- Department of Orthopaedic Surgery, West China Hospital, Sichuan University, Chengdu, China
Background: Septic arthritis with osteomyelitis due to Salmonella enterica serotype Dublin is rare. We reviewed and analyzed cases of septic arthritis with osteomyelitis due to Salmonella enterica serotype Dublin seen at our institution.
Methods: The medical records of all patients diagnosed with Salmonella septic arthritis and/or Salmonella osteomyelitis during 2017–2022 were included. We reviewed the diagnosis, medical history, clinical management, and outcome of all cases.
Results: Five patients with Salmonella septic arthritis or Salmonella osteomyelitis were identified during the 5-year study period. They were all male; the median age was 53 years (range 15–56). Only one was immunodeficient. All five patients were infected at the hip joint and ipsilateral femur, while two suffered bilateral hip septic arthritis with femoral osteomyelitis. Salmonella Dublin was isolated from the hip joint fluid of all patients. Four presented with fever and constitutional signs within four weeks of symptom onset. Four had positive blood cultures, and only one patient had gastrointestinal symptoms. Four patients underwent surgical debridement as the primary surgical plan, and two underwent secondary two-stage exchange after primary surgical debridement failure. The last patient had a two-stage exchange directly as the first surgical treatment. All patients received intravenous antimicrobial therapy for a median duration of 6 (range 4–12) weeks and oral antimicrobial therapy for a median duration of 4 (range 4–6) weeks. All patients had a median duration of follow-up of 12 months (range 9–25), and none had evidence of recurrence of infection.
Conclusions: Septic arthritis due to Salmonella Dublin remains rare. It frequently occurs with ipsilateral femur osteomyelitis adjacent to the infected hip joint in our cases. Surgical debridement or two-stage exchange, along with 4–12 weeks of effective intravenous and followed by 4–6 oral antimicrobial therapy, could successfully eradicate the infection.
Introduction
Septic arthritis is the most rapidly destructive joint disease; it is relatively rare in the general population, and its yearly incidence varies from 4 to 10 per 100,000 in Western Europe to 29 cases per 100,000 in disadvantaged groups from Northern Europe and Australia; however, its mortality rate due to its complications is as high as 11% (1, 2). Moreover, cured patients often suffer from varying degrees of joint dysfunction (2). The most frequent causative organisms are gram-positive bacteria, such as Staphylococcus aureus (1).
Salmonella is one of the Gram-negative bacteria, of which nontyphoidal Salmonella (NTS) is a common pathogen that causes human foodborne infections (3). NTS infection of joints and bones is rarer, accounting for only 0.8% of all Salmonella infections and 0.45% of all types of osteomyelitis, usually occurring in patients with underlying medical conditions, such as sickle cell disease, systemic lupus erythematosus (SLE), immunosuppression, diabetes, etc (4–7). Salmonella enterica Serotype Dublin (S. Dublin) is a host-adapted serotype to cattle (8, 9). Studies have shown that S. Dublin is particularly aggressive in humans, more likely to cause bloodstream infections, and more often lead to severe disease and higher rates of antimicrobial resistance than other serotypes (9, 10). Most of the human risk of infection with Salmonella Dublin is caused by the consumption of raw milk, milk products and raw beef (10, 11). With the increased consumption of these foods worldwide, there have been only infrequent case reports of septic arthritis or osteomyelitis caused by Salmonella Dublin in this time period. Being a rare and reportable disease, and its management protocols and outcomes are also not well defined, we focus on exploring these similar diseases and strive to provide early and effective therapeutic management.
We therefore retrospectively reviewed all patients with a diagnosis of septic arthritis and/or osteomyelitis caused by Salmonella Dublin seen at our institution during 2017–2022 and evaluated the demographics, clinical manifestations, treatment and outcomes of these infections.
Methods
Study design
This is a single-center retrospective case series undertaken at the West China Hospital of Sichuan University. Our study design was approved and was considered exempt by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (approval no. 2022-1494). All patients provided informed consent.
Case ascertainment and data collection
Study patients were evaluated at our institution between 9/1/2017 and 9/1/2022. Cases were ascertained by searching our institution's medical and surgical indices and the microbiology database. Patients who were diagnosed with septic arthritis and/or osteomyelitis due to Salmonella Dublin were included. Information was available for all patients. Patients were followed until the development of surgical treatment failure, death or loss to follow-up. Descriptive statistics were used to summarize the demographic, clinical and surgical treatment details and were analyzed using IBM SPSS version 21 software (IBM Corp., Armonk, NY, United States).
