- Department of Gastroenterology, Affiliated Hospital of Qingdao University, Qingdao, China
Objective: The objective of this study was compare the effects of robot-assisted and laparoscopic-assisted surgery on lymph node dissection and quality of life in upper third gastric cancer patients undergoing radical total gastrectomy.
Methods: The clinical and follow-up data of 409 patients with upper third gastric cancer who underwent total gastrectomy from July 2016 to May 2021 were enrolled. The patients were divided into a robotic group (n = 106) and a laparoscopic group (n = 303). Age, sex, body mass index, American Society of Anesthesiologists score, tumor size and location, pathological type, cT, cN, and cTNM were adjusted to offset selection bias. The patient characteristics, operative procedures, surgical outcomes, oncologic and pathologic outcomes, number of lymph node dissections, quality of life assessment, and nutritional status were compared between the two groups.
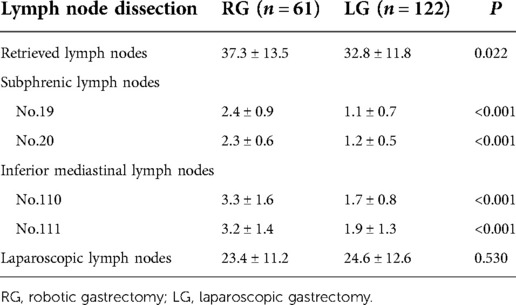
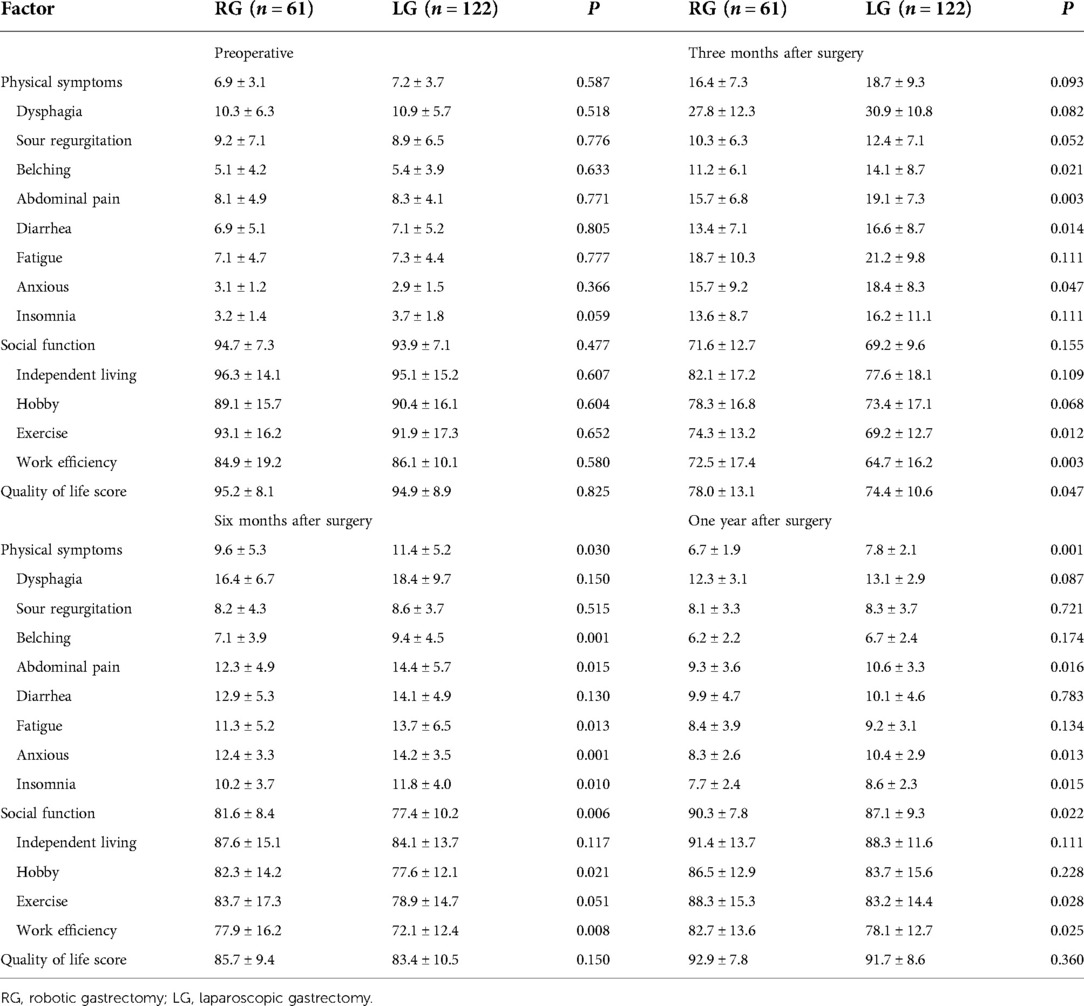
Results: After propensity score matching, 61 cases were included in the robotic group and 122 cases were included in the laparoscopic group. The number of dissected lymph nodes (37.3 ± 13.5 vs. 32.8 ± 11.8, P = 0.022) significantly differed between the two groups. The number of lower mediastinal and subphrenic lymph nodes in the robotic group was greater than that in the laparoscopic group, and the difference was statistically significant (P < 0.001). Compared with the laparoscopic group, the total score of physical symptoms in the robotic group was significantly lower at 6 and 12 months after surgery (P = 0.03 and P = 0.001, respectively). The total social function score at 6 and 12 months after surgery was higher in the robotic group (P = 0.006 and P = 0.022). The quality of life scores were statistically significant only at 3 months after the operation (P = 0.047). A higher patient-generated subjective global assessment (PG-SGA) score is when the score significantly correlated (P < 0.001) with a higher related physical symptoms score, lower social function score, and lower quality of life score.
Conclusion: Compared with laparoscopic radical gastrectomy, robotic radical gastrectomy is safe and feasible. Compared with laparoscopic radical gastrectomy, robotic radical gastrectomy was more refined, was associated with less surgical bleeding, and increased the quality of lymph node dissection. In addition, patients in the robotic group showed better postoperative quality of life.
