
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 06 January 2023
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1072908
This article is part of the Research Topic Advancing Health Equity Through Surgery View all 10 articles
Background: Diastolic dysfunction (DD), one of the earliest signs of cirrhotic cardiomyopathy (CCM), is included in the revised 2019 CCM criteria. Nonetheless, relevant research regarding the effects of revised DD on post-liver transplantation (LT) outcomes remains limited.
Methods: This retrospective study enrolled patients who underwent LT for decompensated cirrhosis, from January 2018 to March 2021. Patients were divided into DD and non-DD groups. Clinical data were collected. Patients were followed up with, for at least 1 year post-LT; cardiovascular adverse events (AEs) and survival status were recorded. Risk factors were identified using 1:2 propensity score matching (PSM), after adjusting for confounding factors. The caliper value was set to 0.02.
Results: Of 231 patients, 153 were diagnosed with DD (male, 81.8%; mean age, 51.5 ± 9.5 years). Nineteen patients with DD died within 1 year, post-LT. After PSM, 97 and 60 patients were diagnosed with and without DD, respectively. Patients with DD had longer intensive care unit (ICU) stays, higher perioperative cardiovascular AEs, and higher mortality rates than those without DD. In a multivariate analysis, interventricular septum (IVS), left atrial volume index (LAVI), and potassium levels were independent prognostic factors of perioperative cardiovascular AEs, while a decreased early diastolic mitral annular tissue velocity (e’), increased neutrophil-to-lymphocyte ratio (NLR) and tumor markers were predictors of mortality within 1 year post-LT after PSM (P < 0.05).
Conclusion: Cardiac DD may contribute to perioperative cardiovascular AEs and mortality post-LT. Clinicians should be aware of decompensated cirrhosis in patients with DD.
Cirrhotic cardiomyopathy (CCM) is defined as impaired contractility due to stress and/or diastolic dysfunction (DD) with electrophysiological abnormalities (1, 2), which is associated with a high incidence of complications and poor survival after liver transplantation (LT) (3, 4). The prevalence of CCM is approximately 33%–53% in patients on the transplant waiting list (5). Some studies have shown that early to late diastolic trans-mitral flow velocity (E/A), isovolumetric relaxation time (IVRT), and time-delay estimation (TDE) are prognostic markers of CCM (6).
DD, as one of the earliest signs of CCM, was entirely updated in the revised 2019 criteria (7) of the Cirrhotic Cardiomyopathy Consortium (CCC), including septal early diastolic mitral annular tissue velocity (e’), early diastolic trans-mitral flow to early diastolic mitral annular tissue velocity (E/e’), left atrial volume index (LAVI), and tricuspid regurgitation maximum velocity (TRV). Nonetheless, relevant research is limited.
This study aimed to investigate the effects and predictive value of revised DD on post-LT outcomes, based on the revised 2019 CCC criteria.
This retrospective study enrolled patients aged 18–70 years, diagnosed with decompensated cirrhosis (8) at the Affiliated Hospital of Qingdao University, Qingdao, China, between January 2018 and March 2021. Patients were divided into two groups (DD or non-DD group), according to echocardiographic examinations of cardiac diastolic function a week before operation, based on the revised 2019 CCC criteria (9–11). DD was determined if three of the following criteria were met: E/e’ > 15, LAVI > 34 ml/m2, e’ < 7 cm/s or TRV > 2.8 m/s. Patients who had known heart disease pre-transplant were excluded. The flow diagram of the study is illustrated in Figure 1. The study was approved by Clinical Trials (NCT04976764) and the Ethics Committee of the Affiliated Hospital of Qing Dao University (QYFYWZLL 26462). All involved persons gave their informed consent (written or verbal, as appropriate) prior to study inclusion.
Enumeration data are presented as frequencies (percentages) and the significance is determined by chi-square test or Fisher's exact test. Intergroup comparisons were performed using a Student's t-test or Mann-Whitney U test. Multivariate logistic regression analysis models were used to identify independent and predictive factors for poor outcomes. Survival status was assessed using Kaplan–Meier curves and the log-rank test. Significant variables in the univariate analysis were tested in a multivariate analysis using Cox's proportional hazard model, to identify the predictors of survival. The caliper value of propensity score matching (PSM), adjusted for confounders, was set to 0.02. A P-value < 0.05 was regarded as statistically significant. SPSS (version 26.0, IBM, New York, USA) was used to analyze the data.
Baseline characteristics of the 231 patients were collected (male, 81.8%; mean age 51.5 ± 9.5 years) (Table 1). A total of 153 (66.2%) patients in the DD group were diagnosed with DD with normal left ventricular ejection fraction, according to the 2019 CCC criteria. Before PSM, the patients with DD tended to be older (P < 0.01) and were more likely to have hypertension (P < 0.01) and diabetes (P < 0.05), in comparison to patients without DD. After PSM, there were 97 patients in the DD group and 69 patients in the non-DD group, with no notable differences in sex, age, smoking status, etiology, and basic diseases, including hypertension and diabetes.