Definitions
We employed the Newman criteria (12) to diagnose septic arthritis. These criteria require 1 of 4 criteria to be met to consider a diagnosis of septic arthritis: (1) Isolation of an organism from an affected joint. (2) Isolation of an organism from another source with an associated hot and swollen joint. (3) Joint pain and swelling and turbid joint fluid in the presence of previous antibiotic therapy. (4) Histologic or radiologic evidence consistent with septic arthritis (1).
Pyogenic osteomyelitis was diagnosed if the following criteria were met (13): (a) clinical presentation consistent with a bone infection (fever, local soft tissue swelling, bone pain and abnormal physical examination); and/or (b) positive blood or puncture fluid culture or positive bone biopsy.; and/or (c) presence of radiological signs consistent with osteomyelitis [especially magnetic resonance imaging (MRI) scan]; and/or (d) surgical finding of pus in the affected marrow cavity.
Salmonella septic arthritis or osteomyelitis was diagnosed if it met the above criteria and if Salmonella species were isolated from two cultures of joint/bone aspirates or intraoperative tissue specimens, purulence in the affected joint/marrow cavity at the time of surgery with one positive culture yielding Salmonella species.
Patients were either classified as having a good outcome or having failed treatment. Treatment failure was defined by one of the following criteria: recurrence of septic arthritis or osteomyelitis due to the same Salmonella strain; death due to septic arthritis or osteomyelitis-related infection and indeterminate clinical failure, defined as clinical, laboratory, or radiological findings suggestive of septic arthritis or osteomyelitis at any time after initial therapy. Patients who did not fulfill the criteria for treatment failure were characterized as having a “good outcome”.
Results
Patient cohort
In our cohort of five patients, the median age at diagnosis of Salmonella septic arthritis with osteomyelitis in our hospital was 53 years (range 15–56). All five patients were male and were diagnosed within the past three years. A summary of the five patients is presented in Table 1.

Table 1. Summary of the 5 patients with 10 episodes of Salmonella septic arthritis with osteomyelitis.
All five patients were infected at the hip joint and ipsilateral femur, while two of them also suffered bilateral hip septic arthritis with femoral osteomyelitis. Salmonella Dublin was isolated from the hip joint fluid of all five patients. No patient had a history of prior septic arthritis or osteomyelitis on the same or different joints and bones. Only one patient suffered from CKD stage 4 requiring long-term oral methylprednisolone and mycophenolate mofetil. This patient's other comorbidities included diabetes mellitus and bilateral osteonecrosis of the femoral head (ONFH). The remaining four patients were in normal immune status and had no underlying comorbidities.
Patients 1 and 2 presented acutely with signs and symptoms present for less than four weeks and were febrile at presentation. Patients 3, 4 and 5 presented with chronic symptoms when coming into our hospital (6–8 months of deep hip pain). The median duration of chronic joint symptoms prior to diagnosis was 7 (range 6–8) months. All five patients initially presented with pain in the involved hip and gradually developed pain in the ipsilateral thigh over the course of the disease. Patient 4 developed a sinus tract adjacent to his left hip incision scar after undergoing two debridement failures outside our hospital.
Patient 5 had a history of eating sashimi, followed by diarrhea in the preceding two months before symptom onset, but he had a negative stool culture when he came to our hospital. Four patients had documented Salmonella bacteremia (positive blood cultures) before hip septic arthritis with femoral osteomyelitis was diagnosed.
Diagnosis
All first episodes of Salmonella septic arthritis or osteomyelitis were diagnosed in accordance with the definitions described in the methodology section. The five patients we reported all had Salmonella enterica Serotype Dublin. All isolates were nonsusceptible to first-generation cephalosporin macrolides, aminoglycosides and nalidixic acid. One of them was nonsusceptible to ciprofloxacin, and the others were susceptible to ciprofloxacin.
