- Department of Neurosurgery, Chongqing General Hospital, Chongqing, China
With the advances in endoscopic technology, endoscopy is widely used in many neurosurgical procedures, such as microvascular decompression, which is an effective method to treat glossopharyngeal neuralgia, trigeminal neuralgia, and facial spasm. The purpose of this study was to determine the efficacy of fully endoscopic microvascular decompression in the treatment of glossopharyngeal neuralgia. We managed a patient with glossopharyngeal neuralgia in our department, whose main clinical manifestation was recurrent left ear and facial pain for 3 years. The patient underwent a fully endoscopic microvascular decompression. The pain in the left ear and face was significantly relieved postoperatively, and there was no recurrence at the 6-month follow-up evaluation. We describe a case of glossopharyngeal neuralgia that was successfully treated by fully endoscopic microvascular decompression, which showed that endoscopy has advantages in microvascular decompression, and fully endoscopic microvascular decompression is an effective method for glossopharyngeal neuralgia.
Introduction
Glossopharyngeal neuralgia (GPN) is a rare cranial nerve compression syndrome that may be caused by the contact or conflict of vessels and nerves. The annual incidence of GPN is 0.2–0.8/100,000 per year (1). The main clinical symptoms of GPN include paroxysmal, temporary, and severe tingling in the distribution of the ninth cranial nerve, such as the root of the tongue, soft palate, tonsils, pharyngeal column, posterior pharyngeal wall, and inner ear (2–5). Therefore, swallowing, coughing, talking, chewing, or yawning induce pain, which severely affects the patient's quality of life (6–11). In addition, the glossopharyngeal nerve is close to the vagus nerve, thus some GPN patients have bradycardia, hypotension, fainting, and even cardiac arrest (12, 13). Currently, the first-line treatment for GPN is drug therapy, including carbamazepine, phenytoin, clonazepam, and gabapentin, of which carbamazepine is the first choice (14, 15). Drug therapy, however, is not always effective for all patients. If the patient's symptoms are not relieved after drug treatment or the patient has drug intolerance, surgical treatment can be considered. The surgical methods available to treat GPN include a percutaneous glossopharyngeal nerve block, microvascular decompression (MVD), gamma knife stereotactic radiosurgery, radiofrequency thermocoagulation, and rhizotomy (16–18). MVD is considered the first-line surgical option for the treatment of GPN (19). When compared with a light microscope, the endoscope has better lighting and vision, thus the surgeon can more effectively visualize the nerves and invading vessels, comprehensively assess the degree of decompression, and reduce the degree of surgical trauma and the associated complications (20–22), thus making the endoscope an important auxiliary tool in patients undergoing MVD. In this study we managed a patient with GPN in our department, the main clinical manifestations of which included recurrent left ear and facial pain for 3 years. Preoperative magnetic resonance imaging of the head showed that the left vertebral artery compressed the posterior group of cranial nerves. The patient underwent fully endoscopic MVD. The pain in the left ear and face was significantly relieved postoperatively, and there was no recurrence at the 6-month follow-up evaluation. Thus, endoscopy has advantages in MVD, and fully endoscopic MVD is a relatively safe, feasible, and effective treatment for GPN.
Case report
Patient
A 57-year-old female was admitted to our hospital in January 2022. The main clinical symptom was paroxysmal, lancinating pain in the left ear and face for approximately 3 years; the pain was induced by cold air. The neurologic examination was normal and the pathologic signs were negative. All laboratory tests, including routine blood, liver and kidney function, and immune and blood coagulation function tests were within normal ranges. The patient had no history of trauma and no family history of genetic diseases. The patient received oral analgesics and acupuncture to the left face, but the effect was not satisfactory. Preoperative magnetic resonance imaging (MRI) of the head showed that the left vertebral artery compressed the posterior group of cranial nerves (Figure 1A). The diagnosis of left GPN was considered based on the patient's clinical manifestations and cranial MRI findings. Due to the increase in severity of pain, intolerance of oral analgesics, and severe impact on the quality of life, the patient underwent fully endoscopic MVD through a suboccipital retrosigmoid approach.
