
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 30 January 2023
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1065715
Background: Obesity is one of the most important public health conditions in the world, and surgical intervention is the only medical treatment recognized by the medical community as a complete and permanent cure for morbid obesity and its complications. The choice of surgical modality is also based more on the experience of the physician or the requirements of people with obesity, rather than on scientific data. In this issue, a thorough comparison of the nutritional deficiencies caused by the three most commonly used surgical modalities is needed.
Objectives: We aimed to use the network meta-analysis to compare the nutritional deficiencies caused by the three most common BS procedures in many subjects who underwent BS to help physicians determine the best BS surgical approach to apply to their clinical people with obesity.
Setting: A systematic review and network meta-analysis of world literature.
Methods: We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, systematically reviewed the literature, and conducted a network meta-analysis using R Studio.
Results: For the four vitamins calcium, vitamin B12, iron and vitamin D, the micronutrient deficiency caused by RYGB is the most serious.
Conclusions: RYGB causes slightly higher nutritional deficiencies in Bariatric surgery, but RYGB remains the most commonly used modality for Bariatric surgery.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022351956, identifier: CRD42022351956.
Obesity is one of the world's most important public health conditions (1). According to the World Health Organization, the global obesity rate has tripled since 1975 (2). Due to rising population numbers and an aging population, in 2019, obesity is responsible for approximately 5.02 million deaths from non-communicable diseases and 102 million disability life-adjusted years, equivalent to 12% of all non-communicable disease deaths (3). Surgical intervention is the only medical treatment recognized by the medical community to completely and permanently treat morbid obesity and its complications (4). Surgical interventions can reduce weight and improve obesity complications through gastrointestinal surgery, achieving a good prognosis (5).
Bariatric surgery (BS) can be divided into two general types: malabsorptive (bypassing certain parts of the gastrointestinal tract to reduce the area of absorption) and restrictive (reducing the gastric volume to allow people with obesity to achieve satiety quickly) (6). Malabsorptive types include Roux-en-Y gastric bypass (RYGB) and biliopancreatic duodenal transposition (BPD), and restrictive types include laparoscopic adjustable gastric banding (LAGB) and laparoscopic sleeve gastrectomy (SG). The three most widely used types in clinical practice are SG, RYGB, and LAGB (7).
However, BS also has its disadvantages. Because surgery alters parts of the gastrointestinal tract, it often results in micronutrient deficiencies (8). Some of these nutrient deficiencies can have severe clinical consequences, including various complications such as Wernicke's encephalopathy and pediculosis due to vitamin B1 deficiency, iron deficiency anemia due to iron deficiency, megaloblastic anemia due to vitamin B12 or folic acid deficiency, bone mineralization and fracture risk due to vitamin D or calcium deficiency, etc (9). In addition to these severe complications, many case reports have reported people with obesity with nutritional deficiencies after BS, but few studies have clarified their incidence after different BS surgical approaches (10). In the published studies, either only one or two BS procedures were evaluated for comparison, only a small number of subjects were included, or the nutritional assessment was not exhaustive (11). The choice of surgical modality was also based more on physician experience and people with obesity requirements than on scientific data. A comprehensive comparison of the nutritional deficiencies caused by the three most commonly used surgical approaches is needed on this topic.
Therefore, we conducted a network meta-analysis of the published literature on BS and nutritional deficiencies. We aimed to use the network meta-analysis to compare the nutritional deficiencies caused by the three most common BS procedures in many subjects who underwent BS to help physicians determine the best BS surgical approach to apply to their clinical people with obesity.
We included studies that should have compared the deficiency rates and complication rates of 4 nutrients (vitamin B12, iron, vitamin D, and calcium) in people with obesity after different BS surgical approach manuscript Formatting. Prior to the start of the study, it was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022351956).
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12). Our search strategy aimed to identify all studies examining the impact of different BS surgical approaches on people with obesity nutrient deficiencies. We used Mesh and did our best to include all synonymous medical terms in the search to avoid omissions. Two co-first authors collaborated with a Ph.D. (the second author of this paper) to identify search terms, as well as relevant search terms and keywords for our primary outcome, nutrient deficiency. We also identified search criteria and strategies and searched PubMed, Embase, Scopus, Google Scholar, and the Cochrane Clinical Trials Register for eligible studies. To ensure comprehensiveness, we also searched the cited literature of relevant reviews on an article-by-article basis.
Several inclusion criteria were used in determining study eligibility.
a. Study design: non-review, non-letters, and other non-research articles.
b. Participants: people with obesity who underwent BS surgery.
c. Interventions and comparisons: studies comparing nutritional deficiencies after two or more BS surgical approaches.
d. Results: these studies reported nutrient deficiency rates as well as complication rates.
e. Data: the data included in the study were not duplicated.
f. Language: the language used in the studies was English.
