
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 23 February 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.769041
This article is part of the Research Topic Tissue Healing Complications in Abdominal Surgery View all 5 articles
Diabetic ketoacidosis (DKA), an acute and life-threatening complication of diabetes, is a metabolic disorder caused by insulin deficiency and an increase in counter-regulatory hormones. Several cases of DKA without marked hyperglycemia have been reported and are defined as euglycemic DKA (eu-DKA). The use of sodium-glucose cotransporter 2 inhibitors (SGLT2is) is associated with the occurrence of eu-DKA, of which, dapagliflozin is one of the agents. In this study, we report a case of dapagliflozin-associated eu-DKA following surgery for pancreatic carcinoma. A 57-year-old woman presented with acute abdominal pain after surgery for pancreatic carcinoma. Emergency exploratory laparotomy was performed because of suspicion of gastrointestinal perforation based on a CT scan. The surgeons observed that the stomach was significantly dilated but not perforated. Meanwhile, the patient developed shock and severe acidosis. A further examination confirmed the diagnosis of dapagliflozin-associated eu-DKA. We reviewed the precipitating factors and mechanisms of SGLT2i-associated eu-DKA and discussed the treatment and prevention of this condition. Clinicians need to be alert of the occurrence of SGLT2i-associated eu-DKA in patients treated with this drug in the perioperative period.
Diabetic ketoacidosis (DKA), an acute and life-threatening complication of diabetes, is a metabolic disorder caused by insulin deficiency and an increase in counter-regulatory hormones. As per the United States and United Kingdom guidelines, DKA is usually diagnosed based on hyperglycemia [blood glucose (BG) threshold of 250 or 200 mg/dl], ketosis, and metabolic acidosis (1, 2). However, several cases of DKA have been reported without marked hyperglycemia and are defined as euglycemic DKA (eu-DKA) (1, 3). Because of the absence of hyperglycemia, the diagnosis or exclusion of eu-DKA appears more challenging in emergencies. Generally, the occurrence of eu-DKA is facilitated by the following precipitating factors: strict diet/starvation, pregnancy, alcohol consumption, and use of sodium-glucose cotransporter 2 inhibitors (SGLT2is) (1, 4). Recently, there have been increasing reports of SGLT2i-related Eu-DKA after the use of drugs such as canagliflozin, ipragliflozin, empagliflozin, and dapagliflozin (5–7). However, the use of these agents has not received sufficient attention. Several cases of misdiagnosis or delayed diagnosis have been reported.
Dapagliflozin, an SGLT2i, prevents the reabsorption of glucose from primary urine by inhibiting renal SGLT2 receptors (3, 8). The pathogenesis of euglycemia in SGLT2i-associated eu-DKA is thought to be associated with increased renal glucose clearance by SGLT2is (1, 6). However, eu-DKA does not occur in all patients receiving SGLT2is. More research is required on precipitating factors and mechanisms of SGLT2i-associated eu-DKA.
In this study, we reported a case of dapagliflozin-associated eu-DKA after surgery for pancreatic carcinoma. A 57-year-old woman presented with acute abdominal pain after surgery for pancreatic carcinoma. Emergency exploratory laparotomy was performed because of suspicion of gastrointestinal perforation based on a CT scan. The surgeons observed that the stomach was significantly dilated but not perforated. In addition, the patient developed shock and severe acidosis. Further examination confirmed the diagnosis of dapagliflozin-associated eu-DKA. We report this case to remind surgeons to be alert of the occurrence of SGLT2i-associated eu-DKA in patients receiving this medication during the perioperative period.