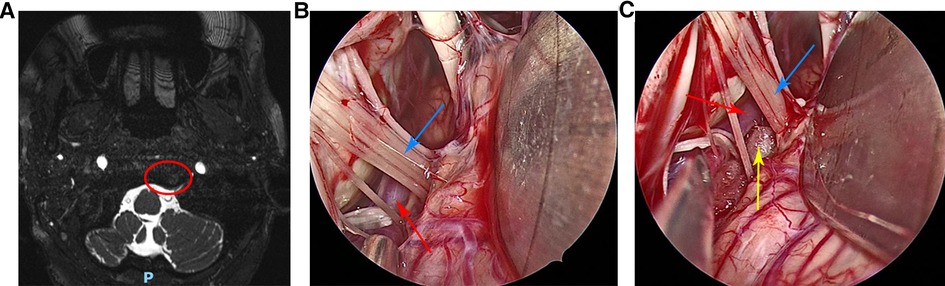
Figure 1. (A) Preoperative head magnetic resonance imaging of the patient. (B) The left vertebral artery and left glossopharyngeal nerve were clearly visible under the endoscopic view. (C) The Teflon pad was placed between the left glossopharyngeal nerve and the left vertebral artery under the endoscopic view. Red circle indicates posterior cranial nerve was compressed by the left vertebral artery, red arrow indicates the left vertebral artery, blue arrow indicates the left glossopharyngeal nerve and the yellow arrow indicates the Teflon pad.
Surgical procedure
After general anesthesia, the patient was placed in the right lateral decubitus position with the head lowered 15° and rotated 10° to the contralateral side. The neck was slightly forward, the mandible was approximately two transverse fingers away from the sternum, and the mastoid on the operating side was roughly parallel to the operating table in the highest position. First, a vertical incision approximately 5 cm in length was made behind the ear, the skin and muscle were incised, and the base of the mastoid was fully exposed. A bone window approximately 3 cm*3 cm in size was removed by a drill, with the upper edge reaching below the transverse sinus and the lower edge reaching the skull base. Then, the dura mater was exposed and incised, and the arachnoid was incised to slowly release cerebrospinal fluid, so that the cerebellar hemisphere naturally collapsed to create sufficient surgical space. Next, under the endoscope, the arachnoid between the glossopharyngeal nerves was completely separated, and the root of the left glossopharyngeal nerve was fully exposed. Intraoperatively it was seen that the left vertebral artery was in close contact with the glossopharyngeal nerve root (Figure 1B), and the root of the glossopharyngeal nerve was compressed. The offending blood vessels in contact with the glossopharyngeal nerve roots were separated and shifted. A Teflon pad was placed between the offending blood vessel and the glossopharyngeal nerve roots (Figure 1C), and hemostatic gauze covered the surface of the cerebellum. Repeated endoscopy was performed to confirm that no offending vessels were missed, the decompression was adequate, and the position of the Teflon pad was appropriate. Finally, the dura mater was sutured, and the artificial meninges were double-layered to prevent cerebrospinal fluid leakage. The skull defect was repaired with a titanium plate and four screws, and the sutures were closed layer-by-layer.
Results
After a full endoscopic MVD for GPN, the left ear and facial pain were completely relieved. On the 4th day postoperatively, the patient began coughing repeatedly with sputum production, thus, hypostatic pneumonia was considered. We asked the patient to expectorate more frequently, and treatment with expectorants and antibiotics were initiated. The symptoms improved significantly after treatment. There was no evidence of recurrence at the 6-month follow-up evaluation. Thus, fully endoscopic MVD is an effective method for the treatment of GPN.
