- 1The First Clinical Medical College, Lanzhou University, Lanzhou, China
- 2Department of General Surgery, The First Hospital of Lanzhou University, Lanzhou, China
- 3Gansu Province Key Laboratory of Biological Therapy and Regenerative Medicine Transformation, Lanzhou, China
Background: The occurrence of postoperative complications of distal cholangiocarcinoma (dCCA) is an indicator of poor patient prognosis. This study aimed to determine the immune-nutritional indexes (INIs) that can predict short-term postoperative complications.
Methods: A retrospective analysis of 148 patients with dCCA who were operated radical pancreaticoduodenectomy at the First Hospital of Lanzhou University from December 2015 to March 2022 was conducted to assess the predictive value of preoperative INIs and preoperative laboratory tests for short-term postoperative complications, and a decision tree model was developed using classification and regression tree (CART) analysis to identify subgroups at risk for overall complications.
Results: In this study, 83 patients (56.08%) experienced overall complications. Clavien-Dindo grade III-V complications occurred in 20 patients (13.51%), and 2 patients died. The areas under curves (AUCs) of the preoperative prognostic nutritional index (PNI), controlling nutritional status (CONUT) score, and neutrophil-to-lymphocyte ratio (NLR) were compared; the PNI provided the maximum discrimination for complications (AUC = 0.685, 95% CI = 0.600–0.770), with an optimal cutoff value of 46.9, and the PNI ≤ 46.9 group had higher incidences of overall complications (70.67% vs. 40.00%, P < 0.001) and infectious complications (28.77% vs. 13.33%, P = 0.035). Multivariate logistic regression analysis identified PNI (OR = 0.87, 95% CI: 0.80–0.94) and total bilirubin (OR = 1.01, 95% CI: 1.00–1.01) were independent risk factors for overall complications (P < 0.05). According to CART analysis, PNI was the most important parameter, followed by the total bilirubin (TBIL) level. Patients with a PNI lower than the critical value and TBIL higher than the critical value had the highest overall complication rate (90.24%); the risk prediction model had an AUC of 0.714 (95% CI, 0.640–0.789) and could be used to stratify the risk of overall complications and predict grade I-II complications (P < 0.05).
Conclusion: The preoperative PNI is a good predictor for short-term complications after the radical resection of dCCA. The decision tree model makes PNI and TBIL easier to use in clinical practice.
Introduction
Cholangiocarcinoma (CCA) is a highly lethal malignancy that may occur anywhere within the biliary tree and/or liver parenchyma (1). The incidence of CCA is gradually increasing worldwide (2). Depending on the anatomical site of origin, CCA is classified as intrahepatic, perihilar, or distal cholangiocarcinoma (dCCA), and dCCA accounts for approximately 20% of CCA cases (3). Due to the location and invasive nature of dCCA, such patients often present with locally advanced or metastatic disease. Surgery through pancreaticoduodenectomy is the only curative option (4). Postoperative complications are one of the factors for poor prognosis in many cancers, and the occurrence of postoperative complications will prolong the postoperative recovery time, increase the economic burden, and result in poor long-term prognosis (5). It has been reported that postoperative complications may lead to systemic inflammation, which may reduce the immune response to cancer (6).
Patients with cancer often suffer from severe nutritional deficiencies, malnutrition, and low immunity will adversely affect the development of cancer patients, increase the incidence of infection, length of hospital stay, and risk of death (7), and may lead to higher rates of complications (8). In addition, We reviewed the relationship between preoperative immuno-nutritional related indicators and the occurrence of complications and prognosis in patients with CCA, where the association of prognostic nutritional index (PNI), nutritional control status (CONUT) score, neutrophil-to-lymphocyte ratio (NLR)and dCCA needs to be further investigated. This research aimed to evaluate the ability of preoperative immune-nutritional indexes (INIs) to predict postoperative complications after radical resection of dCCA and establish a decision tree model subgroups according to risk level to provide a basis for the prevention and treatment of postoperative complications, guide health education and prognosis management for patients, and accelerate patient recovery.
Methods
This retrospective study was conducted in The First Hospital of Lanzhou University in China, the study followed the Declaration of Helsinki and was approved by the Ethics Committee of The First Hospital of Lanzhou University (LDYY2022-412).