A 57-year-old woman was admitted to a hospital with upper abdominal pain for more than half a year. The pain was mild. Physical examination of the patient did not reveal any pathological findings. The patient had been taking appropriate oral medications for diabetes mellitus, hypertension, and stroke for the past 6 years. The oral agents prescribed to the patient at the time of presentation were metformin (0.5 g twice daily), dapagliflozin (5 mg once daily), and aspirin (100 mg once daily). Contrast-enhanced CT revealed an enhanced mass on the tail of the pancreas invading the splenic vessel, which was diagnosed as pancreatic carcinoma (Figure 1). The clinical stage was T2N0M0 according to the American Joint Committee on Cancer tumor node metastasis (TNM) staging (8th edition). Laparoscopic distal pancreatectomy with en bloc splenectomy was conducted. Short gastric arteries were ligated. A hard grayish-white mass of about 4 cm in diameter was observed on the tail of the pancreas, and pathological investigations revealed a poorly differentiated pancreatic ductal adenocarcinoma. Somatostatin was routinely used to suppress pancreatic secretion after surgery. The patient started a clear liquid diet on the third postoperative day. Meanwhile, metformin (0.5 g twice daily) and dapagliflozin (5 mg once daily) were reinitiated, and blood glucose level was monitored. The patient only experienced slight back and incision site pain. Daily drainage volume consistently remained between 10 and 80 ml. The amylase level of the drainage fluid was 50,600 U/L on the 3rd postoperative day. The postoperative pancreatic fistula was diagnosed according to the definition of the International Study Group of Pancreatic Surgery. However, the patient had a sudden onset of severe postprandial abdominal pain on the 8th postoperative day. Physical examination showed abdominal distention, mild tenderness, and suspicious rebound tenderness. The patient had no chills or fever, and her vital signs were stable. A small amount of bloody fluid was drained from the abdominal drainage tube. Peripheral blood investigation revealed a white blood cell (WBC) count of 16,000 μl (neutrophils 87.8%). Serum biochemical examination revealed the following: glucose 223 mg/dl, total bilirubin 8.6 umol/L, alanine aminotransferase (ALT) 9 U/L, albumin 3.61 g/dl, and C-reactive protein 168.1 mg/L. Postoperative BG levels of the patient consistently remained between 86 and 295 mg/dl (Figure 2). Emergency contrast-enhanced CT revealed free intraperitoneal air and suspected discontinuity of the wall of greater gastric curvature (Figure 3). Considering the possibility of gastric perforation, an emergency exploratory laparotomy was performed. However, the surgeons observed that the stomach was significantly dilated but not perforated. Blood supply to the stomach wall was normal, and there was no necrosis in any part of the stomach. Meanwhile, the patient developed shock with severe acidosis (pH 6.8). The patient was transferred to the surgical intensive care unit after the emergency surgery. Arterial blood gas analysis revealed pH of 7.09, partial pressure of carbon dioxide of 56.7 mmHg, of 14 mmol/L, and anion gap of 36.2 mmol/L, indicating high anion gap metabolic acidosis with respiratory acidosis. The patient's temperature was 36.5°C, blood pressure was 114/61 mmHg (with norepinephrine, 0.13 ug/kg*min), and heart rate was 125 bpm. Peripheral blood investigation revealed a white blood cells (WBC) count of 26,800 μl (neutrophils 87.5%). Serum biochemical examination revealed the following: glucose, 270 mg/dl, procalcitonin 2.66 ng/ml, total bilirubin 8.9 umol/l, ALT 28U/l, albumin 3.33 g/dl, and C-reactive protein 137.8 mg/L. Based on the results of serum biochemical investigations, symptoms, medical history, and surgical findings, we suspected eu-DKA, which misguided the surgeons into performing exploratory laparotomy. Then, blood beta-hydroxybutyrate (BOHB) was revealed to be 10.87 mmol/L (normal range 0.03-0.3 mmol/L), and urine ketones were strongly positive (4+). These results confirmed our diagnosis. Through literature review, we identified dapagliflozin as the possible cause of eu-DKA. Dapagliflozin was discontinued, and the patient underwent fluid replacement and insulin therapy. One day later, the shock and severe acidosis were corrected, and the endotracheal tube was removed. Four days later, the patient was transferred to a regular room. The patient was treated with nutritional support, suppression of pancreatic secretion, and insulin to control blood glucose. The patient recovered and was discharged on the 42nd day of hospitalization.
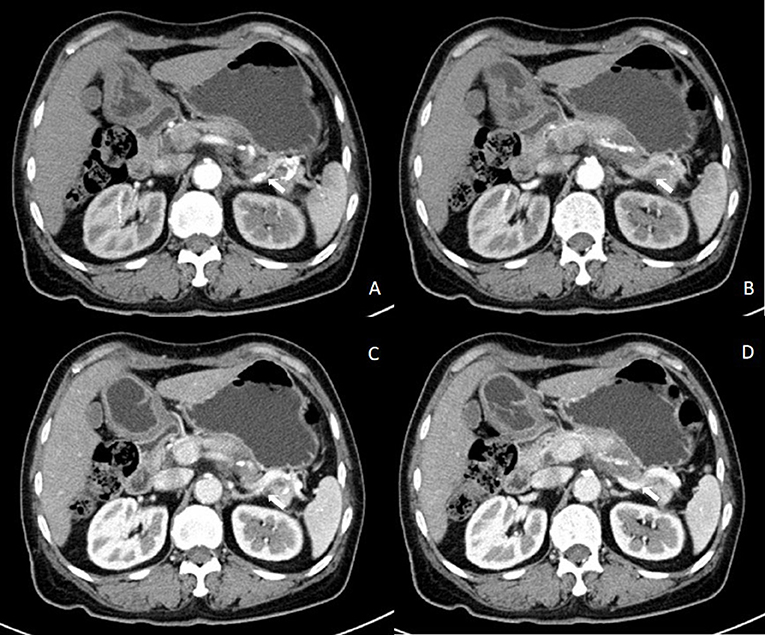
Figure 1. Results of contrast-enhanced abdominal CT before the first surgery. Contrast-enhanced abdominal CT in the (A,B) arterial phase and in the (C,D) venous phase showing an enhanced mass on the tail of the pancreas invading the splenic vessel.
