 10:1758834017749748. doi: 10.1177/1758834017749748
22. Kim MH, Kim YK, Shin DH, Lee HJ, Shin N, Kim A, et al. Yes associated protein is a poor prognostic factor in well-differentiated lung adenocarcinoma. Int J Clin Exp Pathol. (2015) 8:15933–9.
23. Malik SA, Khan MS, Dar M, Hussain MU, Mudassar S. TAZ is an independent prognostic factor in non-small cell lung carcinoma: elucidation at protein level. Cancer Biomark. (2017) 18:389–95. doi: 10.3233/CBM-160263
24. Song Y, Sun Y, Lei Y, Yang K, Tang R. YAP1 promotes multidrug resistance of small cell lung cancer by CD74-related signaling pathways. Cancer Med. (2020) 9:259–68. doi: 10.1002/cam4.2668
25. Sun PL, Kim JE, Yoo SB, Kim H, Jin Y, Jheon S, et al. Cytoplasmic YAP expression is associated with prolonged survival in patients with lung adenocarcinomas and epidermal growth factor receptor tyrosine kinase inhibitor treatment. Ann Surg Oncol. (2014) 21 (Suppl. 4):S610–8. doi: 10.1245/s10434-014-3715-5
26. Wang X, Guo Y, Liu L, Wei J, Zhang J, Xie T, et al. YAP1 protein expression has variant prognostic significance in small cell lung cancer (SCLC) stratified by histological subtypes. Lung Cancer. (2021) 160:166–74. doi: 10.1016/j.lungcan.2021.06.026
27. Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. (2010) 101:1279–85. doi: 10.1111/j.1349-7006.2010.01511.x
28. Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu ZH, et al. Prognostic significance of TAZ expression in resected non-small cell lung cancer. J Thorac Oncol. (2012) 7:799–807. doi: 10.1097/JTO.0b013e318248240b
29. Wu L, Leng D, Cun D, Foged C, Yang M. Advances in combination therapy of lung cancer: rationales, delivery technologies and dosage regimens. J Control Release. (2017) 260:78–91. doi: 10.1016/j.jconrel.2017.05.023
30. Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat. (2010) 13:67–78. doi: 10.1016/j.drup.2010.04.001
31. Crees ZD, Shearrow C, Lin L, Girard J, Arasi K, Bhoraskar A, et al. EGFR/c-Met and mTOR signaling are predictors of survival in non-small cell lung cancer. Ther Adv Med Oncol. (2020) 12:1758835920953731. doi: 10.1177/1758835920953731
32. Lim JS, Ibaseta A, Fischer MM, Cancilla B, O'Young G, Cristea S, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. (2017) 545:360–4. doi: 10.1038/nature22323
33. Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. (2012) 38:292–302. doi: 10.1016/j.ctrv.2011.07.008
34. Wang W, Huang Q, Chen Y, Huang Z, Huang Y, Wang Y, et al. The novel FAT4 activator jujuboside A suppresses NSCLC tumorigenesis by activating HIPPO signaling and inhibiting YAP nuclear translocation. Pharmacol Res. (2021) 170:105723. doi: 10.1016/j.phrs.2021.105723
35. Horie M, Saito A, Ohshima M, Suzuki HI, Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer Sci. (2016) 107:1755–66. doi: 10.1111/cas.13078
36. Yeung B, Yu J, Yang X. Roles of the Hippo pathway in lung development and tumorigenesis. Int J Cancer. (2016) 138:533–9. doi: 10.1002/ijc.29457
37. Feng J, Ren P, Gou J, Li Z. Prognostic significance of TAZ expression in various cancers: a meta-analysis. Onco Targets Ther. (2016) 9:5235–44. doi: 10.2147/OTT.S102616
38. Sun Z, Xu R, Li X, Ren W, Ou C, Wang Q, et al. Prognostic value of Yes-associated protein 1 (YAP1) in various cancers: a meta-analysis. PLoS ONE. (2015) 10:e0135119. doi: 10.1371/journal.pone.0135119
39. Zhu L, Ma G, Liu J, Deng Y, Wu Q, Chen W, et al. Prognostic significance of nuclear Yes-associated protein 1 in patients with nonsmall cell lung cancer: a systematic review and meta-analysis. Medicine. (2019) 98:e15069. doi: 10.1097/MD.0000000000018264
Keywords: non-small cell lung cancer, Hippo pathway, YAP, TAZ, prognosis
Citation: Jiang Y, Xie W-J, Chen R-W, You W-W, Ye W-L, Chen H, Chen W-X and Xu J-P (2022) The Hippo Signaling Core Components YAP and TAZ as New Prognostic Factors in Lung Cancer. Front. Surg. 9:813123. doi: 10.3389/fsurg.2022.813123
Received: 11 November 2021; Accepted: 31 January 2022;
Published: 21 March 2022.
Edited by:
Zeming Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Hou-Qun Ying, Second Affiliated Hospital of Nanchang University, ChinaShulong Zhang, Shanghai Xuhui Central Hospital, China
Copyright © 2022 Jiang, Xie, Chen, You, Ye, Chen, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Xu Chen, Y3d4anlrQDE2My5jb20=; Jian-Ping Xu, amlhbnBpbmd4dTY1QHllYWgubmV0; Yu Jiang, MTM4MDk1NTA5NTFAMTM5LmNvbQ==
 Yu Jiang
Yu Jiang Wen-Jing Xie1
Wen-Jing Xie1