Patient enrollment
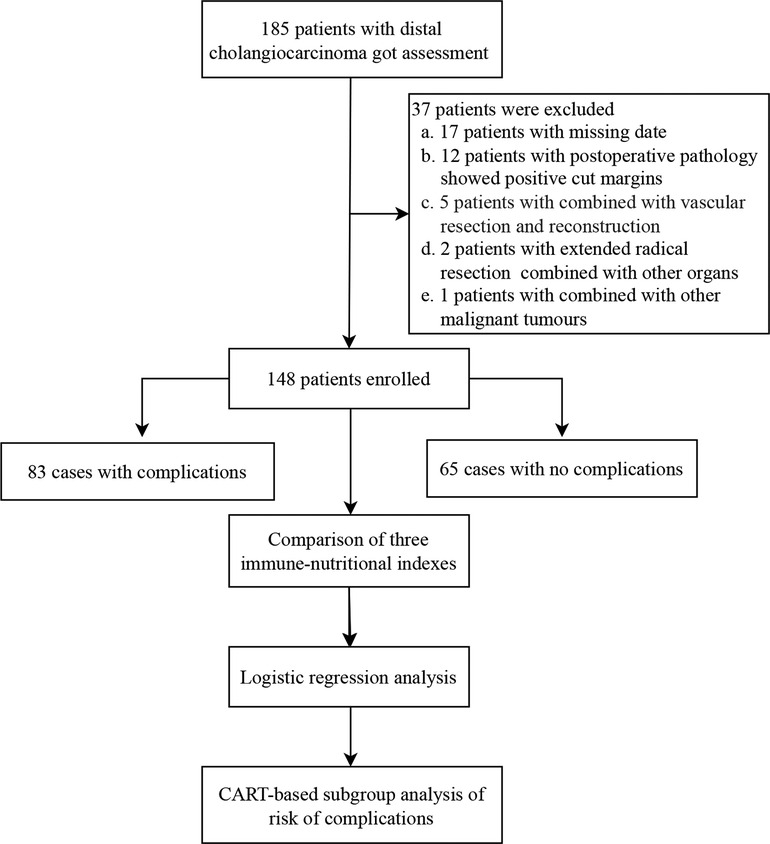
This retrospective study included patients with pancreaticoduodenectomy for dCCA at the First Hospital of Lanzhou University from December 2015 to March 2022. Patients who met the following criteria were included: (1) postoperative pathological examination consistent with a diagnosis of cholangiocarcinoma; (2) no distant metastasis found during the operation; (3) no severe heart, lung, kidney, or brain dysfunction before the operation (without combined organ dysfunction such as heart failure, respiratory failure, renal failure, disorientation and stress disorder, etc.); and (4) complete clinical medical records. Subjects with the following were excluded: (1) combined vascular resection and reconstruction; (2) extended radical resection combined with resection of other organs; (3) postoperative pathology showing a positive cut margin; (4) previously complicated with other malignant tumors; and (5) lack of clinical medical records. Finally, 148 patients were enrolled in this study (Figure 1).
Data collection
Hospital records were retrospectively evaluated for baseline information, laboratory tests, postoperative pathological factors, and postoperative complications, as well as PNI, CONUT score, and NLR when the patient was first admitted to the hospital. PNI was calculated using the following formula: serum albumin (g/L) + 5 × total lymphocyte count (109 L−1) (9). The CONUT score consists of three parameters: serum albumin, total peripheral lymphocyte count, and total cholesterol. Postoperative complications were defined as those occurring within 2 weeks after surgery and were assessed by the Clavien‒Dindo classification (10). Abdominal infection was defined by a positive abdominal culture collection, persistent fever or elevated leucocyte count, and effective antibiotic treatment, with or without bacteremia or a liver abscess, together with the exclusion of other infections including pulmonary and wound infections (11). Postoperatively, delayed gastric emptying was defined as the need for gastric tube placement for more than 3 days after the operation, the need for repeat catheter placement due to vomiting and other reasons after extubation, and an inability to consume solids for 7 days or longer after the operation (12).