The two co-first authors performed data searches in various databases and performed initial screening based on the review of titles and abstracts to determine study eligibility. All references were imported into Zotero and automatically screened for duplicates by this software. Once we identified studies for potential inclusion, we screened the full text of the articles. Disagreements were resolved through discussion; if consensus could not be reached, the second author served as the arbiter. We also searched for disease guidelines, systematic reviews, and survey articles involving nutritional deficiencies after BS or BS and reviewed their reference sections to ensure that no studies were missed. When necessary, the three reviewers discussed any issues related to study inclusion or exclusion with each other and with the senior author. We used Zotero's notes feature to mark the general content of studies or reasons for exclusion to facilitate our full-text review. The manuscript did not involve the use of animal or human subjects.
All three reviewers were involved, and many abstracts were identified for full-text review. All reviewers received training on study eligibility criteria and data extraction. A panel of three reviewers reads and assessed all included full-text articles to determine whether they met the eligibility criteria and, if not, to explain the reasons for exclusion. One reviewer independently extracted the following data elements from each article that met the eligibility criteria table developed by the study team: first author's name, year of publication, journal name, location, BS program model of the study, nutrient deficiency status, study population (including disease), selection of study cohort, sample size, study purpose, primary outcome, and statistical significance (if applicable) (13).
The study used the gemtc package in R (R Studio 2022.02.1 Build 461-Inc version) (https://posit.co) to analyze the data (14, 15). We performed a network meta-analysis (Markov chain Monte Carlo approach in a Bayesian framework) in order to study nutrient deficiencies due to different surgical modalities, pooling the different BS surgical modalities and nutrient deficiencies included in the studies (16). Not all studies could be included in the network meta-analysis, as some of the included studies did not include nutrient deficiency as a dichotomous variable and they only provided data for calculating odds ratios (ORs). We first developed a consistency model using forest plots to describe the comparison of ORs and 95% confidence intervals (CrI) between different BS surgical modalities. The difference in effect between interventions was judged to be statistically significant by whether the Crl of the interventions compared with each other crossed 1 or not. We also performed nodal analysis methods and consistency tests for all included studies to look for sources of heterogeneity and to ensure the assumption of homogeneity in the model.
In the study, we plotted trajectory and density plots to assess the volatility and overlap of MCMC chains and the convergence of the model (17). From the trajectory plots, we know whether the MCMC chain fluctuations are stable and have good overlap when the number of iterations reaches 5,000 or more; from the density plots, we know whether Bandwidth tends to 0 and reaches stability when the number of iterations reaches 20,000. When the requirements are satisfied, the synthesis indicates that the model converges well. We also plotted the Brooks-Gelman-Rubin diagnostic and calculated the potential size reduction factor (PSRF).
A satisfactory convergence model needs to satisfy two conditions at the same time:
a. 97.5% of the scaling factor and the median value of the scaling factor converge to 1 and reach stability after n iterations of calculation.
b. The PSRF value tends to be 1.
When the model satisfies both of the above conditions for convergence, we consider it a satisfactorily convergent model. The results of different BS surgical approaches were then taken and the probability of occurrence of a particular nutrient deficiency after different BS surgical approaches was deduced from the individual ranking results. The logarithms of the indices were then taken and compared with each other, and the results were plotted as bar charts. Although the ranking gives the ranking results for each BS surgical modality, it is not possible to simply conclude that the intervention is superior or inferior, but must be viewed in conjunction with other outcomes. We used the I2 test to assess heterogeneity. Because of the observed heterogeneity across studies, we used random effects models to estimate unadjusted and adjusted pooled rates and to identify sources of heterogeneity.
Final conclusions will be drawn from the following three sources:
a. from the forest plot as to which surgical modality was used with the highest risk of outcome events.
b. From the rank-ordering results, which intervention is ranked first in terms of probability of occurrence.
3,312 potential studies were identified in our literature review (Figure 1).
We reviewed the citations, titles, and abstracts of 3,312 studies and identified 277 articles for inclusion. After a full-text review, 20 of these articles were included (Table 1). Studies were typically excluded because there was no comparison of surgical modalities or nutritional deficiencies were not the primary outcome. We began by mapping the network relationships in preparation for the network meta-analysis (Figure 2). While SG was directly compared with RYGB and LAGB due to the network linkages between trials in Figure 2, comparisons between the remaining two surgical modalities (i.e., RYGB and LAGB) were directly linked through at least two trials.
