- Department of Orthopedics, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Objective: This study aims to report one case of intraspinal epidural cement leakage caused by a novel percutaneous vesselplasty.
Methods: A clinical case report from the Orthopedic center of our hospital and a literature review. A 63-year-old woman with an L2 osteoporotic compression fracture underwent novel kyphoplasty, percutaneous vesselplasty. This rare complication was evaluated through a literature search, and its special types are classified in more detail.
Results: The patient was hospitalized with low back pain two weeks after a fall. After auxiliary examination, a new type of percutaneous vesselplasty was performed. After the intraoperative injection of bone cement, bone cement leakage extended along the posterior longitudinal ligament and epidural space. There were no special compression symptoms of the spinal cord, and the prognosis of conservative treatment was good.
Conclusion: Although percutaneous vesselplasty is relatively safe and frequent, intraspinal leakage may occur, so sufficient preoperative evaluation, intraoperative continuous fluoroscopic monitoring, and timely evaluation of postoperative images are extremely necessary.
Introduction
Percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) are effective methods for osteoporotic vertebral compression fractures. However, bone cement leakage is a common complication. Some types of bone cement leakage will not cause serious consequences and are mostly asymptomatic, but some will cause serious consequences, even irreversible injury (1). Herein, we report a special case in which the bone cement infiltrated the epidural space in the spinal canal during percutaneous vesselplasty and spread along the posterior longitudinal ligament, emphasizing the potential risks of this type of percutaneous vesselplasty.
Case report
The patient was a 63-year-old female who complained of low back pain after a fall for two weeks. The admission examination showed no obvious deformity of the lumbar vertebrae, positive tenderness and percussion pain of the lumbar spinous process, obvious limitation of lumbar movement, no obvious abnormality of sensory muscle strength of the lower extremities, and no pathological signs. The bone scan showed abnormal imaging agent concentration and compressibility in L2 vertebral body. The main diagnosis was osteoporotic vertebral compression fracture (L2), and VAS pain score was 7, so a percutaneous vesselplasty was planned (2, 3).
We used the related instruments of the vertebroplasty system (VCF-XTM Bone Filler Delivery System), including a double-layer bone filling bag BVFX-D20, controllable bone cement injection device, extension tube TmurCCD2murT301, bone drilling needle and working casing TmurN201T, as well as a precision bit T-D401 (4). The patient was lying prone, and the involved vertebral segments were located by Kirschner wire and C-arm x-ray machine fluoroscopy. The entry point was marked, disinfected, and local anesthesia applied. The bone scan and tomography showed abnormal concentration and compressibility of imaging agents in L2 vertebrae (Figure 1). At the beginning of the operation, the puncture needle was inserted through the right pedicle until it reached the midline of the vertebral body in the positive position (Figure 2) (5). For more satisfactory dispersion, we chose to break the bone filling bag. At this time, the patient developed transient low back pain, which was relieved after a few minutes, and fluoroscopy showed that the bone cement extended along the posterior longitudinal ligament space and epidural space (Figure 3). The patient's vital signs were good, with no obvious abnormality in the lower limb movement, and the operation was stopped. After the operation, the patient returned to the ward and was given symptomatic treatment and monitored.
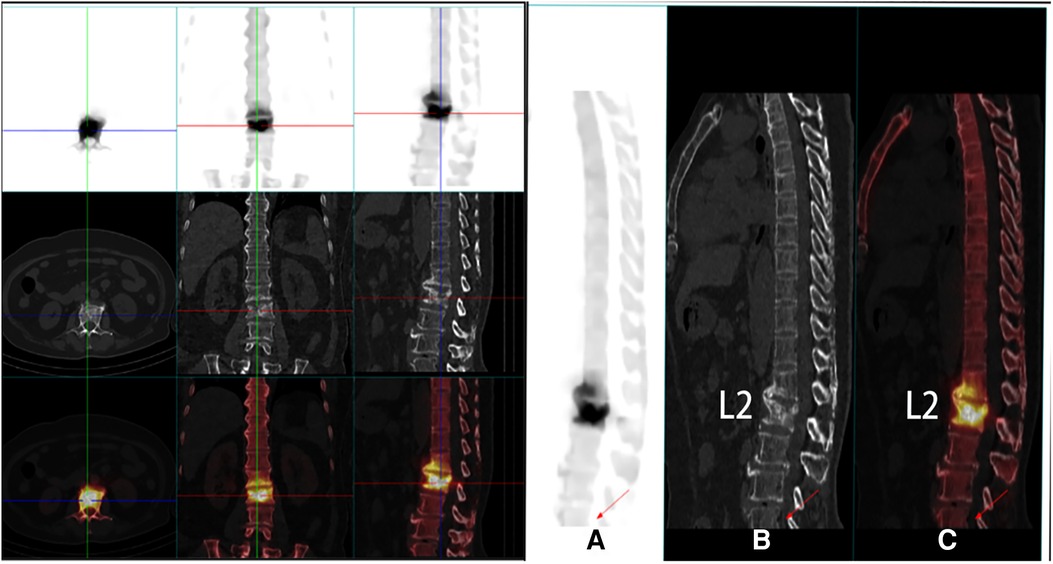
Figure 1. PET-CT images of the patient's preoperative coronal and sagittal vertebral fractures (A–C).