Statistical analysis
All statistical analyses in this study were conducted using R (version 4.1.3). Quantitative data are presented as median (interquartile range) and were compared using the Wilcoxon rank sum test. Qualitative data were compared by the chi-square test and are expressed as frequency and percentage (%). P values < 0.05 were considered statistically significant. The potential predictive value of preoperative INIs for overall complications was assessed by the area under the receiver operating characteristic (ROC) curve. The Youden index was used to select the optimal cutoff value, which was set as the value with the maximum summed sensitivity and specificity value. Logistic regression analysis was used to identify risk factors for overall complications. P values < 0.05 were considered statistically significant, and independent risk factors for overall complications after surgery for dCCA were identified by univariate and multifactorial analysis.
The classification and regression tree (CART) model was constructed using the R package “party” (version 4.1.3) by identifying independent risk factors for overall complications through logistic regression analysis. CART, a machine learning method for constructing predictive models that simulate clinical decision-making processes (13), is easy to understand and implement. The decision tree construction process is recursive. Thus, the parameter most closely related to the result is extracted as the first node, When there is no correlation between the predictor variable and the outcome variable, the algorithm stops, and the p values show the relationship between the preoperative variables and the postoperative complications. Finally, the AUC was used to evaluate the accuracy of the decision tree in predicting overall complication outcomes.
Results
Patient characteristics
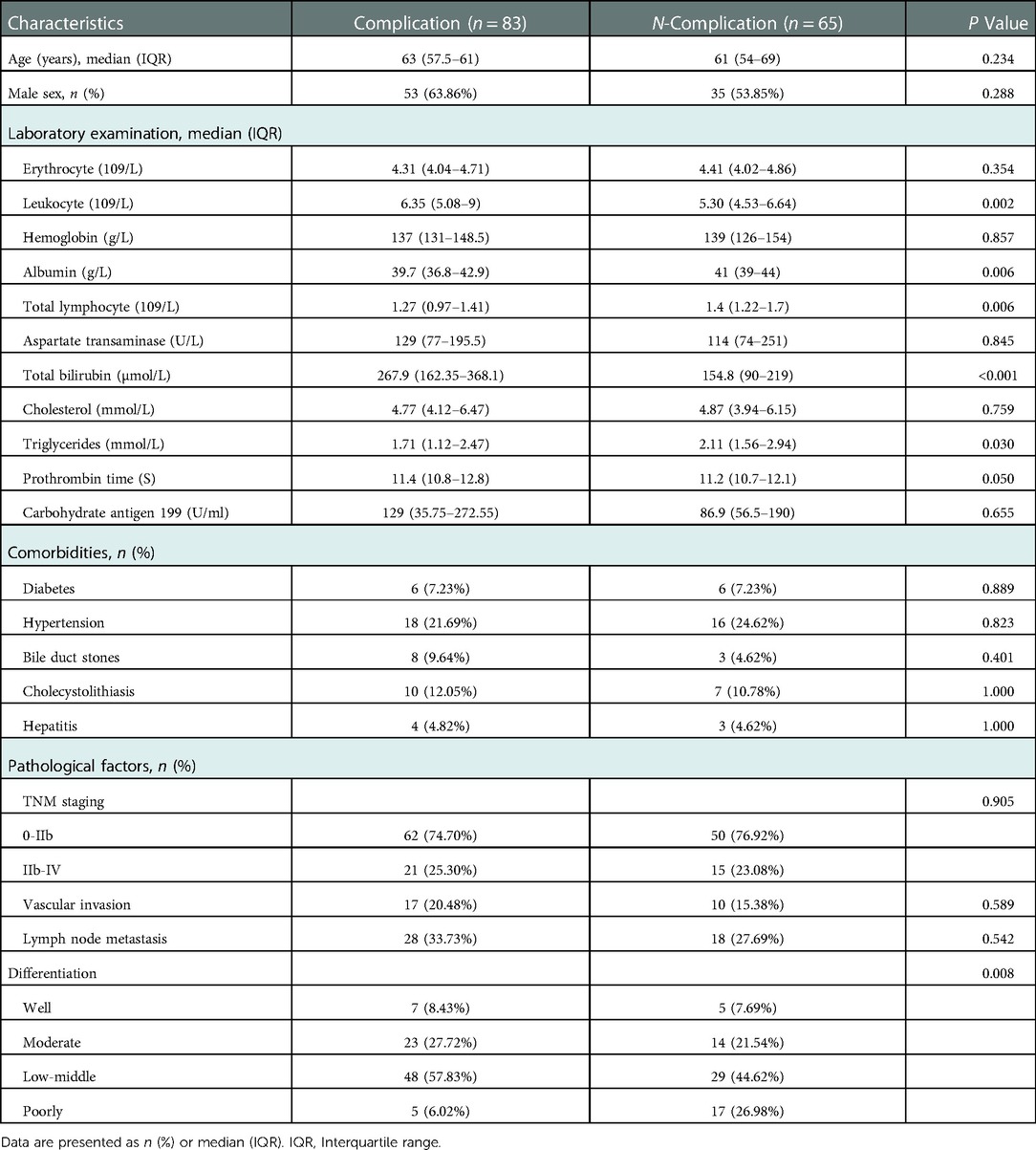
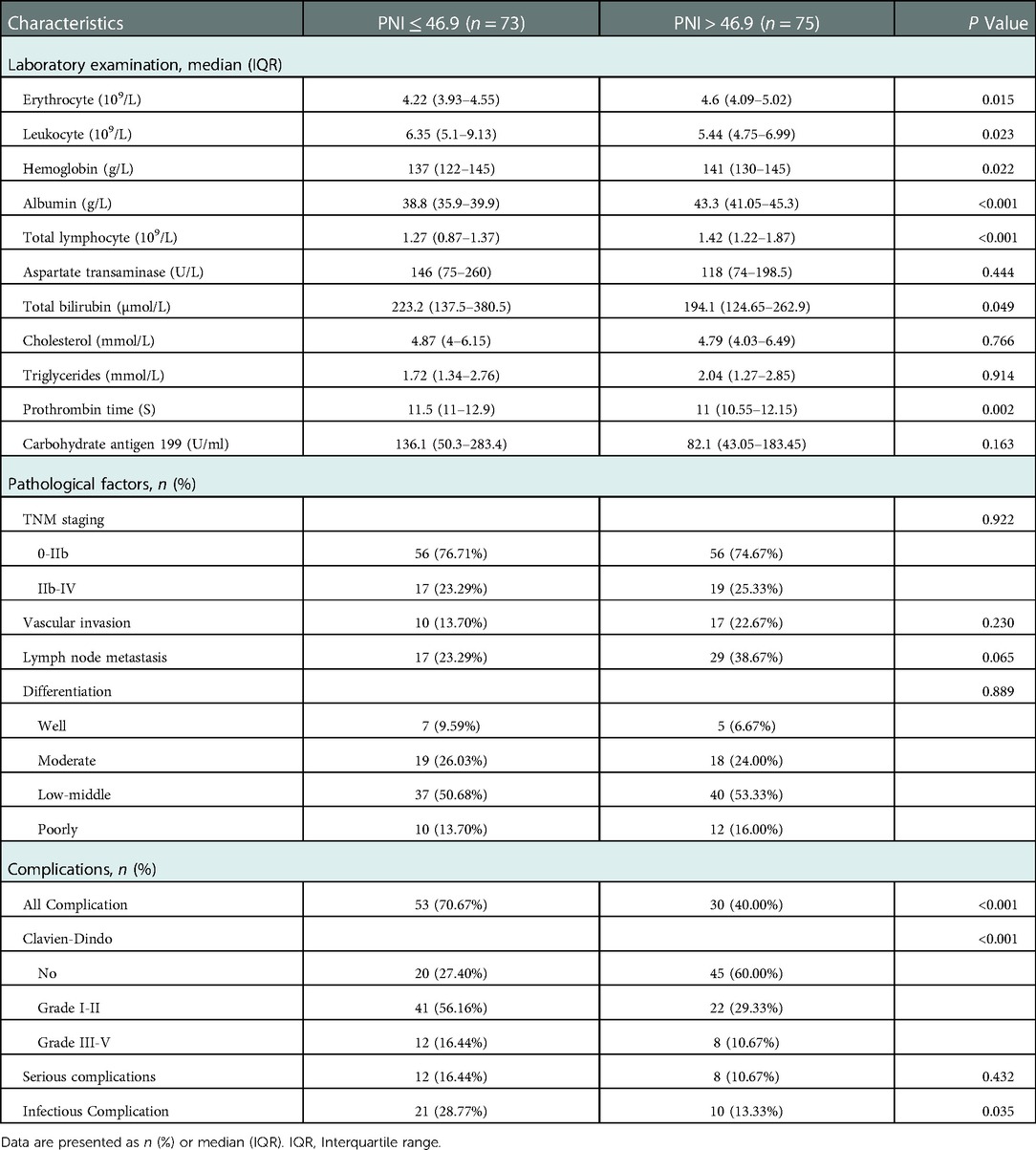
This study included 148 patients, 88 males, and 60 females, the age range of 28–78 years. Clinical characteristics, such as preoperative blood routine, biochemical tests, coagulation indicators, tumor markers, and postoperative pathology, were collected, and the clinical characteristics of the patients were summarized and analyzed (Table 1).