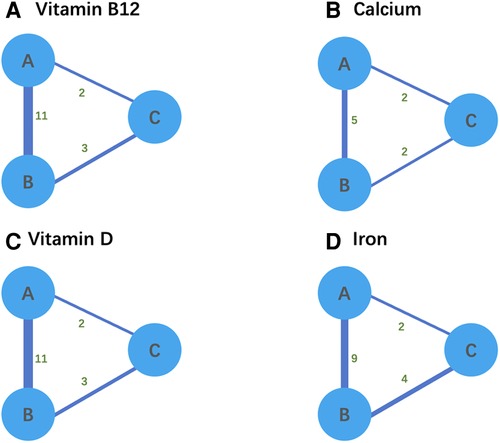
Figure 2. (A–D) Network relationship diagram for network meta-analysis. Thicker lines ndicate a greater cumulative number of enrolled studies per direct comparison. A refers to LSG, B refers to RYGB, and C refers to AGB.
We included 13 trials (n = 2408) that reported vitamin B12 deficiency after BS (Table 1). A network meta-analysis was used to rank the BS surgical modalities in order of frequency of postoperative vitamin B12 deficiency (from A–C). The forest plot in Figure 3 depicts A for SG, B for RYGB, and C for LAGB. We show the ORs and 95% CrI for the comparison by forest plot, where the OR for the outcome of intervention B compared with A was 2.90 and the OR for the outcome of C compared with A was 2.00, indicating that the risk of outcome events was 2.9 times higher for surgical modality B than for intervention modality A and 2 times higher for C. The 95% Crl for ORs were (1.70–5.60) and (0.68–5.80), respectively. Since the Crl of the intervention crossed 1 for C compared to A, the difference in effect between them was not statistically significant. The trajectory and density plots show that the model converged well and satisfied the required conditions.
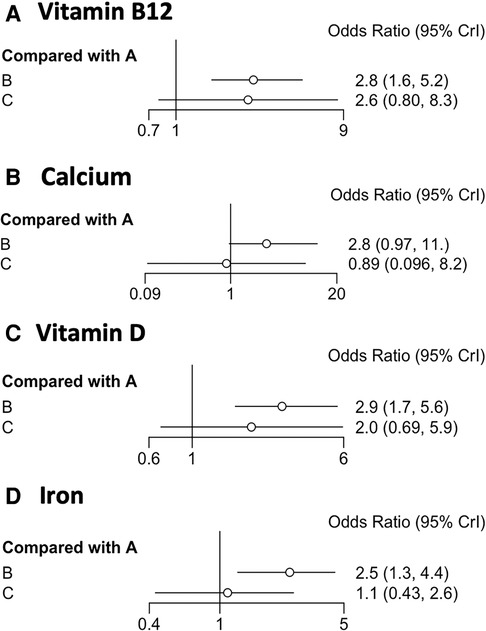
Figure 3. (A–D) Forest plots depicting OR values and 95% confidence intervals (CrI) for comparisons between operative BC and A. A refers to LSG, B refers to RYGB, and C refers to AGB.
We ranked the probability of vitamin B12 deficiency following BS for each of the three surgical procedures (see Figure 4A). Therefore, postoperative SG is better in terms of vitamin B12 absorption than the other two surgical approaches (i.e., RYGB and LAGB). Comparisons of RYGB with LAGB are not statistically significant.
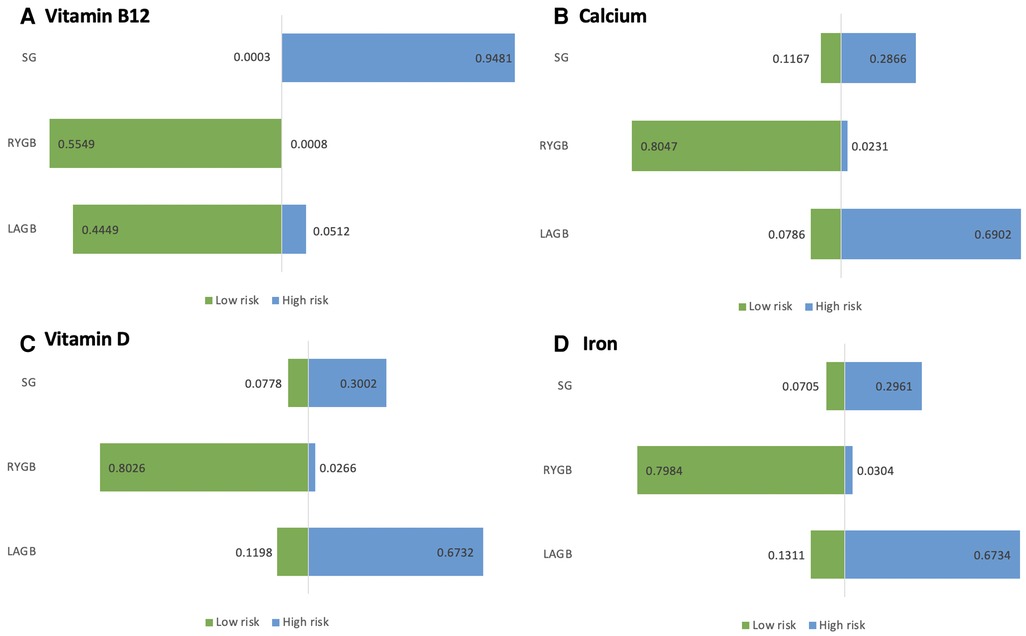
Figure 4. (A–D) Risk ranking chart risk probability ranking of nutrient deficiencies due to bariatric surgery.