Introduction
In the past 40 years, the worldwide incidence rate of upper third gastric cancer (GC) has shown a significant upward trend (1, 2). Upper third gastric cancer is defined as adenocarcinoma of the upper third of the stomach, with or without esophagogastric junction adenocarcinoma, according to the Classification of the Japan Gastric Cancer Association (JGCA) (3). Although great progress has been made in targeting, immunological treatment, perioperative radiotherapy, and chemotherapy based on the molecular classification of upper third gastric cancer, surgery still plays an important role. Surgical methods, including traditional laparotomy and laparoscopy, represented by minimally invasive surgery and the Da Vinci robot, have become the main methods used to treat upper third gastric cancer cases worldwide. In 2002, Hashizume et al. carried out the world's first robot-assisted radical gastrectomy for gastric cancer (4). Robot-assisted surgery has a good 3D field of view, which is more suitable for narrow body cavity operations. The precise anatomy modified by the computer, the excellent suture technology under the microscope, and the movement of the 7-degree-of-freedom robot arm overcome the limits of the human body, which significantly reduced the dependence of the operator on the team. These unique advantages are unmatched by traditional laparoscopy. In addition, robotic surgery also has the advantages of a short learning curve, less surgical bleeding, an increased number of lymph node dissections, and other potential tumor control (5). However, few studies comparing laparoscopic and robotic lymph node dissection in upper third gastric cancer are currently available.
Radical gastrectomy combined with local lymph node dissection is the main treatment strategy for resectable gastric cancer (6). Especially for patients with advanced upper gastric cancer, radical total gastrectomy combined with D2 lymph node dissection is recommended. With the development of minimally invasive surgery and the improvement of postoperative quality of life (QOL), the recovery of postoperative somatic symptoms and social functions has gradually become the focus of attention for gastric cancer patients (7). The purpose of this study was to compare the effects of laparoscopic- and robotic-assisted gastrectomy combined with D2 lymph node dissection on the potential tumor control effect and quality of life of patients with upper third gastric cancer.