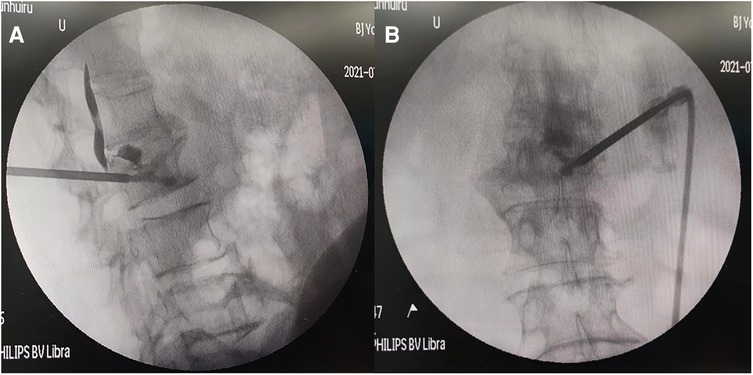
Figure 2. Intraoperative continuous fluoroscopy before the bone cement leakage (A–C front and Side view, D puncture needle entering the vertebral body through the pedicle).
Her low back pain was significantly better than before the operation, and her VAS pain score decreased from 7 to 2. There was no obvious movement and sensory disturbance, and urination and defecation were normal. A routine CT scan after operation showed that L2 vertebral body was filled with bone cement, and there was a small amount of bone cement leakage in L1–2 intervertebral disc, which leaked into the epidural space of the spinal canal and distributed along the posterior longitudinal ligament from T12 to L2. There was no compression of the spinal cord, as shown in Figure 4.
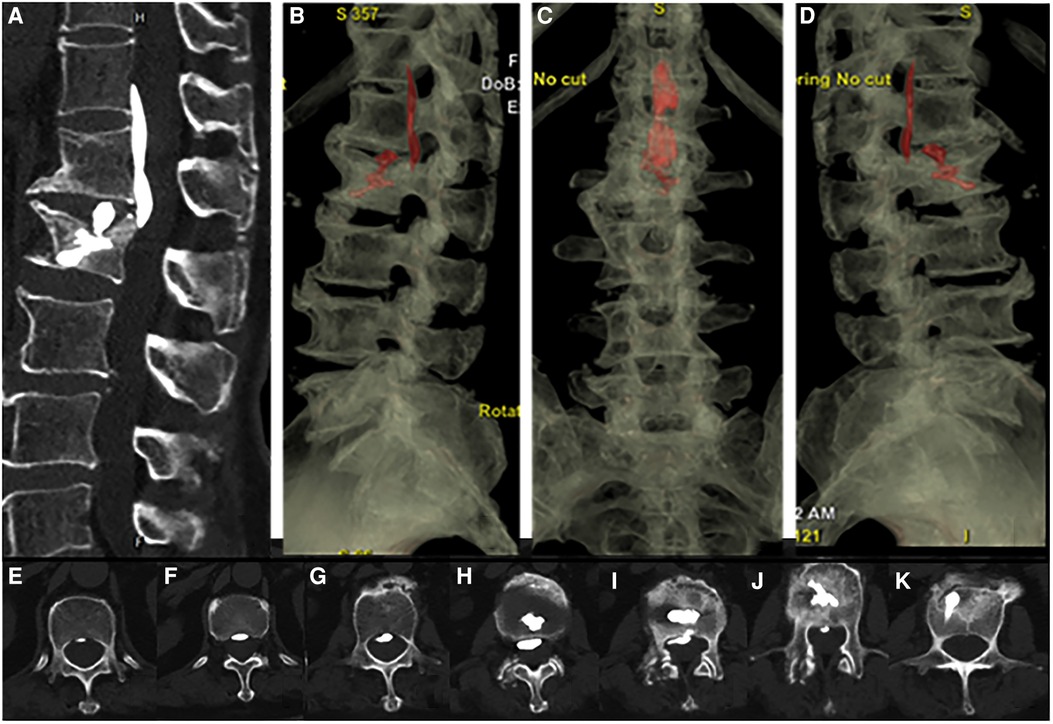
Figure 4. Postoperative CT scan 3D reconstruction showed bone cement infiltrating the epidural space of the spinal canal and distributed along the posterior longitudinal ligament from T12 to L2 (A–D) and the filling of bone cement in the posterior spinal canal of the L2 vertebral body(E–K).