A comparison of the serological indices and inflammatory markers in Table 2 showed that erythrocyte, hemoglobin, and carbohydrate antigen (CA) 19-9 levels differed significantly between the groups, after PSM (P < 0.05). No differences were noted in the systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR) between the groups (P > 0.05). Additional results of routine laboratory tests, including complete blood cell counts, liver function, renal function, blood lipids, electrolytes, glucose, myocardial enzymes, coagulation function, and other tumor markers, are described in Supplementary Table S1 of Appendix 1.
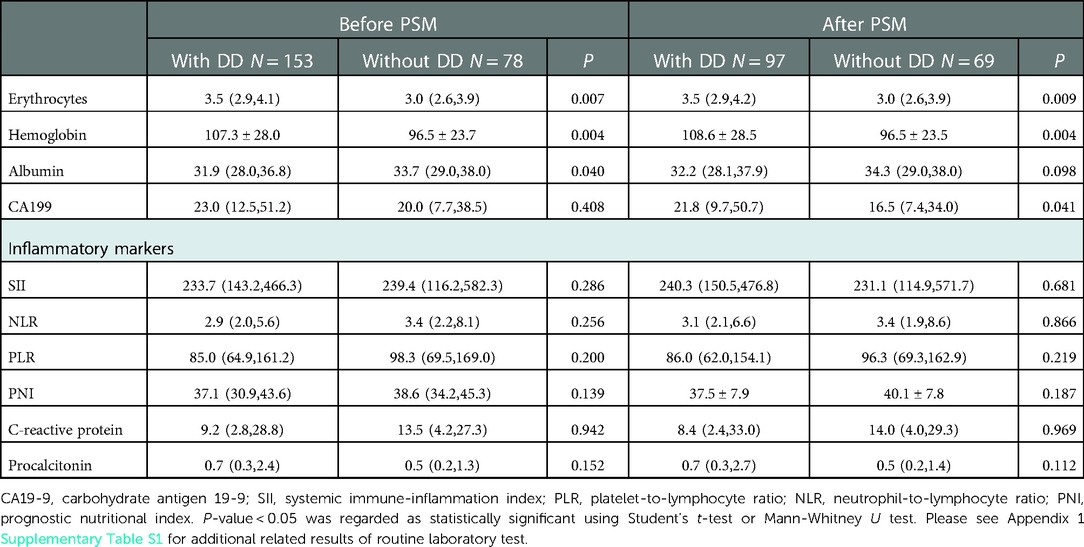
Table 2. Comparisons of routine laboratory test results and inflammatory markers between patients with and without DD.
There were significant differences in the septal e’, E/e’, and tricuspid regurgitation (TR) maximum velocity between the groups (P < 0.01). Moreover, it was found that pulmonary artery systolic pressure, left ventricular pulse wave, and interventricular septum (IVS) were higher in the DD group than in the non-DD group, before and after PSM (P < 0.01). The median QT interval was not prolonged (P > 0.05). Other echocardiographic characteristics are presented in Table 3.
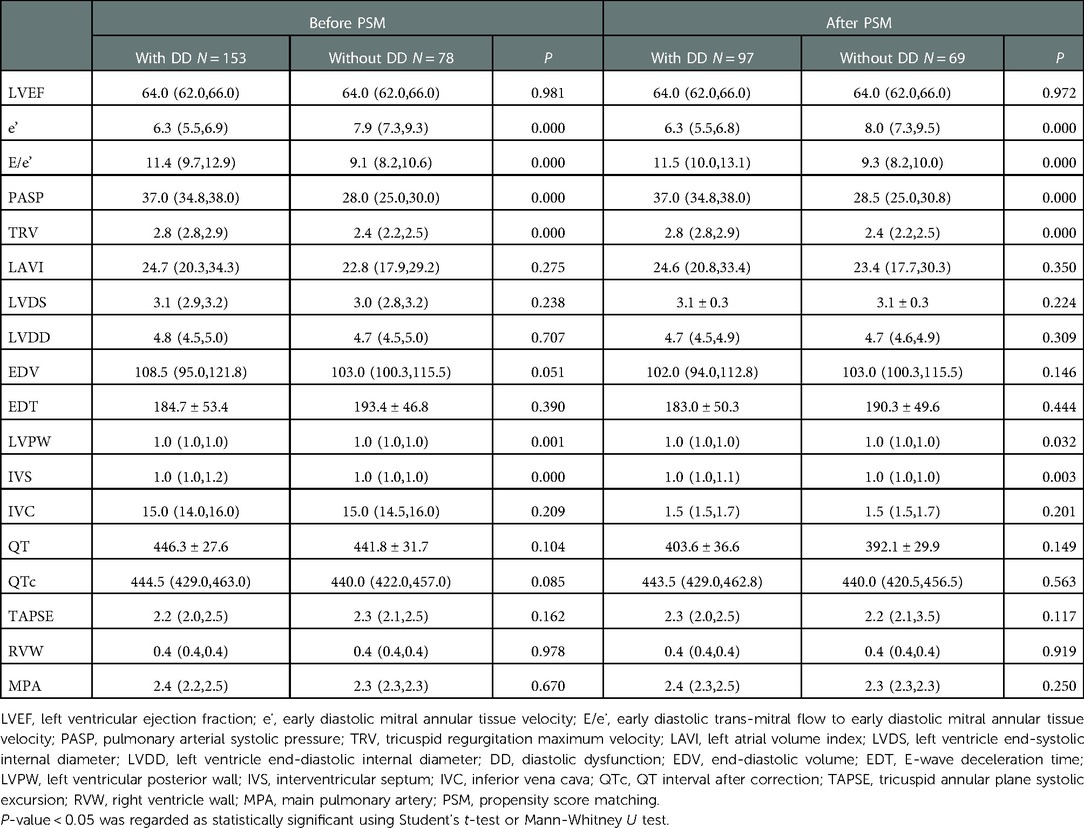
Table 3. Comparisons of echocardiography parameters and QT interval between patients with and without DD.