Six of our included trials (n = 987) reported calcium deficiency after BS (Table 1). We ranked the frequency of postoperative calcium deficiency (from A–C). We show the ORs and 95% CrI for comparison by forest plot, with an outcome OR of 2.80 for intervention B compared to A and 0.89 for C compared to A. This indicates that the risk of an outcome event using surgical modality B is 2.8 times greater than the risk of an outcome event using intervention A and 0.89 times greater than the risk of an outcome event using intervention C. The 95% Crl for ORs was (0.97–11.00), (0.096–8.20). Since the Crl comparisons for the interventions all crossed 1, the difference in effect between them was not statistically significant. It can be seen from the trajectory and density plots that the model converged well and met the required conditions.
We compared each intervention A–C to each other and computed the logarithm of the index outcomes. For each of the three surgical procedures, the probability of postoperative calcium deficiency was ranked (see Figure 4B). As a result, we could only conclude from the ranked results that RYGB had a higher risk of postoperative calcium deficiency symptoms than SG and LAGB.
We ranked the frequency of postoperative iron deficiency (from A–C). We show the ORs and 95% CrI of the comparisons by forest plots, with an OR of 2.50 for the outcome of comparison of intervention B with A and an OR of 1.10 for the outcome of comparison of C with A. This indicates that the risk of occurrence of an outcome event with surgical modality B is 2.5 times greater than the risk of occurrence of an outcome event with intervention A and 1.1 times greater with C. The 95% Crl of the ORs are (1.30–4.40), (0.43–2.60). Since the Crl for the comparison of A and C crossed 1, the difference in effect between them was not statistically significant. The incidence of RYGB is significantly higher than that of SG and LAGB as can be observed by the forest plot. it is clear from the trajectory and density plots that the model converges well and satisfies the required conditions.
The probability of postoperative calcium deficiency was ranked for each of the three surgical procedures (see Figure 4C). Direct comparison between interventions The I2 between pair-wise comparisons (pair-wise) was 0%, and the I2 between the results of network comparisons (network) was also 0%, then there was no heterogeneity between them and the homogeneity assumption was satisfied. Therefore, it can be judged that RYGB is more likely to cause iron deficiency symptoms in the postoperative period compared to SG and LAGB.
We ranked the frequency of postoperative vitamin D deficiency (from A–C). We showed the ORs and 95% CrI of the comparisons by forest plots, with an OR of 2.90 for the outcome of intervention B compared with A and 2.00 for the outcome of C compared with A. This indicates that the risk of an outcome event in surgical approach B is 2.9 times greater than the risk of an outcome event in intervention A and 2.0 times greater in C. The 95% Crl of the ORs was (1.7–5.60), (0.69–5.90). Since the Crl for the comparison of A and C crossed 1, the difference in effect between them was not statistically significant. From the forest plot, it can be seen that the incidence of RYGB is higher than SG and LAGB. from the trajectory and density plots, it can be seen that the model converges well and satisfies the required conditions.
The probability of postoperative calcium deficiency was ranked for each of the three surgical procedures (see Figure 4D). The direct comparison between interventions (pair-wise) is 0%, and the I2 between network comparison (network) results is also 0%, then there is no heterogeneity between them and the homogeneity assumption is satisfied. Therefore, it can be judged that RYGB is more likely to cause postoperative Vitamin D deficiency symptoms compared with SG and LAGB.
The goal of choosing the most appropriate surgical option is always to create the best balance between weight loss, complications, and micronutrient deficiencies. In clinical practice, BS is often performed only with SG, RYGB, and LAGB (38). Current data suggest that RYGB is more likely to cause micronutrient deficiencies than SG and LAGB. Although previous studies have compared the three most commonly used procedures, there is still a lack of more accurate evidence to choose the most scientific procedure.
Compared with RYGB, LAGB has a higher risk of slip/dilation and surgical reversal/conversion, but a lower risk of stricture, ulceration, and hernia compared with RYGB (39). LAGB has a shorter duration of stay compared to RYGB; SG has a higher risk of reoperation compared to RYGB (40). Compared with RYGB, SG and LAGB are associated with a higher failure rate or secondary surgery, which may be the main reason for the current high clinical use of RYGB.