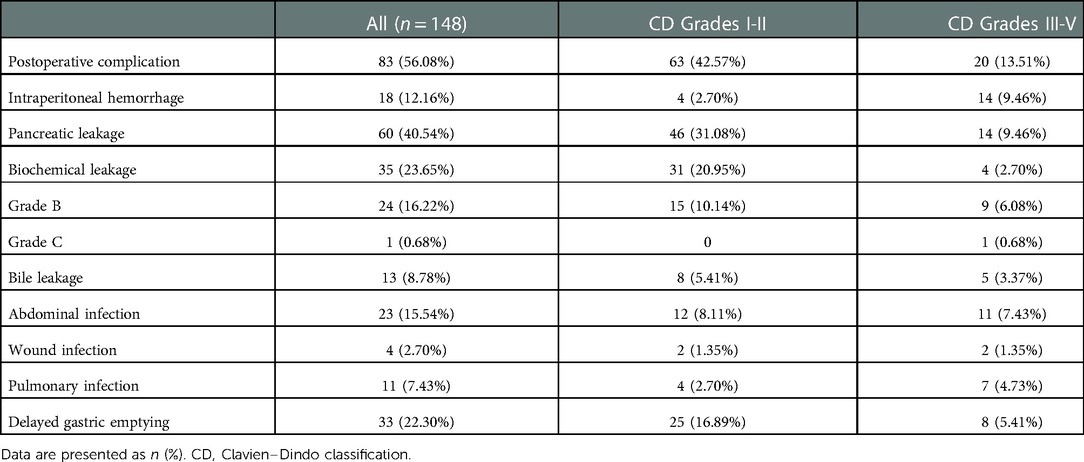
In this study, 83 patients (56.08%) had overall complications, of which 20 patients (13.51%) had Clavien‒Dindo grade III-V complications, and 2 patients died. There were 60 cases (40.54%) of pancreatic leakage, 35 cases (23.65%) of biochemical leakage, 24 cases (16.22%) of Grade B pancreatic leakage, 1 case (0.68%) of Grade C pancreatic leakage, 33 cases (22.30%) of delayed gastric emptying, 23 cases (15.54%) of abdominal infection, and 18 cases (12.26%) of abdominal bleeding. There were 13 cases (8.78%) of bile leakage, 11 cases (7.43%) of pulmonary infection, and 4 cases (2.70%) of wound infection (Table 2).
Predictive values of INIs for postoperative complications
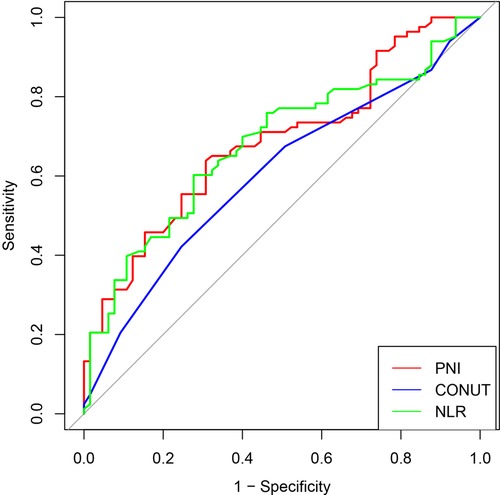
For investigating the predictive value of preoperative INIs for short-term complications after radical surgery for dCCA and evaluating the correlation between the predictor and outcome variables, we plotted ROC curves for PNI, CONUT, and NLR. The results show that PNI has the highest differentiation (AUC = 0.685, 95% CI = 0.600–0.770), followed by NLR (AUC = 0.678, 95% CI = 0.592–0.765) and CONUT (AUC = 0.603, 95% CI = 0.514–0.692). A comparison of the AUC of each curve showed that PNI correlated more strongly with complications than CONUT and NLR. PNI has a better predictive value for postoperative complications, with an optimal cutoff value of 46.9, corresponding to the maximum Youden index (Figure 2).
Correlations between PNI and clinical characteristics
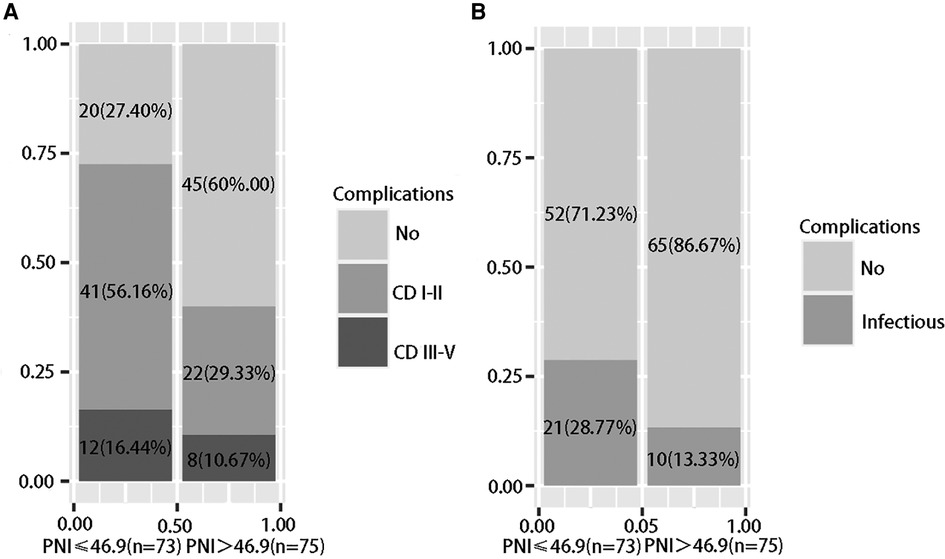
The patients were divided into a low PNI group (PNI ≤ 46.9, n = 73) and a high PNI group (PNI > 46.9, n = 75) based on the optimal cutoff value. The low PNI group had lower erythrocyte counts, higher leukocyte counts, lower hemoglobin levels, lower albumin levels, lower total lymphocyte counts, higher total bilirubin levels, and longer prothrombin times. There were no significant differences in pathological characteristics between the two groups. In terms of postoperative complications, the low PNI group had a higher occurrence of complications (70.67%, P < 0.001), and a low PNI had a stronger ability to predict overall complications [Figure 3(a)], and the low PNI group had a higher occurrence of infectious complications (28.77%, P = 0.035) [Figure 3(b)]. A low PNI had predictive value for overall complications and infectious complications (Table 3).