Patients with DD had an increased Child-Pugh class score before and after PSM (P = 0.003 and P = 0.045, respectively). No significant differences were observed with respect to cardiac function class, model for end-stage liver disease (MELD) score, physician global assessment score, American Society of Anesthesiology score, bleeding volume, and volume of blood transfusion by PSM (Supplementary Table S2 of Appendix 1). Nonetheless, patients with DD had longer intensive care unit stays than those without DD (P < 0.05) (Table 4).
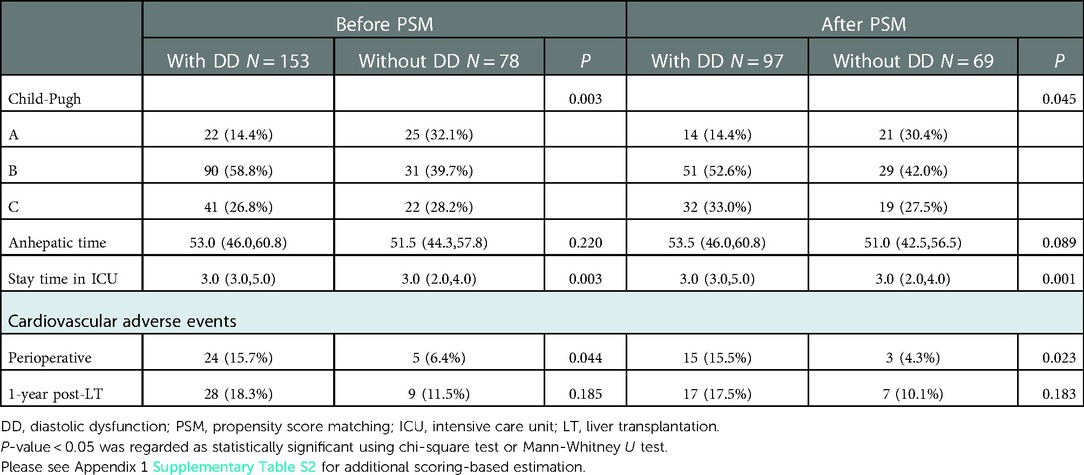
Table 4. Comparison of scoring-based estimation and procedure-related data between patients with and without DD.
Enrolled patients were followed up, for at least 1 year. In total, 29 patients (12.6%) developed perioperative cardiovascular adverse events (AEs), 24 of whom had DD. Patients with DD frequently experienced perioperative cardiovascular events (15.7% vs. 6.4%, P < 0.05) (Table 4). In a multivariate analysis, anhepatic time (P = 0.030), potassium levels (P = 0.024), IVS (P = 0.018), and bleeding volume (P = 0.046) were found to be independent predictors of the incidence of perioperative cardiovascular AEs before PSM. Considering age, sex, etiology, smoking status, and basic diseases, IVS (P = 0.008), LAVI (P = 0.047), and potassium level (P = 0.003) were independently correlated with perioperative cardiovascular AEs after PSM (Table 5).

Table 5. Univariable and multivariable logistic regression analysis of perioperative cardiovascular adverse events.