The median erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) values and interleukin-6 (IL-6) values at presentation were 79 mm/1st hour (range 62–120), 79.3 mg/L (range 6.56–306) and 29.4 pg/ml (range 3.74–282.8), respectively (normal range of ESR 0–21 mm/1 h, CRP <5 mg/L and IL-6 <7 pg/ml). White blood cell counts (WBCs) and neutrophilic granulocyte counts (NEUTs) were within normal limits for each patient on admission. The median WBC and NEUT were 7.34 × 109/L (range 6.51–9.25) and 5.42 × 109/L (range 3.23–6.29), respectively (normal range of WBC 3.5–9.5 × 109/L and NEUT 1.8–6.3 × 109/L). Every patient had available pathology reports; two were confirmed to have acute inflammation manifested as congestion, edema, and massive neutrophil infiltration, while the other three had fibroreactive changes, necrosis and perinecrotic inflammatory cell infiltration without evidence of acute inflammation.
Management and outcome
Clinical management included antimicrobial therapy only, surgical debridement, and two-stage exchange.
Patients 1 and 2 presented with acute septic arthritis. Patient 1 underwent direct hip debridement, catheter irrigation and drainage, and patient 2 underwent bilateral hip debridement, catheter irrigation and drainage after failing four weeks of intravenous antibiotics. As the disease progressed, both patients were found to also co-exist with ipsilateral Salmonella osteomyelitis of the femur and underwent debridement and decompression of the ipsilateral femur.
Patients 3, 4 and 5 presented with chronic septic arthritis. Patient 3 initially underwent debridement and antibiotic treatment of the affected hip in our hospital and was readmitted for two-stage exchange after the prior debridement failure. Patient 4 underwent two-stage exchange in our hospital after failing two hip debridements outside. Patient 5 underwent two-stage exchange in our hospital after failing conservative treatment with antibiotics for 8 weeks outside (Figures 1–3).

Figure 1. Patient 5, a 52-year-old male, anteroposterior radiograph of the hip revealed severe joint space narrowing, disappearance of the acetabular sourcil, and bone destruction in the femoral head and femoral neck. Images were taken six months after the onset of hip pain and fever.
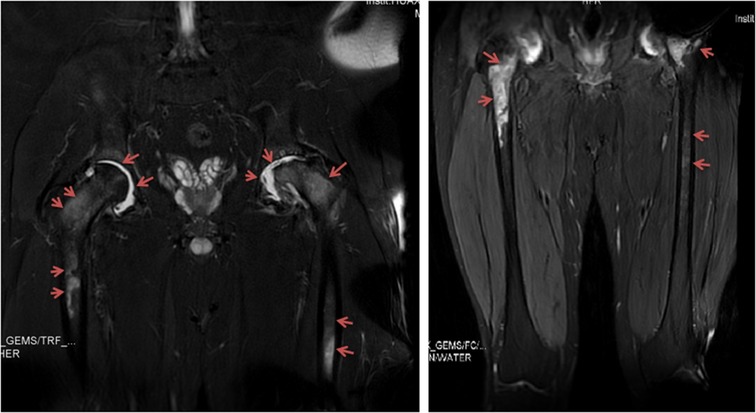
Figure 2. Patient 5: magnetic resonance imaging of the hip joint indicated joint effusion and periarticular bone and femur marrow signal changes. Images were taken six months after the onset of hip pain and fever.

Figure 3. (A) Patient 5, anteroposterior radiograph of the hip taken after first-stage surgery. (B) shows an anteroposterior radiograph of the hip taken six months after stage 2 bilateral THA, indicating the prosthesis in place and good bone ingrowth without osteolysis.
In total, two cases underwent surgical debridement only, and three required final two-stage exchange to eradicate the infection. The first stage entails debridement of the infected tissues of the hip, resection arthroplasty of the proximal femur followed by implantation of an antibiotic-loaded (vancomycin and tigecycline) cement spacer. The second stage involved implantation of a total hip replacement when satisfactory infection control was achieved. The standard of infection control is wound healing and normal erythrocyte sedimentation rate/C-reactive protein level measured at three consecutive follow-up visits, negative clinical signs and symptoms, negative radiological findings, and a negative joint fluid culture (14). All patients received intravenous antimicrobial therapy for a median duration of 6 (range 4–12) weeks and oral antimicrobial therapy for a median duration of 4 (range 4–6) weeks. All patients had a median duration of follow-up of 12 months (range 9–25), and none had evidence of recurrence of infection. The median Pain VAS and Harris hip score of patients were 8 points (range 7–9) and 20 points (range 11–23) respectively at admission. One year after surgery, all patients' pain level was greatly reduced and hip function was significantly improved. The median Pain VAS and Harris hip score were 1 point (range 1–2) and 87 points (range 59–88) respectively. There were significant differences in the two scores before and after the treatment (Table 2).