Logistic regression analysis to identify risk factors for overall complications
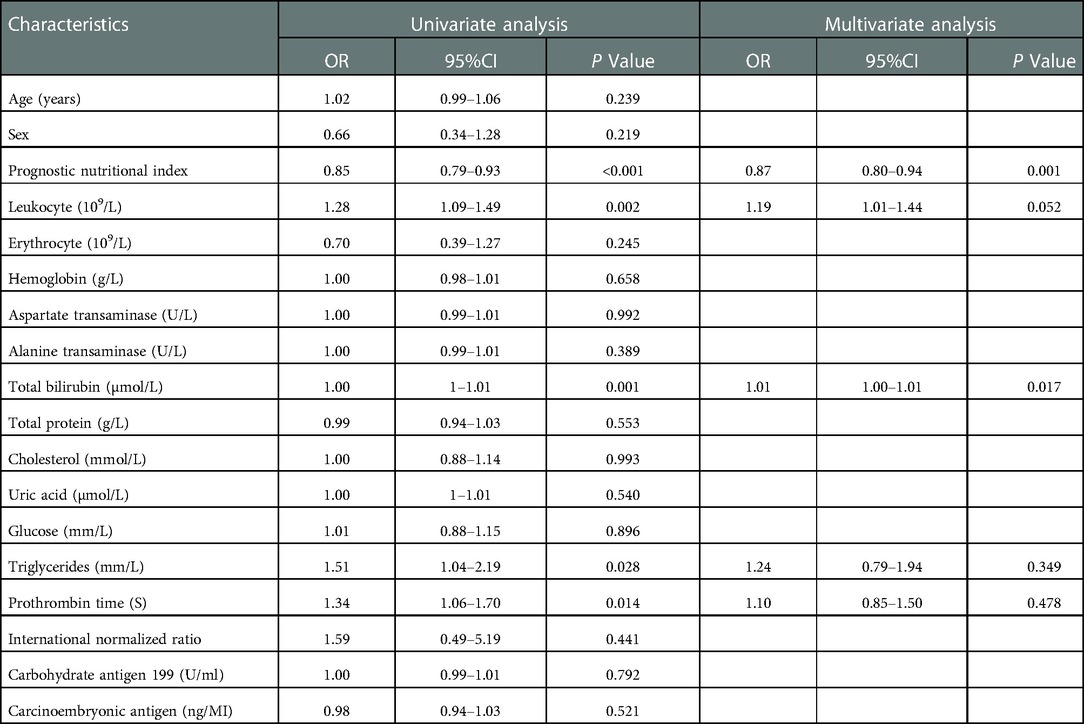
This study used logistic regression analysis to identify risk factors for postoperative complications (Table 4). Univariate analysis showed that prognostic nutritional index (OR = 0.85, 95% CI: 0.79–0.93), total bilirubin (OR = 1.00, 95% CI: 1–1.01), leukocyte count (OR = 1.28, 95% CI: 1.09–1.49), prothrombin time (OR = 1.34, 95% CI: 1.06–1.70) and triglycerides (OR = 1.51, 95% CI: 1.04–2.19) were influencing factors for overall complications (P < 0.05). The above influencing factors were included in multivariate logistic regression analysis, and the results showed that prognostic nutritional index (OR = 0.87, 95% CI: 0.80–0.94) and total bilirubin (OR = 1.01, 95% CI: 1.00–1.01) were independent risk factors for overall complications (P < 0.05).