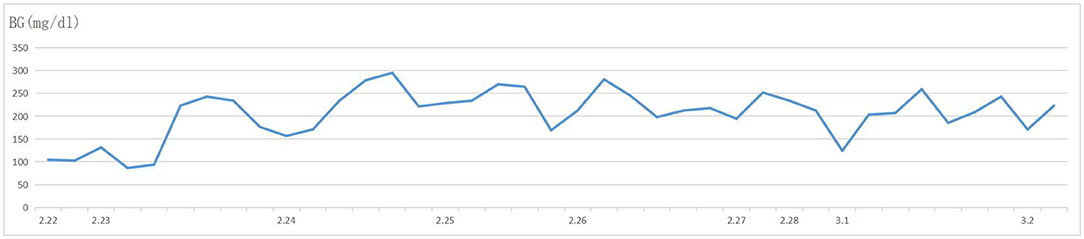
Figure 2. Blood glucose levels of the patient after resection of the pancreatic carcinoma. The level of blood glucose consistently remained between 86 and 295 mg/dl after resection of the pancreatic carcinoma.
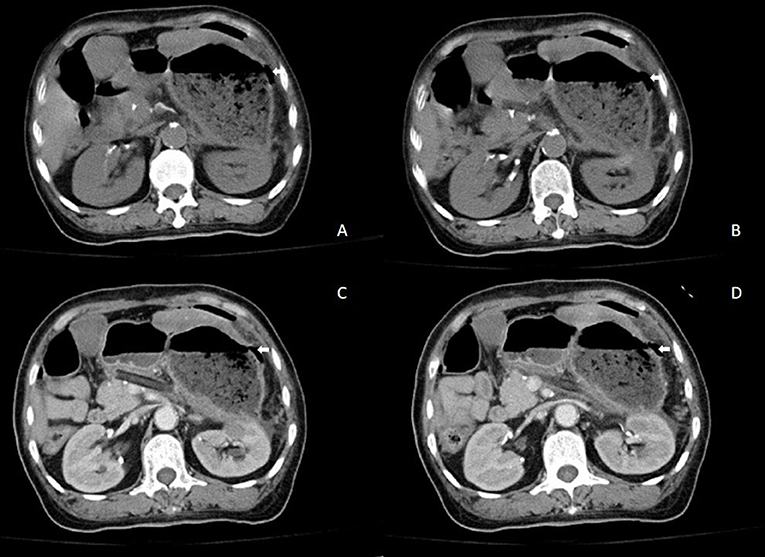
Figure 3. Results of contrast-enhanced abdominal CT when the patient suddenly presented with acute abdominal pain. (A,B) Abdominal CT and (C,D) contrast-enhanced abdominal CT showing free intraperitoneal air and suspected discontinuity of the wall of greater gastric curvature.
Hyperglycemia, ketosis, and metabolic acidosis are classic clinical manifestations of DKA. In 1973, Munro et al. reported 211 episodes of DKA in patients with type 1 diabetes. They found that 37 patients had a BG level of <300 mg/L, and that 16 patients had a level of <200 mg/L. They described this condition as “euglycemic DKA” for the first time (9). Subsequently, in 1993, Jenkins et al. reported a case series of 722 episodes of DKA in Birmingham, United Kingdom. A total of 23 patients were diagnosed with eu-DKA, and eight patients had a BG level of <200 mg/L (10). The DKA, without marked hyperglycemia, is the main feature of eu-DKA. Traditional causes of DKA without marked hyperglycemia vary and include strict diet, starvation, pregnancy, alcohol consumption, and chronic liver disease (1, 4, 11, 12). The SGLT2is was first marketed in 2013 as a newest class of antihyperglycemic medication. In 2015, several studies reported that SGLT2is might predispose ketoacidosis (13). A few cases treated with SGLT2is demonstrated an eu-DKA (14–16). However, SGLT2i-associated eu-DKA has not attracted much attention.