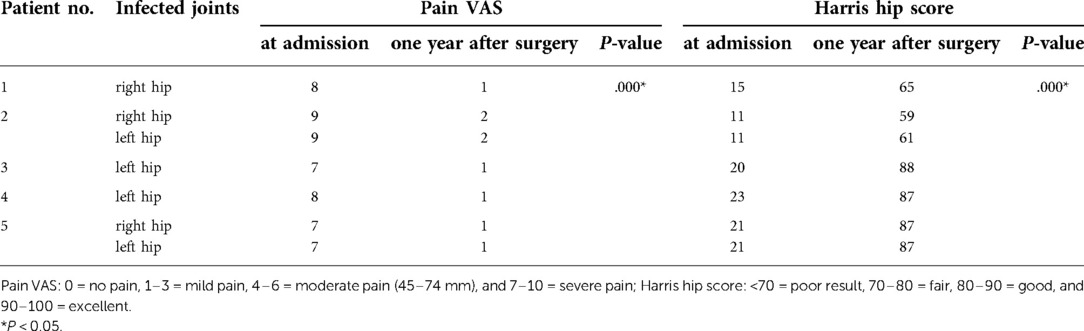
Table 2. Summary and comparison of the VAS pain score and Harris hip score of 5 patients at admission and one year after surgery.
Discussion
Our study showed that septic arthritis due to Salmonella Dublin remains rare, and this serotype may affect bones and joints through bloodstream infection, with fewer gastrointestinal symptoms. In our cases, septic arthritis frequently occurred with osteomyelitis adjacent to the infected joint, and most were immunocompetent adults, which also suggested that S. Dublin is invasive. All patients required surgery combined with sensitive antibiotics to achieve better outcomes.
Salmonella infection is a public health problem worldwide and accounts for at least one-third of all outbreaks in the United States (3, 15). Septic arthritis is an uncommon consequence of NTS infection, with an estimated incidence of less than 0.1%–0.2% (16). Jones et al. revealed 46,639 positive cultures for Salmonella; 5% of those cases were isolated from blood and only 0.06% from bone or joint synovial fluid (17). The pathway by which Salmonella causes septic arthritis is generally considered to be hematogenous (18) and occurs more often in patients with immunodeficiency or underlying diseases (4, 5, 19, 20). Bone and joint infections due to Salmonella in immunocompetent adults are very rare. However, disease severity varies widely between different Salmonella serotypes; Salmonella Dublin is known to be more likely than other NTS species to cause serious problems, such as bloodstream infections (17, 21), and the majority do not have immunodeficiency. In our case, four patients were immunocompetent and presented with joint pain, fever exceeding 38.5°C, and blood cultures found S. Dublin (with the same species identified in synovial fluid and operative cultures). The only patient with a negative blood culture suffered from CKD stage 4 requiring long-term oral immunosuppressive drugs. The study found that the mean age of infection with Salmonella Dublin is 54 years (17). Harvey et al. also reported that 60% of Salmonella Dublin infections occurred in men (10). Our case review is consistent with previous reports, in which all were males and the median age of infection was 53 years. Previous studies have confirmed that S. Dublin is frequently isolated from live cattle and that raw unpasteurized milk or cheeses made from milk may be contaminated with S. Dublin (10, 11). Many studies have found that men consume more undercooked beef than women (22, 23). Occupational exposure to cattle may also lead to an increased frequency of infection in males (10). However, our patient's occupation was not related to cattle, and there was no obvious history of exposure to raw beef or dairy products. We speculated that the initial gastrointestinal salmonellosis may be mild and may be overlooked. Of course, this may also be caused by a long incubation period and parenteral entry route of infection (24, 25).
The S. Dublin serotype has a higher proportion of resistant strains than other serotypes, according to the National Antimicrobial Resistance Surveillance System (NARMS) surveillance data (10). Harvey et al. showed that in 102 clinical isolates of Salmonella Dublin, 41% were pansusceptible, 55% were multidrug resistant, and among these MDR isolates, 84% were resistant to >5 classes of antimicrobials (10). In our case, the clinical isolates were resistant to first-generation cephalosporins, aminoglycosides, penicillins and nalidixic acid. Fortunately, only one isolate was resistant to ciprofloxacin but remained susceptible to third-generation cephalosporins. Both third-generation cephalosporins and fluoroquinolones have been successfully used in cases of Salmonella infection (6, 24). Fluoroquinolones are advocated as excellent choice drugs because of their potent anti-Salmonella activity, good bone penetration and ability to kill stationary phase as well as active phase bacteria (26). In our case review, ceftriaxone, ciprofloxacin, and more broad-spectrum and potent antibacterial drugs, such as carbapenems (imipenem-cilastatin), were the choices for septic arthritis due to S. Dublin. Broad-spectrum antibiotics such as carbapenems are used in some cases because patients still have recurrent bacteremia and fever despite the intravenous infusion of third-generation cephalosporin or fluoroquinolone. In addition, due to the symptoms of high fever, we added vancomycin to prevent possible concomitant gram-positive infection (6, 26).