We used radar plots to show the combined ranking results of nutrient deficiencies (Figure 5). In Figure 5, there are three groups (i.e., SG, RYGB, and LAGB) with four dimensions (i.e., Vitamin B12, calcium, Vitamin D, and Iron). The farther from the center point means the higher risk of deficiency of that nutrient. The results showed that RYGB was more likely to cause vitamin B12 deficiency compared to SG, while LAGB was less pronounced, but LAGB was less deficient than RYGB (41). This may be because RYGB is a malabsorptive procedure, compared with SG and LAGB, Insufficient secretion of endogenous factors due to reduced gastric capacity and intestinal rearrangement, resulting in reduced absorption of vitamin B12, so we recommend postoperative supplementation and long-term, regular laboratory monitoring should have adhered to (42).
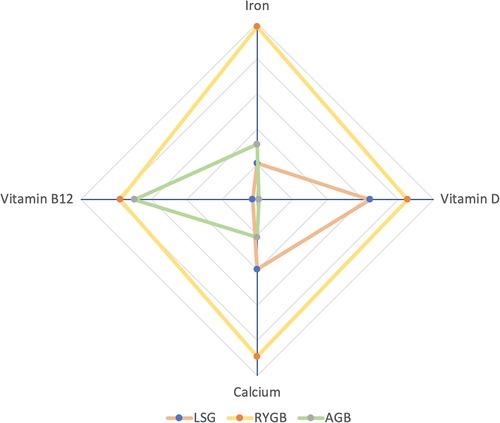
Figure 5. Rank-ordered radar chart: the farther from the center point is, the higher the probability of the corresponding event.
The duodenum and proximal jejunum are the best sites for dietary calcium absorption, where vitamin D is involved. Therefore, calcium deficiency can be further exacerbated if the diet is deficient in vitamin D (43). Food does not pass through the duodenum after RYGB, bypassing these parts of the small intestine, which reduces calcium and vitamin D intake, ultimately leading to calcium deficiency (44). Bone mineral density typically decreases after BS due to altered mechanical loading of the bone, whereas parathyroid hormone production increases. If parathyroid hormone continues to rise and hyperthyroidism occurs, it can lead to osteopenia and osteoporosis. Therefore, metabolic bone disease is a long-term risk factor after BS bypass surgery (45). In contrast, SG and LAGB did not alter duodenal traits, so calcium and magnesium deficiencies were less frequent.
After RYGB, iron deficiency was significantly higher in people with obesity than with SG and LAGB (46). This is because iron needs to be absorbed in the duodenum and proximal jejunum, with the participation of gastric acid (47). Divalent iron is better absorbed than trivalent iron when supplementing with iron after surgery (48). This is because ferrous iron is usually found in animal foods, while ferric iron is found in plant foods (49). Ferric iron needs to be reduced to ferrous iron under the action of gastric acid before it can be absorbed by the intestinal tract (50). Therefore, it is recommended to consume animal food after BS. RYGB is also a procedure with a high rate of vitamin D deficiency because it bypasses part of the duodenum and ileum, which is the main site of vitamin D absorption (51). RYGB is more likely to cause vitamin D deficiency than SG, while the defect in LAGB is not significant. For people with obesity, vitamin D deficiency should be detected early and supplemented in time to avoid the deterioration of the disease.
Regardless of the type of BS, it is inevitable that nutritional deficiencies will result. Our primary goals are to find the most appropriate procedure for people with obesity, minimize complications and nutritional losses, and monitor and supplement postoperatively. When selecting a procedure, people with obesity and doctor must also consider specific differences in postoperative nutritional needs and care (52).
In this study, we found that nutritional deficiencies after BS are widespread, although data from large, robust long-term RCTs are lacking. More studies are needed, such as direct comparisons of the effects of different BS procedures on clinically relevant nutritional deficiencies during the long-term, follow-up-especially for newer procedure types. Studying postoperative nutritional deficiencies in different subgroups (e.g., those with other diseases, special populations, or weaker social determinants of health) of BS should also be a high research priority.
Because our goal was to compare nutritional deficiencies after three commonly used bariatric surgeries at the same time (which can only be accomplished by constructing a network meta-analysis), we included surgical options in our network meta-analysis to help clinicians reduce the incidence of nutritional deficiencies in patients. We included all studies that fulfilled the inclusion criterion for the absence of risk. Small studies, to be sure, can introduce a lot of bias since there is greater uncertainty in the estimations and the outcomes are more likely to be random.