More research is required on precipitating factors and mechanisms of SGLT2i-associated eu-DKA. Insulin deficiency and increase in glucagon levels are usually considered mechanisms of SGLT2i-associated DKA (1). SGLT2 is expressed by human pancreatic alpha cells. Several studies have reported that SGLT2is might have a direct effect on pancreatic alpha cells (1, 17). Recently, Capozzi et al. demonstrated that SGLT2i-associated DKA was not altered by interruption of glucagon signaling and stated that the condition might occur independently of glucagon (18).
Approximately one-third of patients with SGLT2i-associated DKA demonstrate lower BG levels or “euglycemic” status (19). An increase in renal glucose clearance may have contributed to the lower BG levels of patients with SGLT2i-associated DKA (1). Starvation, surgery, acute illness, dehydration, and excessive alcohol intake may be precipitating factors of unremarkable hyperglycemia in SGLT2i-associated DKA. Several studies have reported the occurrence of SGLT2i-associated eu-DKA in patients with acute pancreatitis (15, 20). In our case report, the surgery and the perioperative management might be related to the occurrence of SGLT2-iassociated eu-DKA. However, the question remains whether eu-DKA was precipitated by the insufficiently drained pancreatic fistula, secondary inflammation after surgery, or the pancreatic malignancy itself. Further research is required.
Clinical features of eu-DKA are similar to those of DKA. Classic clinical presentation includes dehydration, tachypnea, deep and sighing (Kussmaul) respiration, nausea, vomiting, abdominal pain that could mimic an acute abdominal condition, confusion, drowsiness, and loss of consciousness (1, 21). Biochemical criteria for the diagnosis of DKA are hyperglycemia (BG threshold of 250 or 200 mg/dl), metabolic acidosis (venous pH <7.3 or serum bicarbonate <15 mmol/L), and ketonemia or ketonuria. Blood BOHB concentration is usually ≥ 3 mmol/L. Urine ketones are typically moderate or largely positive (≥ 2+) (21). The level of BG in eu-DKA is usually normal or unremarkable (<200 or 250 mg/dl). Unremarkable BG level may be the only difference between eu-DKA and DKA, which also makes it challenging to diagnose the disease.
Conventional treatment of SGLT2i-associated eu-DKA includes rehydration, insulin therapy, and glucose administration, and is similar to that of DKA. The mainstay of treatment is rehydration, which can normalize the volume and increase the clearance of ketone bodies (22). Insulin can decrease the synthesis of liver glucose and ketone bodies and increase the utilization of ketone bodies. Moreover, low insulin concentrations can affect lipolysis (22). Therefore, insulin administration should be continued even when the level of BG decreases below 200 mg/dl. Preventing the occurrence of SGLT2i-associated eu-DKA appears to be more important than its treatment. The SGLT2is should be avoided or withheld in the presence of the precipitating factors of SGLT2i-associated eu-DKA. It is recommended to use insulin instead of SGLT2is to control BG. The BG level and arterial blood gas of patients with pancreatic disease should be monitored, especially during the perioperative period.
Euglycemic diabetic ketoacidosis (eu-DKA) is a life-threatening disease, and the diagnosis of and ability to distinguish the condition are challenging during an emergency because of unremarkable hyperglycemia. A case of dapagliflozin-associated eu-DKA has been reported in the present study, which was associated with abdominal pain that mimicked acute surgical abdominal pain after surgery for pancreatic carcinoma. Awareness should be raised when patients are receiving this medication in the perioperative period, because eu-DKA is a potentially life-threatening complication. Further research is required on precipitating factors and mechanisms of SGLT2i-associated eu-DKA. Moreover, prevention of the occurrence of SGLT2i-associated eu-DKA appears to be more important than treatment.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The study were reviewed and approved by appropriate institutional of the Second Affiliated Hospital, School of Medicine, Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JH, XL, and RJ collected the data and drafted the manuscript. WL, HZ, and LL reviewed and modified the manuscript. All authors agreed on the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DKA, diabetic ketoacidosis; eu-DKA, euglycemic diabetic ketoacidosis; SGLT2i, sodium-glucose co-transporter 2 inhibitors; BG, blood glucose; ALT, alanine aminotransferase; NE, norepinephrine; WBC, white blood cell.