After routine treatment, all laboratory indexes were in the normal range at discharge. Routine follow-up was performed three and six months after the operation, and the patient recovered well without discomfort. Because this type of cement leakage is rare, we will continue to follow up the patient, and we will detect other discomfort in time if there is any subsequent discomfort.
Discussion
Percutaneous kyphoplasty (PKP), as minimally invasive spine surgery, has a long history of development and mature technology (6, 7). It is widely used for its advantages, such as less trauma and good postoperative effect (8). Notably, percutaneous vesselplasty is a further surgical approach based on PKP (9, 10). The progress of technical without affecting the diffusion of bone cement greatly reduce the possibility of cement leakage into the spinal canal through the fracture line or the posterior edge of discontinuous vertebral body and reduce the most serious neurological complications (11).
It has been reported that bone cement leakage is one of the most common complications in PKP surgery, with an incidence of about 15%. The different leakage sites and leakage paths can be classified into several types. Among them, the consequences of intraspinal leakage are the most serious and therefore have received more attention (12). Cement protruding into the spinal canal may cause compression of nerves, numbness and pain in the lower limbs, and even paralysis.
There are several common types of bone cement leakage. The first is Yeom classification: via a basivertebral vein (type B), via a segmental vein (type S), and through a cortical defect (type C), in which type C is refined into two subtypes with leakage reaching the intervertebral disc (type D) and leakage not reaching the intervertebral disc (type C) (13). The second classification is the Wang classification, including five types: type A, through a cortical defect into the paraspinal soft tissues; type B, through the basivertebral foramen; type C, via the needle channel; type D, through a cortical defect into the disc space; and type E, via the paravertebral vein (14). According to the existing classification criteria, our case should be included in the compound type D that enters the intervertebral disc space through cortical damage. However, in the above traditional classification, there is no specific classification of intraspinal leakage (15, 16); thus, because of the particularity of this case report, we recommend a more specific classification of bone cement leakage into the bony spinal canal. Division, according to its anatomy, and the results of compression of the dural sac will be of guiding significance to whether and when we should take measures.
The posterior longitudinal ligament is composed of two layers, and the fibers from the deep layer are closely combined with the fibers of the annulus fibrosus of the intervertebral disc (17). There is a potential fascial fissure of considerable size on the dorsal side (18). The posterior part of the superficial layer of the posterior longitudinal ligament is connected to the dura mater, which effectively prevents the leakage of bone cement into the dural space (19). Therefore, according to its leakage path, it is reasonable to classify it as the intraspinal epidural space, and because of its complex leakage path, it is more appropriate to classify it as a mixed leakage.
We believe that it is extremely necessary to determine whether the leakage is located inside or outside the dura and the extent of compression. Only when lower limb symptoms occur, do we recommend acute decompression. Open decompression through a posterior approach or spinal endoscopy should be performed to relieve compression (20). Consequently, given the more uniform shape of the leak in our case, which is located in the epidural intraspinal canal, we believe that conservative treatment can be successful, such as rest immobilization and anti-inflammatory analgesia.
X-rays are routinely used to evaluate the intraoperative process and distribution of bone cement, which may lead to a missed diagnosis. Early and faster intervention is recommended for patients with bone cement leakage; therefore, performing intraoperative CT scanning and neurophysiological examinations when necessary for special types of bone cement leakage rather than simply classifying patients by symptoms (21).
By exploring the reasons for the leakage of bone cement in this case, combined with the current clinical experience, the preventive measures for the type of leakage may include the following: an improved preoperative examination including various necessary imaging and physical examinations to identify the anatomy of the fractured vertebral body, the degeneration of the upper and lower intervertebral discs, and the integrity of the vertebral body (22, 23). Due to the different injection methods of bone cement in the balloon, a more careful operation is required, the amount of bone cement bolus should also be appropriate, and continuous fluoroscopic monitoring is recommended (24–26). We suggest that we should be more cautious in the treatment of special types of fractures or for which the type of injury cannot be fully determined by preoperative examination.