However, the quantity and quality of the identified evidence limit the strength of our conclusions. Only a few studies had sufficiently long follow-ups; however, we also note that the double-blind requirement may have artificially reduced the quality of the studies, which is rarely performed in trials evaluating surgical interventions. In addition, only a few studies reported patient undernutrition at baseline, but we attempted to address this limitation by performing a sensitivity analysis of these studies. Because this was an interim sensitivity analysis, the results should be interpreted cautiously. We included this part of the study in the heterogeneity study. For this study, we first noticed that there was no heterogeneity in these trials (I2 = 0%) and that patients had nearly the same rate of the nutritional deficit as the group reporting baseline values. Secondly, In these trials, the follow-up time was so extensive that researchers may have thought that baseline levels could be ignored during long-term follow-up. As a result of these considerations, we believe that incorporating these trials in the network meta-analysis would have no detrimental consequences.
We followed PRISMA, developed a review protocol in advance, and mapped Brooks-Gelman-Rubin diagnostics charts. We used Mesh, a comprehensive literature search strategy, quality assessment and data extraction by repeat reviewers, and rigorous statistical methods to reduce potential bias. Given that the medical tools used to perform BS have advanced over time, there is very little literature on direct comparisons between different surgical approaches. However, we continue to use state-of-the-art, rigorous natural comparison methods to estimate the relative effectiveness of each procedure compared to others. Therefore, we are confident that our results are valid.
Our network meta-analysis comparing the three most commonly used Bariatric surgery procedures yielded three key conclusions. First, high-quality data from randomized controlled trials remain insufficient—especially on calcium and vitamin D deficiencies after Bariatric surgery. Second, RYGB appears to be more likely to cause nutritional deficiencies after Bariatric surgery. Third, we determined the consequences of nutritional deficiencies between different surgical procedures. Although the nutritional deficiency after RYGB is slightly more severe than the other two procedures (i.e., SG and LAGB), it is still the most commonly used bariatric surgery in clinical practice.
This study suggests that choosing the three most common BS procedures is a trade-off between safety and efficacy. The information synthesized here can help people with obesity and physicians decide on the type of procedure to undergo or perform. We believe that the nutrient comparisons provided by our web-based meta-analysis can also help people with obesity and physicians understand the relative effectiveness of different procedures and incorporate them into their decision-making along with other essential data elements, e.g., local expertise and adverse (Effectiveness profiles).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YC and XL: contributed to the conception and design of the study. YC, LW, and DZ: organized the database. YC and LW: performed the statistical analysis. YC: wrote the first draft of the manuscript. CW, ML, DZ, and ST: wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
The present study was supported by the Medical and Health Science and Technology Development Project of Shandong Province (No. 202005031357).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Finucane MM, Stevens GA, Cowan M, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. (2011) 377:557–67. doi: 10.1016/S0140-6736(10)62037-5
2. Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
3. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
4. Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. (2002) 288:2793–6. doi: 10.1001/jama.288.22.2793
5. Kral JG, Näslund E. Surgical treatment of obesity. Nat Rev Endocrinol. (2007) 3:574–83. doi: 10.1038/ncpendmet0563
6. Sandoval D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes. (2011) 35:S45–9. doi: 10.1038/ijo.2011.148
7. Stein J, Stier C, Raab H, Weiner R. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. (2014) 40:582–609. doi: 10.1111/apt.12872
8. Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. (2006) 331:219–25. doi: 10.1097/00000441-200604000-00009
9. Oudman E, Wijnia JW, van Dam M, Biter LU, Postma A. Preventing wernicke encephalopathy after bariatric surgery. Obes Surg. (2018) 28:2060–8. doi: 10.1007/s11695-018-3262-4
10. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. (2012) 8:544–56. doi: 10.1038/nrendo.2012.48
11. Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. (1999) 9:150–4. doi: 10.1381/096089299765553395
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Zhang W, Fan M, Wang C, Mahawar K, Parmar C, Chen W, et al. Global bariatric research collaborative. Hair loss after metabolic and bariatric surgery: a systematic review and meta-analysis. Obes Surg. (2021) 31:2649–59. doi: 10.1007/s11695-021-05311-2
14. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. (2012) 3:285–99. doi: 10.1002/jrsm.1054
15. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
16. Qian T, Zhao M, Wan Y, Li M, Xu X. Comparison of the efficacy and safety of drug therapies for macular edema secondary to central retinal vein occlusion. BMJ Open. (2018) 8:e022700. doi: 10.1136/bmjopen-2018-022700
17. Thanigaimani S, Phie J, Sharma C, Wong S, Ibrahim M, Huynh P, et al. Network meta-analysis comparing the outcomes of treatments for intermittent claudication tested in randomized controlled trials. J Am Heart Assoc. (2021) 10:e019672. doi: 10.1161/JAHA.120.019672
18. Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. (2014) 10:262–8. doi: 10.1016/j.soard.2013.07.014
19. Antoniewicz A, Kalinowski P, Kotulecka KJ, Kocoń P, Paluszkiewicz R, Remiszewski P, et al. Nutritional deficiencies in patients after roux-en-Y gastric bypass and sleeve gastrectomy during 12-month follow-up. Obes Surg. (2019) 29:3277–84. doi: 10.1007/s11695-019-03985-3
20. Aron-Wisnewsky J, Verger EO, Bounaix C, Dao MC, Oppert J-M, Bouillot J-L, et al. Nutritional and protein deficiencies in the short term following both gastric bypass and gastric banding. PLoS One. (2016) 11:e0149588. doi: 10.1371/journal.pone.0149588
21. Bailly L, Schiavo L, Sebastianelli L, Fabre R, Pradier C, Iannelli A. Anemia and bariatric surgery: results of a national French survey on administrative data of 306,298 consecutive patients between 2008 and 2016. Obes Surg. (2018) 28:2313–20. doi: 10.1007/s11695-018-3143-x
22. Barzin M, Tasdighi E, Ebadinejad A, Khalaj A, Mahdavi M, Valizadeh M, et al. Anemia after sleeve gastrectomy and one-anastomosis gastric bypass: an investigation based on the Tehran obesity treatment study (TOTS). World J Surg. (2022) 46:1713–20. doi: 10.1007/s00268-022-06528-7
23. Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, et al. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. (2009) 19:56–65. doi: 10.1007/s11695-008-9571-2
24. Ferraz ÁAB, Carvalho MRC, Siqueira LT, Santa-Cruz F, Campos JM. Micronutrient deficiencies following bariatric surgery: a comparative analysis between sleeve gastrectomy and roux-en-Y gastric bypass. Rev Col Bras Cir. (2018) 45:e2016. doi: 10.1590/0100-6991e-20182016
25. Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, Gould J. Vitamin D Status of morbidly obese bariatric surgery patients. J Surg Res. (2010) 164:198–202. doi: 10.1016/j.jss.2010.06.029
26. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. (2010) 20:447–53. doi: 10.1007/s11695-009-0068-4
27. Johnson LM, Ikramuddin S, Leslie DB, Slusarek B, Killeen AA. Analysis of vitamin levels and deficiencies in bariatric surgery patients: a single-institutional analysis. Surg Obes Relat Dis. (2019) 15:1146–52. doi: 10.1016/j.soard.2019.04.028
28. Guan B, Yang J, Chen Y, Yang W, Wang C. Nutritional deficiencies in Chinese patients undergoing gastric bypass and sleeve gastrectomy: prevalence and predictors. Obes Surg. (2018) 28:2727–36. doi: 10.1007/s11695-018-3225-9
29. Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI <50 kg/m2. Obes Surg. (2011) 21:1650–6. doi: 10.1007/s11695-011-0479-x
30. Ledoux S, Flamant M, Calabrese D, Bogard C, Sami O, Coupaye M. What are the micronutrient deficiencies responsible for the most common nutritional symptoms after bariatric surgery? Obes Surg. (2020) 30:1891–7. doi: 10.1007/s11695-020-04412-8
31. Lin S, Guan W, Yang N, Zang Y, Liu R, Liang H. Short-Term outcomes of sleeve gastrectomy plus jejunojejunal bypass: a retrospective comparative study with sleeve gastrectomy and roux-en-Y gastric bypass in Chinese patients with BMI ≥ 35 kg/m2. Obes Surg. (2019) 29:1352–9. doi: 10.1007/s11695-018-03688-1
32. Lombardo M, Franchi A, Biolcati Rinaldi R, Rizzo G, D’Adamo M, Guglielmi V, et al. Long-term iron and vitamin B12 deficiency are present after bariatric surgery, despite the widespread use of supplements. Int J Environ Res Public Health. (2021) 18:4541. doi: 10.3390/ijerph18094541