1. Bonora BM, Avogaro A, Fadini GP. Euglycemic ketoacidosis. Curr Diab Rep. (2020) 20:25. doi: 10.1007/s11892-020-01307-x
2. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. (2015) 38:1687–93. doi: 10.2337/dc15-0843
3. Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. (2019) 63:9–14. doi: 10.1016/j.ejim.2019.03.014
4. Joseph F, Anderson L, Goenka N, Vora J. Starvation-induced true diabetic euglycemic ketoacidosis in severe depression. J Gen Intern Med. (2009) 24:129–31. doi: 10.1007/s11606-008-0829-0
5. Roach P, Skierczynski P. Euglycemic diabetic ketoacidosis in a patient with type 2 diabetes after treatment with empagliflozin. Diabetes Care. (2016) 39:e3. doi: 10.2337/dc15-1797
6. Musso G, Saba F, Cassader M, Gambino R. Diabetic ketoacidosis with SGLT2 inhibitors. BMJ. (2020) 371:m4147. doi: 10.1136/bmj.m4147
7. Zhang L, Tamilia M. Euglycemic diabetic ketoacidosis associated with the use of a sodium-glucose cotransporter-2 inhibitor. CMAJ. (2018) 190:E766–8. doi: 10.1503/cmaj.171319
8. Shah NK, Deeb WE, Choksi R, Epstein BJ. Dapagliflozin: a novel sodium-glucose cotransporter type 2 inhibitor for the treatment of type 2 diabetes mellitus. Pharmacotherapy. (2012) 32:80–94. doi: 10.1002/PHAR.1010
9. Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. (1973) 2:578–80. doi: 10.1136/bmj.2.5866.578
10. Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta Diabetol. (1993) 30:251–3. doi: 10.1007/BF00569937
11. Guo RX, Yang LZ, Li LX, Zhao XP. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J Obstet Gynaecol Res. (2008) 34:324–30. doi: 10.1111/j.1447-0756.2008.00720.x
12. McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J. (2006) 23:417–20. doi: 10.1136/emj.2004.017590
13. Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. (2015) 100:2849–52. doi: 10.1210/jc.2015-1884
14. Ata F, Yousaf Z, Khan AA, Razok A, Akram J, Ali EAH, et al. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. (2021) 11:10293. doi: 10.1038/s41598-021-89752-w
15. Badwal K, Tariq T, Peirce D. Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient presenting with acute pancreatitis. Case Rep Endocrinol. (2018) 2018:6450563. doi: 10.1155/2018/6450563
16. Lee IH, Ahn DJ. Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes mellitus: a case report. Medicine. (2020) 99:e20228. doi: 10.1097/MD.0000000000020228
17. Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. (2010) 1:57–92. doi: 10.1007/s13300-010-0006-4
18. Capozzi ME, Coch RW, Koech J, Astapova II, Wait JB, Encisco SE, et al. The limited role of glucagon for ketogenesis during fasting or in response to SGLT2 inhibition. Diabetes. (2020) 69:882–92. doi: 10.2337/db19-1216
19. Bonora BM, Avogaro A, Fadini GP. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diab Obes Metab. (2018) 20:25–33. doi: 10.1111/dom.13012
20. Bouchaala K, Bahloul M, Bradii S, Kallel H, Chtara K, Bouaziz M. Acute pancreatitis induced by diabetic ketoacidosis with major hypertriglyceridemia: report of four cases. Case Rep Critic Care. (2020) 2020:7653730. doi: 10.1155/2020/7653730
21. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. (2018) 19 Suppl 27:155–77. doi: 10.1111/pedi.12701
22. Lapolla A, Amaro F, Bruttomesso D, Di Bartolo P, Grassi G, Maffeis C, et al. Diabetic ketoacidosis: a consensus statement of the Italian Association of Medical Diabetologists (AMD), Italian Society of Diabetology (SID), Italian Society of Endocrinology and Pediatric Diabetoloy (SIEDP). Nutr Metab Cardiovasc Dis. (2020) 30:1633–44. doi: 10.1016/j.numecd.2020.06.006
Keywords: DKA, euglycemic diabetic ketoacidosis, dapagliflozin, pancreatic carcinoma, SGLT2i
Citation: Luo X, Ji R, Lu W, Zhu H, Li L and Hu J (2022) Dapagliflozin-Associated Euglycemic Diabetic Ketoacidosis in a Patient Who Underwent Surgery for Pancreatic Carcinoma: A Case Report. Front. Surg. 9:769041. doi: 10.3389/fsurg.2022.769041
Received: 01 September 2021; Accepted: 21 January 2022;
Published: 23 February 2022.
Edited by:
Robert Kliček, Clinical Hospital Dubrava, CroatiaReviewed by:
Joris Jaekers, University Hospitals Leuven, BelgiumCopyright © 2022 Luo, Ji, Lu, Zhu, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Hu, aHVqdW56ZXl5MjAxNkB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.