Septic arthritis, an urgent disease, may damage cartilage directly through bacterial enterotoxins or indirectly due to the host's immune response to bacteria, requiring surgical intervention in most patients (27, 28). Salmonella often affects a single joint, and the hip joint is the most commonly infected joint (29). Our cases were all infected at the hip joint, and two were affected with bilateral hips. Surgical debridement is the first choice for septic arthritis to eradicate the infection (30). Routine open debridement includes removal of all accessible synovial tissue, capsulectomy, and drain placement (14). Drainage placement is fundamental to reduce intraarticular pressure and to minimize joint cartilage destruction. In our cases, two patients were diagnosed with acute septic arthritis, and preoperative MRI revealed significant articular surface damage; thus, we promptly performed surgical debridement in open procedures. However, they were subsequently found to combine with ipsilateral femoral osteomyelitis. The diagnosis of Salmonella osteomyelitis may be delayed because of insidious and overshadowed early symptoms by severe joint pain. After thorough joint debridement, they still had recurrent fever and began to describe thigh pain. The thigh MRI showed femoral osteomyelitis. Therefore, we performed femoral debridement, decompression, and drainage. Their symptoms were eventually relieved, with no evidence of recurrence of infection. For this type of coexisting and insidious Salmonella osteomyelitis, we recommend performing MRI scan on adjacent areas of the infected joint to identify and minimize harm to patients in early clinical management. The prognosis of septic arthritis after Salmonella infection is unclear; although most patients show resolution of arthritis within four months of onset, chronic symptoms can persist for up to five years (3). Chronic hip infection may gradually lead to joint degeneration, complete joint destruction or even result in chronic osteomyelitis of the proximal femur. If in this situation, we recommend a two-stage exchange. Thorough joint and medullary debridement and cement-loaded spacers can effectively eradicate infection and improve pain (31–33). Then, the second-stage THA can further restore good hip motion. In our case, three patients were diagnosed with chronic septic arthritis with femoral osteomyelitis. They had long-term joint pain and dysfunction and even needed a walker or wheelchair to maintain their normal life. X-ray and MRI showed severe joint destruction, joint space narrowing, and erosion of the proximal femur. Considering the above situation and that they were all over 50 years old, we performed two-stage exchange surgery and achieved satisfactory outcomes.
Success rates described in the literature may vary due to limited follow-up time, different definitions of successful outcomes, and the propensity for publication bias of successfully treated case reports. In our cohort, the median duration of follow-up was twelve months. There are several limitations to this study. First, this study was retrospective and had the same biases inherent in other retrospective studies. Second, the limited sample size is a function of the low incidence of the disease. Third, all of our cases occurred within the past three years; thus, the follow-up time was relatively short, and the long-term clinical outcome could not be determined. Nevertheless, this series of septic arthritis with osteomyelitis due to Salmonella Dublin supports the value of early joint debridement or two-stage exchange combined with susceptible antibiotic therapy in these patients.