Twenty-one patients (9.10%) with DD died within the first year of follow-up. The causes were mainly AEs, graft rejection, progression of liver disease and sepsis (Table 6). But we found no statistically significant association between DD and each clinical event. The Kaplan–Meier curves in Figure 2 show that patients with DD had lower survival rates than those without DD. In a multivariable Cox regression analysis, carcinoembryonic antigen, e’, and left ventricle end-systolic internal diameter were correlated with the occurrence of death, within 1-year post-LT. In the model adjusted by PSM, decreased e’, increased NLR and tumor markers were associated with a greater 1-year mortality rate (Table 7).
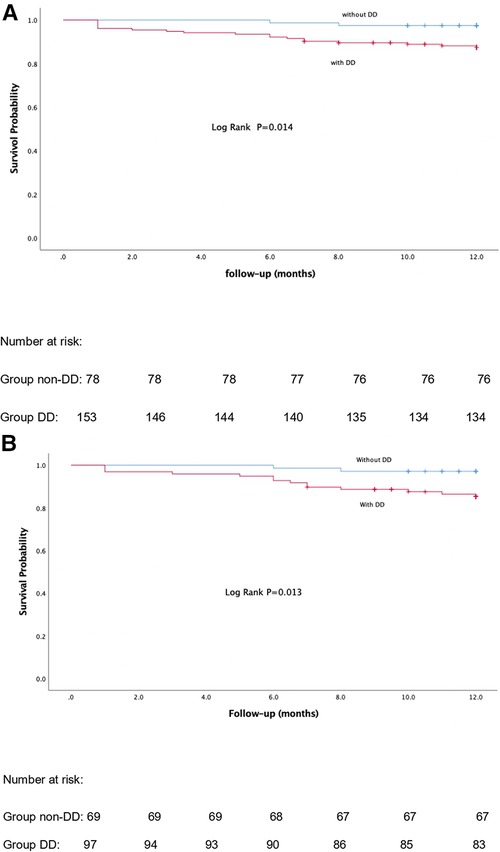
Figure 2. Kaplan-Meier curves of 1-year mortality post-LT before and after propensity score matching analysis. (A) Before-PSM. (B) After-PSM. P-value < 0.05 was regarded as statistically significant.
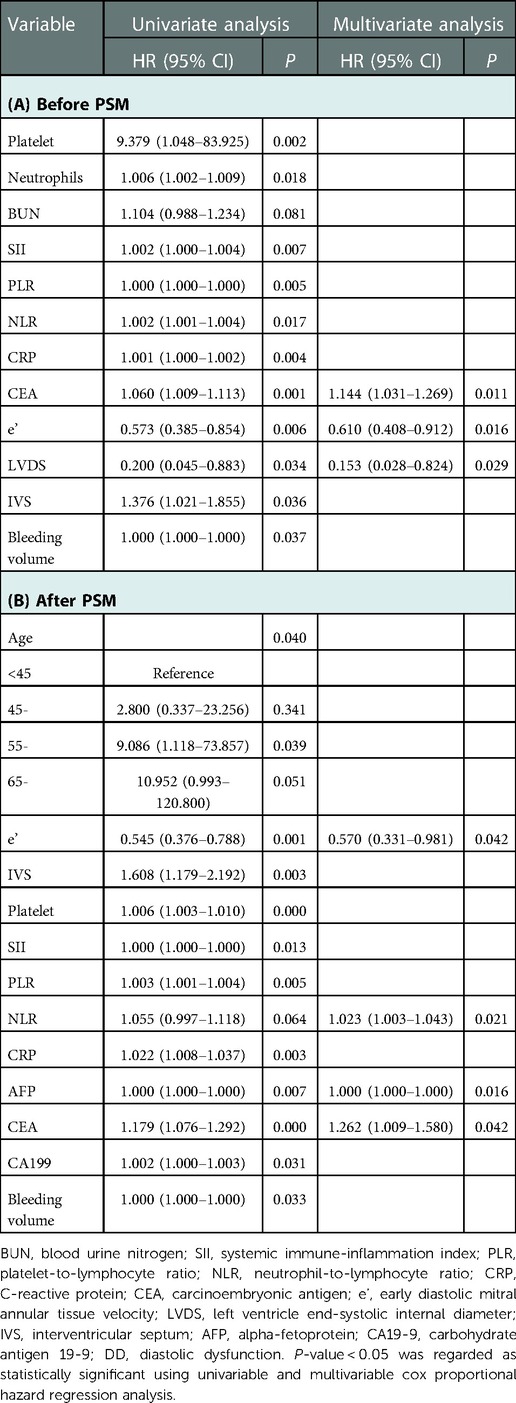
Table 7. Univariable and multivariable cox proportional hazard regression analysis of 1-year mortality post-LT.
IVS and e’ were the strongest independent prognostic factors for perioperative cardiovascular AEs and mortality, respectively.