CART-based subgroup analysis of overall complication risk
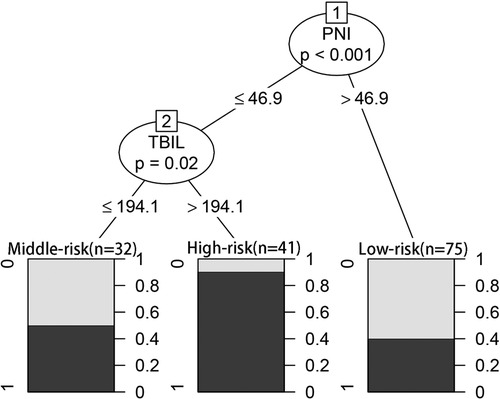
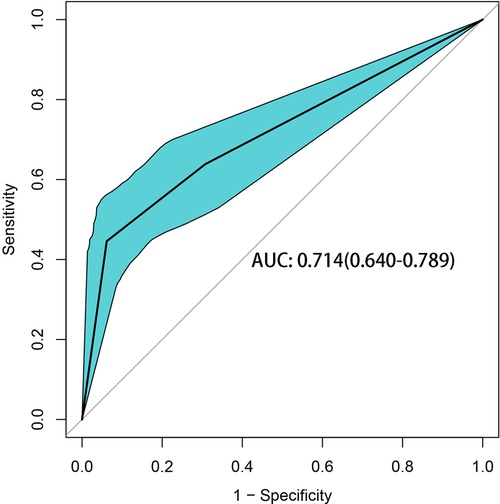
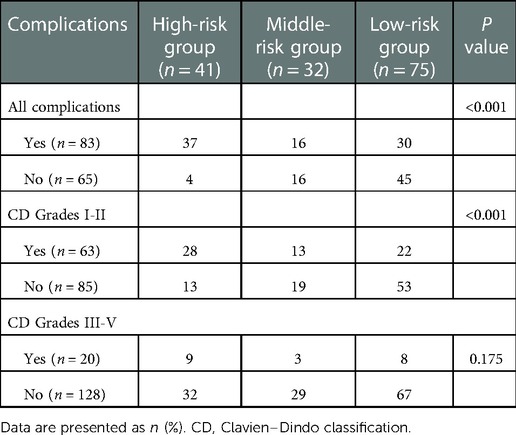
In the CART algorithm, we used the important variables PNI and total bilirubin (TBIL). The PNI was the most critical parameter for overall complications, and the optimal cutoff value was 46.9. Based on the correlation between the predictor and outcome variables, the CART algorithm selected the optimal cut-off point for TBIL levels for all patients, with an optimal cut-off value of 194.1 µmol/L (Figure 4). Therefore, patients with a PNI lower than the cutoff value and TBIL higher than the cutoff value had the highest complication rate (high-risk group: 37/41 patients, 90.24%), followed by the medium-risk group and low-risk group having overall complication rates of 50% (16/32 patients) and 40% (30/75 patients), respectively. The risk prediction model had an AUC of 0.714 (95% CI, 0.640–0.789) and had a good effect in predicting the overall risk of complications (Figure 5). We analyzed the relationship between risk groups and Clavien‒Dindo grades established by the decision tree model (Table 5), which could stratify overall complication risk groups and predict grade I-II complications (P < 0.05).
Discussion
The prognosis of dCCA is poor, and the median survival time of patients with the unresectable disease is less than one year (14). Patients with dCCA who underwent radical pancreaticoduodenectomy, especially R0 resection, can achieve long-term survival (15). However, postoperative complications may lead to the deterioration of long-term survival, more use of hospital resources, and an increase in the postoperative readmission rate (16). Effective preoperative differentiation and intervention of high-risk patients can be of great benefit to both clinicians and patients, and this study focuses on identifying preoperative PNI and TBIL levels as valid indicators for predicting short-term complications after radical surgery for dCCA.
The PNI was first proposed in 1980 by Onodera et al. (17) for preoperative nutritional status assessments and surgical risk predictions in gastrointestinal surgery. Serum albumin has been recognized as a prognostic indicator of various diseases and has been widely used to assess nutritional status and prognosis (18,19). Lymphocytes play an important role in tumor-related immunology and have strong antitumor immune functions (20). Therefore, PNI reflects the nutritional and immune status of patients and has been widely used as a prognostic indicator for various cancer patients (21–24). Some previous studies have found that a low PNI is positively correlated with poor prognosis in patients with biliary tract cancer, and the use of this parameter has shown the potential to improve prediction and identify high-risk patients more accurately and precisely (25). A meta-analysis found that PNI was an independent prognostic factor for overall survival and postoperative complications in cancer patients. In addition, subgroups with cutoff values less than 45 reported higher OR values, which indirectly confirmed that a low PNI was indeed associated with poor prognosis in some cancers (26).
However, this value has not been well-studied in patients with dCCA. We compared the correlation and discrimination between the INIs and complications and selected the PNI to distinguish between malnourished and well-nourished patients. Malnutrition is associated with an increased risk of perioperative morbidity and mortality (27) and is a risk factor for postoperative complications (28). A valid and objective preoperative nutritional risk assessment is of great benefit to both clinicians and patients, helping clinical doctors establish the corresponding diagnosis and treatment strategy to achieve improved clinical outcomes. The mechanism by which PNI affects complications after radical pancreaticoduodenectomy for dCCA is not very clear and may be related to albumin and lymphocyte levels, thus reflecting the nutritional and immune status of patients.
The TBIL level can be used to assess the degree of jaundice and hepatobiliary damage. Hyperbilirubinemia is considered to be a potential high-risk factor associated with postoperative mortality in hilar cholangiocarcinoma (29). A recent retrospective, single-center study once again confirmed that preoperative bilirubin concentrations were an important risk factor for postoperative severe complications and mortality in perihilar cholangiocarcinoma, with optimal cutoff values of 219.23 µmol/L and 548.08 µmol/L, respectively (30). A multicenter European study also showed that a high preoperative TBIL (≥265.2 µmol/L) was significantly associated with increased complications after the major resection of perihilar cholangiocarcinoma (31). Jaundice is associated with immune dysfunction, increased bacterial translocation, and deterioration of nutritional status and liver function (32).
Normally, bilirubin levels need to be less than twice the upper limit of normal before surgery, and preoperative biliary drainage is recommended for patients requiring decompression (TBIL > 250 µmol/L); otherwise, preoperative biliary drainage should be avoided (33). We do not have level 1 evidence for individuals with high serum bilirubin levels. Based on this study, preoperative jaundice reduction is recommended for patients with a TBIL > 194.1 µmol/L to improve performance status and survival, and preoperative biliary drainage is recommended for patients with high bilirubin (TBIL > 250 µmol/L) who require decompression.