Discussion
GPN is a rare cranial nerve compression syndrome characterized by paroxysmal transient severe pain in the distribution area of the ninth cranial nerve, which severely reduces the quality of life. In 1910, Weisenberg first described a 35-year-old man with severe pain in the glossopharyngeal nerve distribution due to a right cerebellopontine angle tumor compressing the ninth cranial nerve (23). Sicard and Robineau reported three patients with severe pain in the glossopharyngeal nerve distribution for unknown reasons, which was cured by surgical treatment of extracranial nerve avulsion (24). Harris referred to these symptoms as “glossopharyngeal neuralgia” to describe the disorder (25). The first successful intracranial resection for GPN was performed by Dandy in 1927, which was considered the gold standard for the treatment of GPN at the time (9). Until 1977, Laha and Jannetta proposed that MVD could be used to treat GPN and reported three patients with GPN who underwent MVD with good surgical outcomes, concluding that compression of the ninth nerve was the cause of GPN (26). Since that time, surgical methods, such as percutaneous glossopharyngeal nerve block, gamma knife stereotactic radiosurgery, and radiofrequency thermocoagulation, have also been used to treat GPN. Currently, MVD is the most widely used surgical method to relieve blood vessel compression of nerves (15). Patel et al. reviewed >200 GPN patients who underwent MVD, and the overall success rate was >90% (1). Similarly, in a retrospective analysis by Zhao et al. there were 35 patients with GPN who underwent MVD. Thirty-three patients (94.3%) had complete relief of pain immediately after MVD treatment, only 6.6% of the patients relapsed, and none of the patients had long-term surgical complications (13). These results showed that MVD is a safe and effective way to treat GPN. Currently, MVD is generally performed under a microscope; however, the microscope has a direct field of view that makes it difficult for the surgeon to visualize the compressed nerve and the offending blood vessel. Dabey et al. estimated that approximately 23% of dual-offending vessels are lost under a microscope (27). With the rapid advances in endoscopic techniques, the endoscope has been adopted for use in MVD surgery. Compared with a microscope, an endoscope can more accurately detect offending blood vessels. Therefore, according to the type of the optical device, MVD can generally be divided into microscopic MVD, endoscopic-assisted MVD, and fully endoscopic MVD (28–30). Furthermore, depending on whether to use conventional instruments, endoscopic neurosurgery can be divided into pure endoscopic neurosurgery (EN) without conventional instruments, and endoscope-controlled microneurosurgery (ECM) with conventional instruments (31). It is worth noting that the endoscopic MVD in this study has not been further classified and is called “fully endoscopic MVD”. In fact, the “fully endoscopic MVD” mentioned in this study belongs to ECM. Magana and Duntze et al. reported 87 cases of endoscopic-assisted MVD and were of the opinion that an endoscope more accurately identifies the offending vessel (20, 32). At the same time, several studies have reported that an endoscope accurately assesses whether the decompression is sufficient (33, 34). In a large meta-analysis endoscopic MVD was compared with microscopic MVD with respect to the short-term remission, offending blood vessel detection, and long-term remission rates, and concluded that an endoscope is superior to a microscope for MVD (21). In 2002, Jarrahy et al. reported the first GPN patient who was treated by fully endoscopic MVD during which all offending vessels were visualized intraoperatively, and the clinical symptoms were completely relieved after the operation (12). Cureus et al. reported two patients with vagal-glossopharyngeal neuralgia who failed medical therapy and subsequently underwent fully endoscopic MVD (35). The vagal-glossopharyngeal neuralgia symptoms were completely relieved in both patients postoperatively (35). Komatsu et al. also reported how they treated GPN with MVD underwent full endoscopic MVD, and believed that endoscopic MVD is a relevant and minimally invasive method for GPN (36). In the present study we reported a female patient with GPN whose symptoms were refractory to oral analgesics and underwent fully endoscopic MVD. The GPN symptoms were completely relieved and there was no evidence of recurrence at the 6-month follow-up evaluation. These results suggest that fully endoscopic MVD is an effective treatment for GPN. The two key factors for successful MVD surgery are to identify all offending vessels and accurately evaluate decompression. Because the lighting, visual angle, and field of vision provided by an endoscope are superior to a microscope, an endoscope has become an important auxiliary tool in MVD surgery. An endoscope not only reduces the probability of overlooking offending blood vessels, but also determines whether the decompression is sufficient. Moreover, an endoscope also provides surgeons with a more comprehensive view of the relevant anatomic structures, avoids damage to the surrounding brain tissue, blood vessels, and nerves, and reduces the postoperative complications. Therefore, we believe that an endoscope is particularly suitable for MVD. At the same time, we performed a 3*3-cm bone flap intraoperatively. Although a relatively large bone window may increase patient injury, we believed at the time that performing an operation under a relatively large bone window could make the operation more flexible and reduce iatrogenic injuries, and the patient could achieve a better functional outcome. When we perform endoscopic surgery under a small bone window, due to the small space, the instruments used during the surgery cannot reach some angles. The larger bone window increases the flexibility of the operation, protects the brain tissue more effectively, and reduces the risk of damage to surrounding tissues, blood vessels, and nerves. At present, we use a straight skin incision. We suggest that if we use a C-type skin incision and muscle flaps, the integrity of the surrounding soft tissues would be preserved, and the risk of occipital muscle/cutaneous nerve injuries would be decreased, thus obtaining better functional outcomes (37, 38).