In CCM, an occult onset process upon encountering environmental stress, such as transplantation, may contribute to rapid progression and even significant mortality rates. The primary outcome of this study showed that DD was related to the occurrence of perioperative cardiovascular AEs and mortality, before and after PSM. Among the echocardiographic parameters, IVS, and e’ were the strongest independent prognostic factors for perioperative cardiovascular AEs and mortality, respectively. Upon encountering environmental stress, such as transplantation, impaired contractility fails to adapt to initial changes. Decreased vascular resistance and increased cardiac output increase cardiac preload, resulting in abnormal filling of the ventricle and blood redistribution (12, 13). Histological examination of a heart with CCM revealed edema, cardiomyocyte hypertrophy, nuclear vacuolization, and fibrosis, which occurred in conjunction with enhanced accumulation of collagen. However, we observed that ventricular wall stiffness would partially recover (14–16). Studies have demonstrated that DD increases the risk of cardiovascular AEs post-LT (5, 17). Nonetheless, few studies have reported such correlation based on the 2005 criteria (18).
Furthermore, elevated IVS was the strongest cardiac predictor, increasing the risk of perioperative cardiovascular AEs by 2.4-fold. These results may be attributed to myocardial remodeling, resulting in alterations in the cardiac structure, as onset was earliest in the IVS (19, 20). Decreased e’ aided in confirming which patients were at an increasing risk of poor survival, in both univariate and multivariate regression analysis (6, 21). This may reflect progressive stiffness of the myocardium and deteriorating cardiac function (22). Prior studies have revealed association of E/A, E/e’, and LAVI with poor survival post-LT (17, 23–26); however, it is not well-suited to apply the E/A ratio owing to its load- and age-dependence (27, 28). In comparison, some previous studies have failed to find any relevance (4, 29). The above mentioned studies did not rule out the influence of confounding factors. The present study adopted the 2019 criteria and performed PSM for risk factors, which reduced the bias in the results.
A prolonged QT interval is the main electrophysiological signature of CCM, which is associated with 30-day cardiovascular AEs and mortality, post-LT (30, 31). In the 2019 CCC consensus, QT prolongation was not warranted, because of its limited value in the diagnosis of CCM (32, 33). Potassium is the major predictive factor of perioperative cardiovascular AEs. Ischemia-reperfusion injury post-LT increase the level of extracellular potassium and reduce the concentration gradient between the inside and outside of the cell. Shortening action potential and impaired conductivity was attributed to altered gating of ion channels, predisposing to malignant arrhythmias (34, 35). Because of the limitations of current study population, possibilities of impaired ion channels cannot be completely excluded (11). Pulmonary arterial hypertension has been shown to be involved in liver fibrosis at the gene level and confers higher mortality (36–38). Cardiac dysfunction was first attributed to the direct effect of alcohol; however, it was revealed to be independent of the etiology (39, 40). Cardiac function deteriorated with the progression of cirrhosis, but showed limited progression with stable cirrhosis within 2 years (25, 28, 41). Pro-B-type natriuretic peptides and troponins have also been described as prognostic markers (42, 43). Nevertheless, this was contradicted by the results of our study. In contrast to the Child-Pugh score, the MELD score showed no impact on the presence of DD because of underestimation of the severity of end-stage liver disease on the waiting list for LT (44).
Given the increased risk of infectious complications, upregulation of inflammatory markers and downregulation of cnidarian complements in both the advancement of cirrhosis and development of CCM were associated with poor survival (21, 45–47). Similarly, NLR was considered an effector, along with SII and PLR, in this study. In addition, all enrolled patients had near-normal systolic function at a resting state maintained by the compensatory pathways of hyperdynamic circulatory state and low systemic vascular resistance (48). Additionally, a very low left ventricular ejection fraction is regarded as a contraindication to LT. Prior studies have revealed associations between global longitudinal strain and poor survival (42, 49). Due to the limitations of diagnostic tools, we were unable to further confirm the influence of global longitudinal strain.
Our study has several limitations. First, this was a single-center study, thus lacks representativeness. Moreover, it was limited by its retrospective and observational study design. Therefore, prospective and multicenter validation studies are required.
Decompensated cirrhosis with DD accelerates perioperative cardiovascular AEs and 1-year post-transplantation mortality rates. Appropriate precedence in decompensated cirrhosis with DD on the waiting list should be considered to ensure timely diagnosis. In PSM analysis, multiple risk factors including IVS, LAVI, e’, potassium, and NLR collectively contributed to perioperative cardiovascular AEs and 1-year mortality, highlighting the need for closer post-LT monitoring and management.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Qing Dao University (QYFYWZLL 26462). The patients/participants provided their written informed consent to participate in this study.
SB made the major contribution to draft the manuscript. YJ obtained institutional review board approval. XN contributed to the data acquisition. WZ and XL assisted in statistical analysis. XJ is the guarantor of the study and made a major contribution to the conception and design of the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of Shandong Province [grant numbers ZR202103040311].
We thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1072908/full#supplementary-material.