Conclusions
Septic arthritis due to Salmonella Dublin remains rare, but this serotype is more invasive and more frequently infects immunocompetent adults. The presentation is often acute with fever, local signs and elevated inflammatory biomarkers. It frequently occurs with ipsilateral femur osteomyelitis adjacent to the infected hip joint in our cases. Treatment requires an early surgical approach, such as surgical debridement or two-stage exchange, combined with 4–12 weeks of effective intravenous and followed by 4–6 oral susceptible antibiotic drug therapy was most often associated with successful eradication of infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BYJ and HX organized ethical approval and registered this study; drafted the work and revised it critically for important intellectual content; collected the data and analyzed and interpreted the data for this work. ZKZ contributions to the conception and design of the work and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by and 1•3•5 project for disciplines of excellence of Sichuan University West China Hospital (grant number: ZYJC18039), the Regional Innovation & Cooperation program of Science & Technology Department of Sichuan Province (grant number: 2021YFQ0028), West China Nursing Discipline Development Special Fund Project, Sichuan University (HXHL20003) and Key Research & Development program of Science & Technology Department of Sichuan Province (grant number: 2021YFS0167).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goldenberg DL. Septic arthritis. Lancet. (1998) 351(9097):197–202. doi: 10.1016/S0140-6736(97)09522-6
2. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. (2010) 375(9717):846–55. doi: 10.1016/S0140-6736(09)61595-6
3. Shin YR, Park KS, Cho KJ, Yoon TR. Bilateral septic arthritis of the hip caused by nontyphoidal salmonella: a case report. Acta Orthop Traumatol Turc. (2020) 54(2):217–20. doi: 10.5152/j.aott.2020.02.278
4. Anand AJ, Glatt AE. Salmonella osteomyelitis and arthritis in sickle cell disease. Semin Arthritis Rheum. (1994) 24(3):211–21. doi: 10.1016/0049-0172(94)90076-0
5. Huang JL, Hung JJ, Wu KC, Lee WI, Chan CK, Ou LS. Septic arthritis in patients with systemic lupus erythematosus: salmonella and nonsalmonella infections compared. Semin Arthritis Rheum. (2006) 36(1):61–7. doi: 10.1016/j.semarthrit.2006.04.003
6. Salem KH. Salmonella osteomyelitis: a rare differential diagnosis in osteolytic lesions around the knee. J Infect Public Health. (2014) 7(1):66–9. doi: 10.1016/j.jiph.2013.07.004
7. Sanchez AA, Mazurek MT, Clapper MF. Salmonella osteomyelitis presenting as fibrous dysplasia. A case report. Clin Orthop Relat Res. (1996) 330:185–9. doi: 10.1097/00003086-199609000-00023
8. McDonough PL, Fogelman D, Shin SJ, Brunner MA, Lein DH. Salmonella enterica serotype Dublin infection: an emerging infectious disease for the northeastern United States. J Clin Microbiol. (1999) 37(8):2418–27. doi: 10.1128/JCM.37.8.2418-2427.1999
9. Taylor DN, Bied JM, Munro JS, Feldman RA. Salmonella Dublin infections in the United States, 1979–1980. J Infect Dis. (1982) 146(3):322–7. doi: 10.1093/infdis/146.3.322
10. Harvey RR, Friedman CR, Crim SM, Judd M, Barrett KA, Tolar B, et al. Epidemiology of Salmonella enterica serotype Dublin infections among humans, United States, 1968-2013. Emerg Infect Dis. (2017) 23(9):1493–501. doi: 10.3201/eid2309.170136
11. Ung A, Baidjoe AY, Van Cauteren D, Fawal N, Fabre L, Guerrisi C, et al. Disentangling a complex nationwide Salmonella Dublin outbreak associated with raw-milk cheese consumption, France, 2015 to 2016. Euro Surveill. (2019) 24(3):1700703. doi: 10.2807/1560-7917.ES.2019.24.3.1700703
12. Sharff KA, Richards EP, Townes JM. Clinical management of septic arthritis. Curr Rheumatol Rep. (2013) 15(6):332. doi: 10.1007/s11926-013-0332-4
13. Spellberg B, Aggrey G, Brennan MB, Footer B, Forrest G, Hamilton F. Use of novel strategies to develop guidelines for management of pyogenic osteomyelitis in adults: a WikiGuidelines group consensus statement. JAMA Netw Open. (2022) 5(5):e2211321. doi: 10.1001/jamanetworkopen.2022.11321