Conclusion
Herein we reported a patient with GPN who was successfully treated using a fully endoscopic MVD, which showed that endoscopy has advantages in performing MVD. Fully endoscopic MVD should be considered safe and effective in the treatment of GPN. We believe that with the development of science and technology and the continuous application and innovation of endoscopy in various fields, endoscopy will play an increasingly important role in neurosurgery in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the committee ethics of Chongqing General Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HTJ, DWZ, PW and LWZ contributed to the study conception and design. Material preparation, data collection and analysis were performed by HTJ, JL, CT, GZ, XRT and NW. The first draft of the manuscript was written by HTJ and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery. (2002) 50(4):705–11. doi: 10.1097/00006123-200204000-00004
2. Greene KA, Marciano FF, Lieberman AN. Trigeminal and glossopharyngeal neuralgia. In: Spetzler RF, editors. Neurovascular surgery. New York, NY: McGraw Hill (1995). p. 1091–100.
3. Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: a study of 217 cases. Arch Neurol. (1981) 38(4):201–5. doi: 10.1001/archneur.1981.00510040027002
4. Haller S, Etienne L, Kövari E, Varoquaux AD, Urbach H, Becker M. Imaging of neurovascular compression syndromes: trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. AJNR Am J Neuroradiol. (2016) 37(8):1384–92. doi: 10.3174/ajnr.A4683
5. Revuelta-Gutiérrez R, Morales-Martínez AH, Mejías-Soto C, Martínez-Anda JJ, Ortega-Porcayo LA. Microvascular decompression for glossopharyngeal neuralgia through a microasterional approach: a case series. Surg Neurol Int. (2016) 7:51. doi: 10.4103/2152-7806.181824
6. Fischer L, Ludin SM, Puente de la Vega K, Sturzenegger M. Neuralgia of the glossopharyngeal nerve in a patient with posttonsillectomy scarring: recovery after local infiltration of procaine-case report and pathophysiologic discussion. Case Rep Neurol Med. (2015) 2015:560546. doi: 10.1155/2015/560546
7. Krasoudakis A, Anyfantakis D, Hadjipetrou A, Kastanakis M, Symvoulakis EK, Marathianos S. Glossopharyngeal neuralgia associated with cardiac syncope: two case reports and literature review. Int J Surg Case Rep. (2015) 12:4–6. doi: 10.1016/j.ijscr.2015.05.007
8. Wang C, Kundaria S, Fernandez-Miranda J, Duvvuri U. A description of the anatomy of the glossopharyngeal nerve as encountered in transoral surgery. Laryngoscope. (2016) 126(9):2010–5. doi: 10.1002/lary.25706
9. Dandy WE. Glossopharyngeal neuralgia (tic douloureux): its diagnosis and treatment. Arch Surg. (1927) 15(2):198–214. doi: 10.1001/archsurg.1927.01130200046002
10. Kondo A. Follow-up results of using microvascular decompression for treatment of glossopharyngeal neuralgia. J Neurosurg. (1998) 88(2):221–5. doi: 10.3171/jns.1998.88.2.0221
11. Yomo S, Arkha Y, Donnet A, Régis J. Gamma knife surgery for glossopharyngeal neuralgia. J Neurosurg. (2009) 110(3):559–63. doi: 10.3171/2008.8.17641
12. Jarrahy R, Cha ST, Eby JB, Berci G, Shahinian HK. Fully endoscopic vascular decompression of the glossopharyngeal nerve. J Craniofac Surg. (2002) 13(1):90–5. doi: 10.1097/00001665-200201000-00021
13. Zhao H, Zhang X, Zhu J, Tang YD, Li ST. Microvascular decompression for glossopharyngeal neuralgia: long-term follow-up. World Neurosurg. (2007) 102:151–6. doi: 10.1016/j.wneu.2017.02.106
14. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38(1):1–211. doi: 10.1177/0333102417738202