AEs, adverse events; AFP, alpha-fetoprotein; BUN, blood urine nitrogen; CCM, cirrhotic cardiomyopathy; CCC, cirrhotic cardiomyopathy consortium; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRP, C-reactive protein; DD, diastolic dysfunction; e’, early diastolic mitral annular tissue velocity; E/e’, early diastolic trans-mitral flow to early diastolic mitral annular tissue velocity; EDV, end-diastolic volume; EDT, E-wave deceleration time; GLS, global longitudinal strain; ICU, intensive care unit; IVRT, isovolumetric relaxation time; IVS, interventricular septum; IVC, inferior vena cava; LT, liver transplantation; LAVI, left atrial volume index; LVDS, left ventricle end-systolic internal diameter; LVDD, left ventricle end-diastolic internal diameter; LVPW, left ventricular posterior wall; LVEF, left ventricular ejection fraction; MPA, main pulmonary artery; MELD, model for end-stage liver disease; NLR, neutrophil-to-lymphocyte ratio; PSM, propensity score matching; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PASP, pulmonary arterial systolic pressure; QTc, QT interval after correction; RVW, right ventricle wall; SII, systemic immune-inflammation index; TRV, tricuspid regurgitation maximum velocity; TAPSE, tricuspid annular plane systolic excursion; TDE, time-delay estimation; TRV, tricuspid regurgitation maximum velocity.
1. Vanwagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, et al. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am J Transplant. (2016) 16:2684–94. doi: 10.1111/ajt.13779
2. Cesari M, Frigo AC, Tonon M, Angeli P. Cardiovascular predictors of death in patients with cirrhosis. Hepatology. (2018) 68:215–23. doi: 10.1002/hep.29520
3. Chahal D, Liu H, Shamatutu C, Sidhu H, Lee SS, Marquez V. Review article: comprehensive analysis of cirrhotic cardiomyopathy. Aliment Pharmacol Ther. (2021) 53:985–98. doi: 10.1111/apt.16305
4. Falletta C, Filì D, Nugara C, Di Gesaro GD, Minà C, Baravoglia CMH, et al. Diastolic dysfunction diagnosed by tissue Doppler imaging in cirrhotic patients: prevalence and its possible relationship with clinical outcome. Eur J Intern Med. (2015) 26:830–4. doi: 10.1016/j.ejim.2015.10.009
5. Izzy M, Soldatova A, Sun X, Angirekula M, Mara K, Lin G, et al. Cirrhotic cardiomyopathy predicts posttransplant cardiovascular disease: revelations of the new diagnostic criteria. Liver Transpl. (2021) 27:876–86. doi: 10.1002/lt.26000
6. Izzy M, Oh J, Watt KD. Cirrhotic cardiomyopathy after transplantation: neither the transient nor innocent bystander. Hepatology. (2018) 68:2008–15. doi: 10.1002/hep.30040
7. Spann A, Coe C, Ajayi T, Montgomery G, Shwetar M, Oje A, et al. Cirrhotic cardiomyopathy: appraisal of the original and revised criteria in predicting post-transplant cardiac outcomes. Liver Transpl. (2022) 28:1321–31. doi: 10.1002/lt.26460
8. European Association for the Study of the Liver. EASL Clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
9. Liu H, Yoon KT, Zhang J, Lee SS. Advances in cirrhotic cardiomyopathy. Curr Opin Gastroenterol. (2021) 37:187–93. doi: 10.1097/MOG.0000000000000733
10. Desai MS. Mechanistic insights into the pathophysiology of cirrhotic cardiomyopathy. Anal Biochem. (2022) 636:114388. doi: 10.1016/j.ab.2021.114388
11. Bodys-Pełka A, Kusztal M, Raszeja-Wyszomirska J, Główczyńska R, Grabowski M. What’s new in cirrhotic cardiomyopathy?-review article -review article. J Pers Med. (2021) 11:1285. doi: 10.3390/jpm
12. Guglin M, Nazif K. New onset nonischemic cardiomyopathy post liver transplantation. Heart Fail Rev. (2022) 27:1829–36. doi: 10.1007/s10741-021-10196-5
13. Rahman S, Mallett SV. Cirrhotic cardiomyopathy: implications for the perioperative management of liver transplant patients. World J Hepatol. (2015) 7:507–20. doi: 10.4254/wjh.v7.i3.507
14. Are VS, Vuppalanchi R, Vilar-Gomez E, Chalasani N. Enhanced liver fibrosis score can be used to predict liver-related events in patients with nonalcoholic steatohepatitis and compensated cirrhosis. Clin Gastroenterol Hepatol. (2021) 19:1292–1293.e3. doi: 10.1016/j.cgh.2020.06.070