In summary, we suggest that the classification of intraspinal bone cement leakage should be more specific and clinical, and adequate preparations should be made before surgery to standardize intraoperative operations to prevent such complications as much as possible. Once such complications occur, the detailed types and ways of leakage can be mastered to predict the results more accurately. Patients can benefit more by taking appropriate measures according to the situation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Beijing Friendship Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Each author made substantial contributions to this work. NA, SJG, JSL and QF: contributed to the conception and design of the work. SJG, NA and JSL: contributed to the acquisition of study data. HXJ, NS and HM: revised this article. All authors have drafted the work or substantively revised it. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu KZ, Chen SC, Xu L. Comparison of percutaneous balloon dilation kyphoplasty and percutaneous vertebroplasty in treatment for thoracolumbar vertebral compression fractures. Eur Rev Med Pharmacol Sci. (2018) 22(1 Suppl):96–102. doi: 10.26355/eurrev_201807_15370
2. Klingler JH, Sircar R, Deininger MH, Scheiwe C, Kogias E, Hubbe U. Vesselplasty: a new minimally invasive approach to treat pathological vertebral fractures in selected tumor patients—preliminary results. Rofo. (2013) 185(4):340–50. doi: 10.1055/s-0032-1330443
3. Yang Y, Tian Q, Wang T, Lu Y, Li W, Wu C. Vessel-plasty using bone-filling mesh container for treatment of malignant severe compression fractures in cervical vertebrae. J Pain Res. (2022) 15:1173–82. doi: 10.2147/JPR.S360195
4. Raina DB, Qayoom I, Larsson D, Zheng MH, Kumar A, Isaksson H, et al. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials. (2019) 188:38–49. doi: 10.1016/j.biomaterials.2018.10.004
5. Chang X, Lv YF, Chen B, Li HY, Han XB, Yang K, et al. Vertebroplasty versus kyphoplasty in osteoporotic vertebral compression fracture: a meta-analysis of prospective comparative studies. Int Orthop. (2015) 39(3):491–500. doi: 10.1007/s00264-014-2525-5
6. Huang S, Zhu X, Xiao D, Zhuang J, Liang G, Liang C, et al. Therapeutic effect of percutaneous kyphoplasty combined with anti-osteoporosis drug on postmenopausal women with osteoporotic vertebral compression fracture and analysis of postoperative bone cement leakage risk factors: a retrospective cohort study. J Orthop Surg Res. (2019) 14(1):452. doi: 10.1186/s13018-019-1499-9
7. Yang H, Chen L, Zheng Z, Yin G, Lu WW, Wang G, et al. Therapeutic effects analysis of percutaneous kyphoplasty for osteoporotic vertebral compression fractures: a multicentre study. J Orthop Translat. (2017) 11:73–7. doi: 10.1016/j.jot.2017.04.003
8. Lu Q, Gao S, Zhou M. The effect of bone cement on the curative effect of percutaneous kyphoplasty in the treatment of osteoporotic vertebral compression fracture. Ann Palliat Med. (2021) 10(10):11013–23. doi: 10.21037/apm-21-2767
9. Li J, Yuan X, Li F, Ding Y, Ma G, Song C, et al. A randomized trial comparing the clinical efficacy and safety of a novel steerable percutaneous kyphoplasty with traditional PKP in osteoporotic vertebral fractures. Ann Transl Med. (2021) 9(12):1024. doi: 10.21037/atm-21-1880
10. Wang D, Li Z, Yin S, Liu R, Sun F, Hu Y, et al. Modified kyphoplasty with controllable balloon dilatation for treatment of thoracolumbar osteoporotic vertebral compression fractures. Int Orthop. (2020) 44(7):1401–8. doi: 10.1007/s00264-020-04592-z
11. Chen Z, Liu L, Wang Z, Gong J, Xia N, Huang W, et al. A retrospective study of the use of percutaneous vesselplasty for pathological vertebral compression fractures. J Cancer Res Ther. (2021) 17(7):1725–9. doi: 10.4103/jcrt.jcrt_2145_21
12. Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones CA, Golmohammadi K, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. (2018) 4(4):Cd006349. doi: 10.1002/14651858.CD006349.pub3
13. Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. (2003) 85(1):83–9. doi: 10.1302/0301-620X.85B1.13026