Preoperative nutritional treatment is increasingly recognized as an important part of surgical care. Nearly 50% of hospitalized patients are malnourished or at risk of malnutrition, and hospitalized and surgical patients who are malnourished have significantly worse clinical outcomes (34). Screening for malnutrition before major surgery is essential because it can identify patients at risk of malnutrition who may benefit from preoperative nutritional intervention (35), which can improve patient outcomes (36). This study shows that preoperative PNI and TBIL levels were independent risk factors for short-term postoperative complications of dCCA. Based on the CART analysis, a high complication rate (90.24%) was found in patients with PNI ≤ 46.9 and TBIL > 194.1 µmol/L, which was useful for us to distinguish patients at potentially high risk preoperatively. Therefore, preoperative evaluations of patient nutritional status and TBIL level to help guide clinicians in formulating corresponding diagnoses and treatment strategies are expected to reduce the incidence of postoperative complications, improve quality of life, and improve the long-term prognosis of patients with dCCA.
This study has some limitations. Since the study was a retrospective analysis of data from a single center, these results may be affected by selection bias, and no further follow-up visit, more prospective, large-sample, multicenter studies are needed for further validation.
Conclusions
Nutritional assessments are necessary before radical pancreaticoduodenectomy for dCCA. Preoperative PNI and TBIL levels can be used as predictive markers for the risk of postoperative complications. Effective perioperative intervention for patients with low PNI and high TBIL levels can further improve the surgical outcomes of patients with dCCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YLH and HRL make the same contribution to this work. Correspondence Auther: PY and WBM make the same contribution to this work. BB, YZ, YYL, PY, and WBM: constructing an idea, formulating research objectives, and research implications, developing a proposal, and critically revising the content of the manuscript. YLH, HRL, YYM, and JLL: researching literature, collecting data, collating data, and analyzing data. JDZ, YXR, CLD, BB, YZ, YYL, PY, and WBM: environmental support, providing relevant research tools and software, taking responsible for pathology, revising the content of the manuscript before submission. YLH, HRL, PY, and WBM: Taking responsibility for the raw data, taking responsibility for the logical interpretation, and presentation of the results, and deciding to submit the manuscript for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82060551); The Foundation of The First Hospital of Lanzhou University (Grant No. ldyyyn2019-20); Natural Science Foundation of Gansu Province (Grant No. 20JR10RA676); Science and Technology Planning Project of Chengguan District in Lanzhou (Grant No. 2020JSCX0043) and Natural Science Foundation of Gansu Province (Grant No. 20JR10FA703).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1091534/full#supplementary-material
Abbreviations
CCA, cholangiocarcinoma; dCCA, distal cholangiocarcinoma; INIs, immune-nutritional indexes; PNI, prognostic nutritional index; CONUT, nutritional control status; NLR, neutrophil-to-lymphocyte ratio; CART, Classification And Regression Tree; TBIL, total bilirubin; ROC, receiver operating characteristic; AUC, area under the curve.
References
1. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. (2021) 7:65. doi: 10.1038/s41572-021-00300-2
2. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. (2018) 15:95–111. doi: 10.1038/nrclinonc.2017.157
3. Bahra M. Operative therapie distaler cholangiokarzinome [surgical treatment of distal cholangiocarcinoma]. Chirurg. (2021) 92:788–95. doi: 10.1007/s00104-021-01453-2
4. Lee RM, Maithel SK. Approaches and outcomes to distal cholangiocarcinoma. Surg Oncol Clin N Am. (2019) 28:631–43. doi: 10.1016/j.soc.2019.06.014
5. Chen Q, Zheng Y, Chen J, Zhou J, Zhao J, Bi X, et al. Differences of intraoperative outcomes and postoperative complications between intrahepatic cholangiocarcinoma and colorectal liver metastasis in different surgical methods. Transl Cancer Res. (2021) 10:4020–32. doi: 10.21037/tcr-21-553
6. Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, et al. Prognostic impacts of postoperative complications in patients with intrahepatic cholangiocarcinoma after curative operations. Int J Clin Oncol. (2017) 22:526–32. doi: 10.1007/s10147-017-1099-9
7. Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, Grande E, Álvarez-Hernández J, Sendrós-Madroño MJ, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. (2018) 20:619–29. doi: 10.1007/s12094-017-1757-4
8. European Association for the Study of the Liver. Electronic address:ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1; European Association for the Study of the Liver. EASL Clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. (2019) 70:172–93. doi: 10.1016/j.jhep.2018.06.024
9. Chen L, Bai P, Kong X, Huang S, Wang Z, Wang X, Fang Y, et al. Prognostic nutritional Index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol. (2021) 9:656741. doi: 10.3389/fcell.2021.656741
10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
11. Li Y, Chen L, Xing C, Ding C, Zhang H, Wang S, et al. Changes in Serum lactate level predict postoperative intra-abdominal infection after pancreatic resection. World J Surg. (2021) 45:1877–86. doi: 10.1007/s00268-021-05987-8
12. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surgery. (2007) 142:761–8. doi: 10.1016/j.surg.2007.05.005