15. Park JG, Jung J, Verma KK, Kang MK, Madamba E, Lopez S, et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. (2021) 53:1030–7. doi: 10.1111/apt.16324
16. Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int. (2017) 37:700–6. doi: 10.1111/liv.13287
17. Dowsley TF, Bayne DB, Langnas AN, Dumitru I, Windle JR, Porter TR, et al. Diastolic dysfunction in patients with end-stage liver disease is associated with development of heart failure early after liver transplantation. Transplantation. (2012) 94:646–51. doi: 10.1097/TP.0b013e31825f0f97
18. Parnham S, Gleadle JM, Leong D, Grover S, Bradbrook C, Woodman RJ, et al. Myocardial perfusion is impaired in asymptomatic renal and liver transplant recipients: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. (2015) 17:56. doi: 10.1186/s12968-015-0166-5
19. Yalçin F, Yalçin H, Küçükler N, Arslan S, Akkuş O, Kurtul A, et al. Basal septal hypertrophy as the early imaging biomarker for adaptive phase of remodeling prior to heart failure. J Clin Med. (2021) 11:75. doi: 10.3390/jcm11010075
20. Yalçin F, Abraham R, Abraham TP. Basal septal hypertrophy: extremely sensitive region to variety of stress stimuli and stressed heart morphology. J Hypertens. (2022) 40:626–7. doi: 10.1097/HJH.0000000000003043
21. Izzy M, Vanwagner LB, Lin G, Altieri M, Findlay JY, Oh JK, et al. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology. (2020) 71:334–45. doi: 10.1002/hep.30875
22. Liu HQ, Jayakumar S, Traboulsi M, Lee SS. Cirrhotic cardiomyopathy: implications for liver transplantation. Liver Transpl. (2017) 23:826–35. doi: 10.1002/lt.24768
23. Khan MA, Yang EY, Zhan Y, Judd RM, Chan W, Nabi F, et al. Association of left atrial volume index and all-cause mortality in patients referred for routine cardiovascular magnetic resonance: a multicenter study. J Cardiovasc Magn Reson. (2019) 21:4. doi: 10.1186/s12968-018-0517-0
24. Liu H, Lee SS. Diagnostic criteria of cirrhotic cardiomyopathy: out with the old, in with the new? Hepatology. (2021) 74:3523–5. doi: 10.1002/hep.32021
25. Wiese S, Hove JD, Mo S, Mygind ND, Tønnesen J, Petersen CL, et al. Cardiac dysfunction in cirrhosis: a 2-yr longitudinal follow-up study using advanced cardiac imaging. Am J Physiol Gastrointest Liver Physiol. (2019) 317:G253–63. doi: 10.1152/ajpgi.00402.2018
26. Moon YJ, Kim JW, Bang YS, Lim YS, Ki Y, Sang BH. Prediction of all-cause mortality after liver transplantation using left ventricular systolic and diastolic function assessment. PLoS One. (2019) 14:e0209100. doi: 10.1371/journal.pone.0209100
27. Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, et al. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Int. (2013) 33:1158–65. doi: 10.1111/liv.12187
28. Giannelli V, Roux O, Laouénan C, Manchon P, Ausloos F, Bachelet D, et al. Impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation. J Hepatol. (2020) 72:463–71. doi: 10.1016/j.jhep.2019.10.002
29. Voiosu AM, Daha IC, Voiosu TA, Mateescu BR, Dan GA, Băicuş CR, et al. Prevalence and impact on survival of hepatopulmonary syndrome and cirrhotic cardiomyopathy in a cohort of cirrhotic patients. Liver Int. (2015) 35:2547–55. doi: 10.1111/liv.12866
30. Kim KS, Kwon HM, Jung KW, Sang BH, Moon YJ, Kim B, et al. Markedly prolonged QTc interval in end-stage liver disease and risk of 30-day cardiovascular event after liver transplant. J Gastroenterol Hepatol. (2021) 36:758–66. doi: 10.1111/jgh.15179
31. Li SH, Hao XW, Liu SM, Gong YX, Niu W, Tang YP. Prolonged QTc interval predicts long-term mortality in cirrhosis: a propensity score matching analysis. Scand J Gastroenterol. (2021) 56:570–7. doi: 10.1080/00365521.2021.1901307
32. Koshy AN, Gow PJ, Testro A, Teh AW, Ko J, Lim HS, et al. Relationship between QT interval prolongation and structural abnormalities in cirrhotic cardiomyopathy: a change in the current paradigm. Am J Transplant. (2021) 21:2240–5. doi: 10.1111/ajt.16500
33. Ko J, Koshy AN, Han HC, Weinberg L, Gow P, Testro A, et al. Effect of liver transplantation on QT-interval prolongation and impact on mortality. Int J Cardiol. (2021) 326:158–63. doi: 10.1016/j.ijcard.2020.11.017
34. Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. (2017) 10:e004667. doi: 10.1161/CIRCEP.116.004667
35. Hegyi B, Chen-Izu Y, Izu LT, Banyasz T. Altered K(+) current profiles underlie cardiac action potential shortening in hyperkalemia and β-adrenergic stimulation. Can J Physiol Pharmacol. (2019) 97:773–80. doi: 10.1139/cjpp-2019-0056
36. Jose A, Shah SA, Anwar N, Jones CR, Sherman KE, Elwing JM. Pulmonary vascular resistance predicts mortality and graft failure in transplantation patients with portopulmonary hypertension. Liver Transpl. (2021) 27:1811–23. doi: 10.1002/lt.26091
37. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53:1801913. doi: 10.1183/13993003.01913-2018
38. Hamberger F, Legchenko E, Chouvarine P, Mederacke YS, Taubert R, Meier M, et al. Pulmonary arterial hypertension and consecutive right heart failure lead to liver fibrosis. Front Cardiovasc Med. (2022) 9:862330. doi: 10.3389/fcvm.2022.862330
39. Carvalheiro F, Rodrigues C, Adrego T, Viana J, Vieira H, Seco C, et al. Diastolic dysfunction in liver cirrhosis: prognostic predictor in liver transplantation? Transplant Proc. (2016) 48:128–31. doi: 10.1016/j.transproceed.2016.01.010
40. Rimbaş RC, Baldea SM, Guerra RDGA, Visoiu SI, Rimbaş M, Pop CS, et al. New definition criteria of myocardial dysfunction in patients with liver cirrhosis: a speckle tracking and tissue Doppler imaging study. Ultrasound Med Biol. (2018) 44:562–74. doi: 10.1016/j.ultrasmedbio.2017.11.013
41. Sundaram V, Patel S, Shetty K, Lindenmeyer CC, Rahimi RS, Flocco G, et al. Risk factors for posttransplantation mortality in recipients with grade 3 acute-on-chronic liver failure: analysis of a north American consortium. Liver Transpl. (2022) 28:1078–89. doi: 10.1002/lt.26408
42. Gallegos-Orozco JF, Charlton MR. Predictors of cardiovascular events after liver transplantation. Clin Liver Dis. (2017) 21:367–79. doi: 10.1016/j.cld.2016.12.009
43. Kwon HM, Moon YJ, Kim KS, Shin WJ, Huh IY, Jun IG, et al. Prognostic value of B-type natriuretic peptide in liver transplant patients: implication in posttransplant mortality. Hepatology. (2021) 74:336–50. doi: 10.1002/hep.31661
44. Chang J, Matheja A, Krzycki S, Lutz P, Böhling N, Glückert K, et al. Model for end-stage liver disease underestimates mortality of patients with acute-on-chronic liver failure waiting for liver transplantation. Dig Liver Dis. (2022) 54:784–90. doi: 10.1016/j.dld.2021.12.011
45. Mittal C, Qureshi W, Singla S, Ahmad U, Huang MA. Pre-transplant left ventricular diastolic dysfunction is associated with post transplant acute graft rejection and graft failure. Dig Dis Sci. (2014) 59:674–80. doi: 10.1007/s10620-013-2955-8
46. Fukui S, Hidaka M, Fukui S, Morimoto S, Hara T, Soyama A, et al. The contribution of serum complement component 3 levels to 90-day mortality in living donor liver transplantation. Front Immunol. (2021) 12:652677. doi: 10.3389/fimmu.2021.652677
47. Wiese S, Voiosu A, Hove JD, Danielsen KV, Voiosu T, Grønbaek H, et al. Fibrogenesis and inflammation contribute to the pathogenesis of cirrhotic cardiomyopathy. Aliment Pharmacol Ther. (2020) 52:340–50. doi: 10.1111/apt.15812
48. Dimitroglou Y, Aggeli C, Alexopoulou A, Mavrogeni S, Tousoulis D. Cardiac imaging in liver transplantation candidates: current knowledge and future perspectives. J Clin Med. (2019) 8:2132. doi: 10.3390/jcm8122132
Keywords: decompensated cirrhosis, cirrhotic cardiomyopathy, liver transplantation, adverse events, mortality
Citation: Bi S, Jiang Y, Zhao W, Niu X, Liu X and Jing X (2023) The predictive value of revised diastolic dysfunction in outcomes of liver transplantation: A propensity score matching analysis. Front. Surg. 9:1072908. doi: 10.3389/fsurg.2022.1072908
Received: 18 October 2022; Accepted: 12 December 2022;
Published: 6 January 2023.
Edited by:
Muhammed Elhadi, University of Tripoli, LibyaReviewed by:
Daniela Kniepeiss, Medical University of Graz, Austria© 2023 Bi, Jiang, Zhao, Niu, Liu and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Jing amluZ3h1ZUBxZHUuZWR1LmNu
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.