14. Wang C, Fan S, Liu J, Suyou L, Shan Z, Zhao F. Basivertebral foramen could be connected with intravertebral cleft: a potential risk factor of cement leakage in percutaneous kyphoplasty. Spine J. (2014) 14(8):1551–8. doi: 10.1016/j.spinee.2013.09.025
15. Zou P, Gong HL, Wei JM, Wei DM, Qian LX, Liu P, et al. Spinal epidural hematoma after percutaneous kyphoplasty: case report and literature review. J Pain Res. (2020) 13:2799–804. doi: 10.2147/JPR.S280650
16. Wiltse LL, Fonseca AS, Amster J, Dimartino P, Ravessoud FA. Relationship of the dura, Hofmann's Ligaments, Batson's plexus, and a fibrovascular membrane lying on the posterior surface of the vertebral bodies and attaching to the deep layer of the posterior longitudinal ligament. An anatomical, radiologic, and clinical study. Spine. (1993) 18(8):1030–43. doi: 10.1097/00007632-199306150-00013
17. Tang B, Xu S, Chen X, Cui L, Wang Y, Yan X, et al. The impact of intravertebral cleft on cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a case-control study. BMC Musculoskelet Disord. (2021) 22(1):805. doi: 10.1186/s12891-021-04685-9
18. Wiltse LL. Anatomy of the extradural compartments of the lumbar spinal canal. Peridural membrane and circumneural sheath. Radiol Clin North Am. (2000) 38(6):1177–206. doi: 10.1016/S0033-8389(08)70003-4
19. Hostin R, Carr J, Gupta MC, Hazelwood S, Dublin A. Importance of the peridural membrane in percutaneous vertebroplasty. J Spinal Disord Tech. (2005) 18(1):34–9. doi: 10.1097/01.bsd.0000127704.83334.1a
20. Hsieh MK, Kao FC, Chiu PY, Chen LH, Yu CW, Niu CC, et al. Risk factors of neurological deficit and pulmonary cement embolism after percutaneous vertebroplasty. J Orthop Surg Res. (2019) 14(1):406. doi: 10.1186/s13018-019-1459-4
21. Chen H, Jia P, Bao L, Feng F, Yang H, Li JJ, et al. Depression of the thoracolumbar posterior vertebral body on the estimation of cement leakage in vertebroplasty and kyphoplasty operations. Chin Med J. (2015) 128(23):3158–62. doi: 10.4103/0366-6999.170264
22. Gao C, Zong M, Wang WT, Xu L, Cao D, Zou YF. Analysis of risk factors causing short-term cement leakages and long-term complications after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Radiol. (2018) 59(5):577–85. doi: 10.1177/0284185117725368
23. Zhong BY, He SC, Zhu HD, Pan T, Fang W, Chen L, et al. Nomogram for predicting intradiscal cement leakage following percutaneous vertebroplasty in patients with osteoporotic related vertebral compression fractures. Pain Physician. (2017) 20(4):E513–e520. PMID: 2853556028535560
24. Nieuwenhuijse MJ, Bollen L, van Erkel AR, Dijkstra PD. Optimal intravertebral cement volume in percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures. Spine. (2012) 37(20):1747–55. doi: 10.1097/BRS.0b013e318254871c
25. Yu WB, Jiang XB, Liang D, Xu WX, Ye LQ, Wang J, et al. Risk factors and score for recollapse of the augmented vertebrae after percutaneous vertebroplasty in osteoporotic vertebral compression fractures. Osteoporos Int. (2019) 30(2):423–30. doi: 10.1007/s00198-018-4754-8
26. Zhang ZF, Yang JL, Jiang HC, Lai Z, Wu F, Pan YQ, et al. An updated comparison of high- and low-viscosity cement vertebroplasty in the treatment of osteoporotic thoracolumbar vertebral compression fractures: a retrospective cohort study. Int J Surg. (2017) 43:126–30. doi: 10.1016/j.ijsu.2017.05.067
Keywords: cement leakage, complication, percutaneous kyphoplasty (PKP), epidural, classification standard
Citation: An N, Guo S, Lin J, Zhuang H, Meng H, Su N and Fei Q (2023) Continuous cement leakage along the posterior longitudinal ligament of the intraspinal epidural during a percutaneous vesselplasty: A case report and literature review. Front. Surg. 9:1087591. doi: 10.3389/fsurg.2022.1087591
Received: 2 November 2022; Accepted: 16 December 2022;
Published: 9 January 2023.
Edited by:
Lifeng Lao, Shanghai Jiao Tong University, ChinaReviewed by:
Liangbin Gao, Sun Yat-sen Memorial Hospital, ChinaXiaoyang Liu, Shandong Provincial Hospital, China
Xing Zejun, Shanxi Medical University, China
© 2023 An, Guo, Lin, Zhuang, Meng, Su and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Fei c3BpbmVmZWlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Ning An†
Ning An† Sijia Guo
Sijia Guo Hai Meng
Hai Meng Qi Fei
Qi Fei