15. Shah RJ, Padalia D. Glossopharyngeal neuralgia. Treasure Island, FL: StatPearls Publishing (2022).
16. Isamat F, Ferrán E, Acebes JJ. Selective percutaneous thermocoagulation rhizotomy in essential glossopharyngeal neuralgia. J Neurosurg. (1981) 55(4):575–80. doi: 10.3171/jns.1981.55.4.0575
17. Rey-Dios R, Cohen-Gadol AA. Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery. Neurosurg Focus. (2013) 34(3):E8. doi: 10.3171/2012.12.FOCUS12391
18. Xiong NX, Zhao HY, Zhang FC, Liu RE. Vagoglossopharyngeal neuralgia treated by microvascular decompression and glossopharyngeal rhizotomy: clinical results of 21 cases. Stereotact Funct Neurosurg. (2012) 90(1):45–50. doi: 10.1159/000333828
19. Zheng X, Wei XY, Zhu J, Yuan Y, Ying TT, Li ST. Microvascular decompression alone without rhizotomy is an effective way of treating glossopharyngeal neuralgia: clinical analysis of 46 cases. Stereotact Funct Neurosurg. (2020) 98(2):129–35. doi: 10.1159/000505712
20. Duntze J, Litré CF, Eap C, Théret E, Bazin A, Chays A, et al. Apport de l'endoscopie pour la décompression microvasculaire dans l'angle ponto-cérébelleux: à propos de 27 cas [adjunctive use of endoscopy during microvascular decompression in the cerebellopontine angle: 27 case reports]. Neurochirurgie. (2011) 57(2):68–72. doi: 10.1016/j.neuchi.2011.03.003
21. Li Y, Mao F, Cheng F, Peng C, Guo D, Wang B. A meta-analysis of endoscopic microvascular decompression versus microscopic microvascular decompression for the treatment for cranial nerve syndrome caused by vascular compression. World Neurosurg. (2019) 126:647–655.e7. doi: 10.1016/j.wneu.2019.01.220
22. Jiang HT, Wang P, Zhou W, Zeng LW, Lin B, Wu N. Fully endoscopic microvascular decompression for hemifacial spasm. Exp Ther Med. (2022) 24(1):483. doi: 10.3892/etm.2022.11410
23. Weisenberg TH. Cerebello-pontine tumor diagnosed for six years as tic doloureux; the symptoms of irritation of the ninth and twelfth cranial nerves. J Am Med Ass. (1910) 54:1600–4. doi: 10.1001/jama.1910.92550460001001g
24. Sicard R, Robineau V. Algie velo-pharyngee-essentielle: traitment chirurgical. Revue Neurol (Paris). (1920) 36:256–7.