33. Silva RdA, Malta FMF, Correia MFFSC, Burgos MdA. Serum vitamin B12, iron and folic acid deficiencies in obese individuals submitted to different bariatric techniques. Arq Bras Cir Dig. (2016) 29:62–6. doi: 10.1590/0102-6720201600s10016
34. Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. (2009) 25:1150–6. doi: 10.1016/j.nut.2009.03.012
35. Verger EO, Aron-Wisnewsky J, Dao MC, Kayser BD, Oppert J-M, Bouillot J-L, et al. Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: a 1-year follow-up. Obes Surg. (2016) 26:785–96. doi: 10.1007/s11695-015-1803-7
36. Shipton MJ, Johal NJ, Dutta N, Slater C, Iqbal Z, Ahmed B, et al. Haemoglobin and hematinic Status before and after bariatric surgery over 4 years of follow-up. Obes Surg. (2021) 31:682–93. doi: 10.1007/s11695-020-04943-0
37. Lombardo M, Franchi A, Padua E, Guglielmi V, D’Adamo M, Annino G, et al. Potential nutritional deficiencies in obese subjects 5 years after bariatric surgery. Bariatr Surg Pract Patient Care. (2019) 14:125–30. doi: 10.1089/bari.2019.0009
38. Toh S, Rasmussen-Torvik LJ, Harmata EE, Pardee R, Saizan R, Malanga E, et al. The national patient-centered clinical research network (PCORnet) bariatric study cohort: rationale, methods, and baseline characteristics. JMIR Res Protoc. (2017) 6:e222. doi: 10.2196/resprot.8323
39. Sheppard CE, Lester ELW, Chuck AW, Birch DW, Karmali S, de Gara CJ. The economic impact of weight regain. Gastroenterol Res Pract. (2013) 2013:379564. doi: 10.1155/2013/379564
40. Davies SW, Efird JT, Guidry CA, Penn RI, Sawyer RG, Schirmer BD, et al. Twenty-first century weight loss: banding versus bypass. Surg Endosc. (2015) 29:947–54. doi: 10.1007/s00464-014-3758-5
41. Muhuri D, Nagy GM, Rawlins V, Sandy L, Bellot P. Exploring vitamin B12 deficiency in sleeve gastrectomy from a histological study of a cadaveric stomach and ileum. J Diet Suppl. (2017) 14:514–20. doi: 10.1080/19390211.2016.1269864
42. Korakas E, Kountouri A, Raptis A, Kokkinos A, Lambadiari V. Bariatric surgery and type 1 diabetes: unanswered questions. Front Endocrinol. (2020) 11:525909. doi: 10.3389/fendo.2020.525909
43. Frediani JK, Reilly CM, Higgins M, Clark PC, Gary RA, Dunbar SB. Quality and adequacy of dietary intake in a southern urban heart failure population. J Cardiovasc Nurs. (2013) 28:119–28. doi: 10.1097/JCN.0b013e318242279e
44. Niu A, Carpenter TO, Grams JM, Bozorgmehri S, Tommasini SM, Schafer AL, et al. High dose vitamin D supplementation does not rescue bone loss following roux-en-Y gastric bypass in female rats. Bone. (2019) 127:172–80. doi: 10.1016/j.bone.2019.06.015
45. Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. (2002) 12:685–92. doi: 10.1381/096089202321019693
46. Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. (2014) 6:3587–600. doi: 10.3390/nu6093587
47. Sandvik J, Bjerkan KK, Græslie H, Hoff DAL, Johnsen G, Klöckner C, et al. Iron deficiency and Anemia 10 years after roux-en-Y gastric bypass for severe obesity. Front Endocrinol. (2021) 12:679066. doi: 10.3389/fendo.2021.679066
48. Björn-Rasmussen E, Hallberg L, Rossander L. Absorption of ‘fortification’ iron: Bioavailability in man of different samples of reduced Fe, and prediction of the effects of Fe fortification. Br. J. Nutr. (1977) 37:375–88. doi: 10.1079/BJN19770041
49. Zhu Z, Wu F, Lu Y, Wu C, Wang Z, Zang J, et al. Total and nonheme dietary iron intake is associated with metabolic syndrome and its components in Chinese men and women. Nutrients. (2018) 10:E1663. doi: 10.3390/nu10111663
50. Milman NT. Managing genetic hemochromatosis: an overview of dietary measures, which may reduce intestinal iron absorption in persons with iron overload. Gastroenterology Res. (2021) 14:66–80. doi: 10.14740/gr1366
51. Allo Miguel G, García Fernández E, Martínez Díaz-Guerra G, Valero Zanuy MÁ, Pérez Zapata A, de la Cruz Vigo F, et al. Recalcitrant hypocalcaemia in a patient with post-thyroidectomy hypoparathyroidism and roux-en-Y gastric bypass. Obes Res Clin Pract. (2016) 10:344–7. doi: 10.1016/j.orcp.2015.09.001
Keywords: bariatric surgeries, malnutrition, obesity, micronutrients, RYGB, LAGB, SG
Citation: Cui Y, Zhang D, Wang L, Liu X, Wang C, Tian S and Li M (2023) Which nutritional prognosis is better? comparison of the three most commonly performed bariatric surgeries: A systematic review and network meta-analysis. Front. Surg. 9:1065715. doi: 10.3389/fsurg.2022.1065715
Received: 10 October 2022; Accepted: 21 November 2022;
Published: 30 January 2023.
Edited by:
Tomas Poskus, Vilnius University, Lithuania© 2023 Cui, Zhang, Wang, Liu, Wang, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Di Zhang emhhbmdkaTEyMTZAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.