13. Hosaka H, Takeuchi M, Imoto T, Yagishita H, Yu A, Maeda Y, et al. Machine learning-based model for predicting postoperative complications among patients with colonic perforation: a retrospective study. J Anus Rectum Colon. (2021) 5:274–80. doi: 10.23922/jarc.2021-010
14. Zhou W, Qian L, Rong Y, Zhou Q, Shan J, Li P, et al. Prognostic factors and patterns of recurrence after curative resection for patients with distal cholangiocarcinoma. Radiother Oncol. (2020) 147:111–7. doi: 10.1016/j.radonc.2020.03.017
15. Chua TC, Mittal A, Arena J, Sheen A, Gill AJ, Samra JS. Resection margin influences survival after pancreatoduodenectomy for distal cholangiocarcinoma. Am J Surg. (2017) 213:1072–6. doi: 10.1016/j.amjsurg.2016.09.049
16. Liu H, Cen X, Suo T, Cai X, Yuan X, Shen S, et al. Trends and hospital variations in surgical outcomes for cholangiocarcinoma in New York state. World J Surg. (2017) 41:525–37. doi: 10.1007/s00268-016-3733-5
17. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85(9):1001–5. PMID: 6438478
18. Li D, Yuan X, Liu J, Li C, Li W. Prognostic value of prognostic nutritional index in lung cancer: a meta-analysis. J Thorac Dis. (2018) 10:5298–307. doi: 10.21037/jtd.2018.08.51
19. Zhang F, Liu Z, Liang J, Liu S, Wu K, Zhang F, et al. Association between preoperative serum albumin and prognosis in patients with adrenocortical carcinoma after primary resection: a retrospective study. BMC Cancer. (2021) 21:961. doi: 10.1186/s12885-021-08689-5
20. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18:148. doi: 10.1186/s12876-018-0877-9
21. Watanabe H, Yamada T, Komori K, Hara K, Kano K, Takahashi K, et al. Effect of prognostic nutrition index in gastric or gastro-oesophageal junction cancer patients undergoing nivolumab monotherapy. In Vivo. (2021) 35:563–9. doi: 10.21873/invivo.12292
22. Kanda M. Preoperative predictors of postoperative complications after gastric cancer resection. Surg Today. (2020) 50:3–11. doi: 10.1007/s00595-019-01877-8
23. Jiang AM, Zhao R, Liu N, Ma YY, Ren MD, Tian T, et al. The prognostic value of pretreatment prognostic nutritional index in patients with small cell lung cancer and it's influencing factors: a meta-analysis of observational studies. J Thorac Dis. (2020) 12:5718–28. doi: 10.21037/jtd-20-1739
24. Wang X, Wang Y. The prognostic nutritional index is prognostic factor of gynecological cancer: a systematic review and meta-analysis. Int J Surg. (2019) 67:79–86. doi: 10.1016/j.ijsu.2019.05.018
25. Wang J, Bo X, Li M, Nan L, Wang C, Gao Z, et al. Prediction efficacy for clinical outcome of prognostic nutritional index in patients with resectable biliary tract cancer depends on sex and obstructive jaundice status. Ann Surg Oncol. (2021) 28:430–8. doi: 10.1245/s10434-020-08728-8
26. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. (2014) 140:1537–49. doi: 10.1007/s00432-014-1714-3
27. Matsuda T, Umeda Y, Matsuda T, Endo Y, Sato D, Kojima T, et al. Preoperative prognostic nutritional index predicts postoperative infectious complications and oncological outcomes after hepatectomy in intrahepatic cholangiocarcinoma. BMC Cancer. (2021) 21:708. doi: 10.1186/s12885-021-08424-0
28. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. (2015) 152:S3–7. doi: 10.1016/S1878-7886(15)30003-5
29. Neuhaus H. Preoperative biliary drainage in hilar cholangiocarcinoma: when and how? Endosc Int Open. (2020) 08:E211–3. doi: 10.1055/a-0990-9912
30. Wronka KM, Grat M, Stypulkowski J, Bik E, Patkowski W, Krawczyk M, et al. Relevance of preoperative hyperbilirubinemia in patients undergoing hepatobiliary resection for hilar cholangiocarcinoma. J Clin Med. (2019) 8:458. doi: 10.3390/jcm8040458
31. Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. (2013) 100:274–83. doi: 10.1002/bjs.8950
32. Lleo A, Colapietro F, Maisonneuve P, Aloise M, Craviotto V, Ceriani R, et al. Risk stratification of cholangiocarcinoma patients presenting with jaundice: a retrospective analysis from a tertiary referral center. Cancers (Basel). (2021) 13:2070. doi: 10.3390/cancers13092070
33. Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. Guidelines for perioperative care for pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) recommendations 2019. World J Surg. (2020) 44:2056–84. doi: 10.1007/s00268-020-05462-w
34. Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia. (2019) 74:27–35. doi: 10.1111/anae.14506
35. Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, et al. American Society for enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. (2018) 126:1883–95. doi: 10.1213/ANE.0000000000002743
Keywords: distal cholangiocarcinoma, postoperative complication, prognostic nutritional index, total bilirubin, decision tree
Citation: He Y, Liu H, Ma Y, Li J, Zhang J, Ren Y, Dong C, Bai B, Zhang Y, Lin Y, Yue P and Meng W (2023) Preoperative prognostic nutritional index predicts short-term complications after radical resection of distal cholangiocarcinoma. Front. Surg. 9:1091534. doi: 10.3389/fsurg.2022.1091534
Received: 7 November 2022; Accepted: 2 December 2022;
Published: 10 January 2023.
Edited by:
Zoltan Czigany, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Tevfiktolga Sahin, İnönü University, TurkeyKatharina Jöchle, University Hospital RWTH Aachen, Germany
Decan Jiang, Charité Universitätsmedizin Berlin, Germany
© 2023 He, Liu, Ma, Li, Zhang, Ren, Dong, Bai, Zhang, Lin, Yue and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbo Meng bWVuZ3diQGx6dS5lZHUuY24= Ping Yue ZHJ5dWVwaW5nQHNpbmEuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yulong He
Yulong He Haoran Liu1,2,†
Haoran Liu1,2,† Yuhu Ma
Yuhu Ma Yanyan Lin
Yanyan Lin Ping Yue
Ping Yue Wenbo Meng
Wenbo Meng