14. D’Angelo F, Monestier L, Zagra L. Active septic arthritis of the hip in adults: what’s new in the treatment? A systematic review. EFORT Open Rev. (2021) 6(3):164–72. doi: 10.1302/2058-5241.6.200082
15. Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. (2006) 43(4):512–7. doi: 10.1086/505973
16. Ray U, Dutta S, Sutradhar A. An unusual case of septic arthritis of the hip. J Clin Diagn Res. (2016) 10(11):Dd03–5. doi: 10.7860/JCDR/2016/22613.8933;19
17. Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, et al. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. (2008) 198(1):109–14. doi: 10.1086/588823
18. Ortiz-Neu C, Marr JS, Cherubin CE, Neu HC. Bone and joint infections due to Salmonella. J Infect Dis. (1978) 138(6):820–8. doi: 10.1093/infdis/138.6.820
19. Ramos JM, García-Corbeira P, Aguado JM, Alés JM, Fernández-Guerrero ML, Soriano F. [Osteoarticular infections by Salmonella non-typhi]. Enferm Infecc Microbiol Clin. (1995) 13(7):406–10. PMID: 8519817
20. Yeargan SA 3rd, Perry JJ, Kane TJ 3rd, Richardson AB. Hematogenous septic arthritis of the adult hip. Orthopedics. (2003) 26(8):771–6. doi: 10.3928/0147-7447-20030801-14
21. Blaser MJ, Feldman RA. From the centers for disease control. Salmonella bacteremia: reports to the Centers for Disease Control, 1968–1979. J Infect Dis. (1981) 143(5):743–6. doi: 10.1093/infdis/143.5.743
22. Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. (2011) 14(4):575–83. doi: 10.1017/S1368980010002077
23. Shiferaw B, Verrill L, Booth H, Zansky SM, Norton DM, Crim S, et al. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey, 2006–2007. Clin Infect Dis. (2012) 54(Suppl 5):S453–7. doi: 10.1093/cid/cis247
24. Huang ZD, Wang CX, Shi TB, Wu BJ, Chen Y, Li WB, et al. Salmonella osteomyelitis in adults: a systematic review. Orthop Surg. (2021) 13(4):1135–40. doi: 10.1111/os.12912
25. Ramos JM, García-Corbeira P, Aguado JM, Alés JM, Soriano F. Classifying extraintestinal non-typhoid Salmonella infections. QJM. (1996) 89(2):123–6. doi: 10.1093/qjmed/89.2.123
26. Gupta A, Berbari EF, Osmon DR, Virk A. Prosthetic joint infection due to Salmonella species: a case series. BMC Infect Dis. (2014) 14:633. doi: 10.1186/s12879-014-0633-x
27. Choi IH, Pizzutillo PD, Bowen JR, Dragann R, Malhis T. Sequelae and reconstruction after septic arthritis of the hip in infants. J Bone Joint Surg Am. (1990) 72(8):1150–65. doi: 10.2106/00004623-199072080-00005
28. Wada A, Fujii T, Takamura K, Yanagida H, Urano N, Surijamorn P. Operative reconstruction of the severe sequelae of infantile septic arthritis of the hip. J Pediatr Orthop. (2007) 27(8):910–4. doi: 10.1097/bpo.0b013e31815a606f
29. Muñoz-Mahamud E, Casanova L, Font L, Fernández-Valencia JA, Bori G. Septic arthritis of the hip caused by nontyphi Salmonella after urinary tract infection. Am J Emerg Med. (2009) 27(3):373.e5–e8. doi: 10.1016/j.ajem.2008.07.024
30. Hassan AS, Rao A, Manadan AM, Block JA. Peripheral bacterial septic arthritis: review of diagnosis and management. J Clin Rheumatol. (2017) 23(8):435–42. doi: 10.1097/RHU.0000000000000588
31. Cho YJ, Patel D, Chun YS, Shin WJ, Rhyu KH. Novel antibiotic-loaded cement femoral head spacer for the treatment of advanced pyogenic arthritis in adult hip. J Arthroplasty. (2018) 33(6):1899–903. doi: 10.1016/j.arth.2017.12.028
32. Fleck EE, Spangehl MJ, Rapuri VR, Beauchamp CP. An articulating antibiotic spacer controls infection and improves pain and function in a degenerative septic hip. Clin Orthop Relat Res. (2011) 469(11):3055–64. doi: 10.1007/s11999-011-1903-1
Keywords: septic arthritis, osteomyelitis, debridement, twostage exchange, salmonella dublin
Citation: Jiang B, Xu H and Zhou Z (2023) Septic arthritis with osteomyelitis due to Salmonella enterica serotype Dublin: A case series. Front. Surg. 9:1069141. doi: 10.3389/fsurg.2022.1069141
Received: 13 October 2022; Accepted: 21 November 2022;
Published: 6 January 2023.
Edited by:
Chunxi Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Mingzhu Zhang, Capital Medical University, ChinaLin Du, Shanghai Jiao Tong University, China
© 2023 Jiang, Xu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongke Zhou emhvdXpvbmdrZUBzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Boyi Jiang†
Boyi Jiang† Zongke Zhou
Zongke Zhou