25. Harris W. Persistent pain in lesions of the peripheral and central nervous system. Br Med J. (1921) 2(3178):896–900. doi: 10.1136/bmj.2.3178.896
26. Laha RK, Jannetta PJ. Glossopharyngeal neuralgia. J Neurosurg. (1977) 47(3):316–20. doi: 10.3171/jns.1977.47.3.0316
27. Dubey A, Yadav N, Ratre S, Parihar VS, Yadav YR. Full endoscopic vascular decompression in trigeminal neuralgia: experience of 230 patients. World Neurosurg. (2018) 113:e612–7. doi: 10.1016/j.wneu.2018.02.108
28. Magnan J, Chays A, Caces F, Lepetre-Gillot C, Cohen JM, Belus JF, et al. Place de l'endoscopie et de la décompression vasculaire dans le traitement du spasme de l'hémiface [role of endoscopy and vascular decompression in the treatment of hemifacial spasm]. Ann Otolaryngol Chir Cervicofac. (1994) 111(3):153–60.7840488
29. Eby JB, Cha ST, Shahinian HK. Fully endoscopic vascular decompression of the facial nerve for hemifacial spasm. Skull Base. (2001) 11(3):189–97. doi: 10.1055/s-2001-16607
30. Zagzoog N, Attar A, Takroni R, Alotaibi MB, Reddy K. Endoscopic versus open microvascular decompression for trigeminal neuralgia: a systematic review and comparative meta-analysis. J Neurosurg. (2018) 7:1–9. doi: 10.3171/2018.6.JNS172690
31. Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery. (1998) 43(6):1330–6. doi: 10.1097/00006123-199812000-00037
32. Magnan J, Chays A, Lepetre C, Pencroffi E, Locatelli P. Surgical perspectives of endoscopy of the cerebellopontine angle. Am J Otol. (1994) 15(3):366–70.8579141
33. Barker FG, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. (1996) 334(17):1077–83. doi: 10.1056/NEJM199604253341701
34. Miyazaki H, Deveze A, Magnan J. Neuro-otologic surgery through minimally invasive retrosigmoid approach: endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal. Laryngoscope. (2005) 115(9):1612–7. doi: 10.1097/01.mlg.0000172038.22929.63
35. Blue R, Spadola M, McAree M, Kvint S, Lee JYK. Endoscopic microvascular decompression for vagoglossopharyngeal neuralgia. Cureus. (2020) 2(12):e12353. doi: 10.7759/cureus.12353
36. Komatsu F, Kishore K, Sengupta R. How I do it: endoscopic microvascular decompression for glossopharyngeal neuralgia. Acta Neurochir (Wien). (2020) 162(11):2833–5. doi: 10.1007/s00701-020-04456-w
37. Chibbaro S, Cebula H, Zaed I, Gubian A, Todeschi J, Scibilia A, et al. A laboratory investigation on a tailored skin and muscle flap variant for the retrosigmoid approach. J Neurol Surg B Skull Base. (2021) 83(Suppl 2):e438–42. doi: 10.1055/s-0041-1730890
38. Chibbaro S, Cebula H, Scibilia A, Spatola G, Todeschi J, Gubian A, et al. Retrosigmoid approach: investigating the role of a C-shaped skin incision and muscle flaps in improving functional outcome and reducing postoperative pain. World Neurosurg. (2018) 111:e340–7. doi: 10.1016/j.wneu.2017.12.050
Keywords: endoscopy, microvascular decompression, glossopharyngeal neuralgia, suboccipital retrosigmoid approach, functional neurosurgery
Citation: Jiang H, Zhou D, Wang P, Zeng L, Liu J, Tang C, Zhang G, Tan X and Wu N (2023) Case report: Fully endoscopic microvascular decompression for glossopharyngeal neuralgia. Front. Surg. 9:1089632. doi: 10.3389/fsurg.2022.1089632
Received: 4 November 2022; Accepted: 2 December 2022;
Published: 6 January 2023.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Salvatore Chibbaro, Neurosurgery Department Strasbourg University Hospital, FranceMukesch Johannes Shah, University of Freiburg, Germany
© 2023 Jiang, Zhou, Wang, Zeng, Liu, Tang, Zhang, Tan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Wu d3VuYW44ODFAdG1tdS5lZHUuY24=
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Haotian Jiang
Haotian Jiang Gang Zhang
Gang Zhang Nan Wu
Nan Wu