Materials and methods
Study design and participants
From July 2016 to May 2021, 409 patients with upper third gastric cancer consecutively received surgical treatment at the Gastrointestinal Surgery Department of Qingdao University Affiliated Hospital. Upper third gastric cancer is defined as adenocarcinoma of the upper third of the stomach, with or without esophagogastric junction adenocarcinoma, according to the Classification of the JGCA (3). The location of the primary carcinoma was determined by esophagogastroscopy.
Patients were divided into two groups based on whether they underwent robotic-assisted or laparoscopic-assisted surgery. Patients underwent propensity score matching analysis, and age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, tumor size, pathological type, cT, cN, and cTNM were adjusted to offset selection bias. The matched robot-assisted group was compared with the laparoscopic-assisted group based on preoperative basic information, perioperative complications, histopathological features, number of lymph nodes dissected during operation, postoperative quality of life, and nutritional status.
Preoperative tumor staging was assessed by computed tomography (CT) and gastroscopy. T stage and N stage were determined using the latest AJCC/UICC TNM staging system (8), and histological types were consistent with the Japanese classification of gastric cancer (3).
Patients’ eligibility criteria
The patients’ eligibility criteria were as follows: (1) 12-month follow-up was completed and the follow-up data were complete; (2) gastric cancer in the upper third of the stomach; (3) first-time gastrectomy; (4) age >20 years for both sexes; (5) R0 gastrectomy; (6) no recurrence or distant metastasis; (7) performance status (PS) 0 or 1 based on the Eastern Cooperative Oncology Group scale; (8) sufficient capacity to understand and respond to the questionnaire; (9) no history of other diseases or operations that might influence the responses to the questionnaire; and (10) no organ failure or mental illness.
In addition, patients with a history of abdominal surgery, double primary tumors, and simultaneous resection of other organs were excluded.
Surgery procedure
All patients had undergone curative resection and D1+/D2 lymphadenectomy in accordance with the Japanese guidelines for treating GC (3).
To obtain a better prognosis, a sufficient tumor edge should be ensured by the surgeons before specimen resection. If the tumor margins cannot meet the requirement and may be positive, intraoperative frozen pathology of the margin needs to be performed to exclude positive results. We stipulate performing uncut Roux-en-Y reconstruction after tumor resection.
The surgical procedure of robotic gastrectomy (RG) was very similar to that of laparoscopic gastrectomy (LG) in terms of trocar placement, surgical anatomical sequence, and anastomosis technique. The surgeons chose the extracorporeal method and stapling instrument methods for anastomosis to reinforce the hand-sewing according to the intraoperative conditions and extracorporeal anastomosis using a minilaparotomy.
Perioperative management
The application of enhanced recovery after surgery (ERAS) has widely gained acceptance, and all patients were managed with the ERAS protocol during the perioperative period (9).
Before surgery, patients received education and exercise in lung function and prerehabilitation. For daily smokers and alcohol abusers, 1 month of abstinence was required before surgery. Chest, abdominal, and pelvic CT were performed to confirm the size and location of the tumor and imaging staging. Neoadjuvant chemotherapy was an option in cases with a large tumor or bulky lymph node metastasis. Cardiac ultrasound and pulmonary function tests were used to evaluate the tolerance of cardiopulmonary function for gastric cancer surgery. Lower extremity vascular ultrasound was used to evaluate the thrombus. Nutrition Risk Screening (NRS) 2002 was used to assess the nutritional status of patients: if the score was ≥3, the patient was given nutritional support (10). The correct evaluation of the patient's tolerance to surgery, reasonable treatment of other combined diseases, correction of anemia and water, and electrolyte disorders improved the patient's general condition.
On the day of surgery, the patients were allowed clear fluids for up to 2 h and solids for up to 6 h before the induction of anesthesia (11). A complex clear carbohydrate-rich drink designed for use within 2 h before anesthesia reduced hunger, thirst, anxiety and the length of stay, as well as postoperative insulin resistance.
After the surgery, the following measures were employed: (1) multimodal analgesia, including epidural analgesia and intravenous analgesia; (2) anasogastric tube, which should be removed on postoperative day 1 (POD1); (3) an abdominal drainage tube, which can be removed on POD3 when drainage fluid is clear and <100 ml/day, when the anastomotic status is good, or when no abdominal infection is found; (4) a urinary catheter, which should be removed on POD1; (5) venous thromboembolism (VTE) prevention, which included mechanical measures (intermittent pneumatic leg compression and elastic stockings) for patients with increased VTE risk; (6) oral feeding: we stipulated that patients can consume a clear fluid diet on POD1–2, semiliquid diet on POD3–4, and soft blended diet on POD5 if tolerable and then gradual transition to a normal diet based on the premise of patient's tolerance and no severe complication (including anastomosis leakage, ileus, high risk of gastroplegia, etc.); (7) movement: we encouraged early ambulation for 1 h/day on POD1 and prepared an appropriate scheme of movement for patients. The time of ambulation should properly increase to 4 h/day on POD7 based on the patient's status and need; (8) discharge: patients could be discharged from the hospital on POD7 according to the discharge criteria (without postoperative complications and primary disease that requires current intervention).
According to the postoperative pathological stage and Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of gastric cancer, patients with stage II/III gastric cancer were treated with 5-fluorouracil-based regimens, either XELOX or S-1, for six to eight cycles. The patients were followed up for 1 year and once at 3, 6, and 12 months. The follow-up included physical examination and laboratory examination. At each follow-up, the diet, physical symptoms, and social function recovery were collected by telephone contact. The deadline for follow-up was May 2022.
Data collection and clinical analysis
Patients were enrolled in this study, and two data management staff members were assigned to collect relevant data. The basic characteristics of patients collected before surgery were age, sex, body mass index, ASA score, and hematologic indices (complete blood count, blood biochemistry, tumor biomarkers, etc.). The operation was characterized by estimated blood loss, operative time, and cost. Postoperative outcomes were mean maximum body temperature during the first 3 days, pain Numerical Assessment Scale (NAS) score on the first 3 days after surgery, days of bowel function recovery, time to start soft diet, complications, and adverse events. Morbidity was described based on the Clavien–Dindo classification of JOCG criteria for postoperative complications and according to the Common Terminology Criteria for Adverse Events (CTCAE 5.0) (12–15). The oncologic and pathologic outcomes were the number of lymph nodes removed, pathological proximal and distal margins, TNM stage, tumor size and location, Lauren classification, and tumor cell differentiation. After discharge, a 1-year follow-up, which included QOL questionnaires, periodic physicals, laboratory examinations, and abdominal CT every 3 months at the outpatient department, began on time.
Assessment of postoperative quality of life: The quality of life assessment scale was constructed with reference to the quality of life questionnaire-core 30 (QLQ-C30) (16, 17) and the gastric cancer specific module scale (quality of life questionnaire-stomach 22, qlq-sto22) (18, 19) designed by the European Organization for Research and Treatment of Cancer (EORTC) in Chinese. The new scale combines the advantages of the above two scales, mainly including physical symptom scores and social function evaluations. According to the scoring procedure of the EORTC scoring manual, the score is linearly converted into a score of 0–100. The higher the function score is, the better the function, and the higher the symptom score is, the more serious the symptoms (20). Quality of life score = (100 − score of physical symptom scale + score of social function scale)/2.
Nutritional parameters after gastrectomy were assessed on the basis of changes in serum prealbumin, albumin, hemoglobin, meal size and times, foods with different degrees of hardness and softness, prognostic nutritional index (PNI), and body weight at 3, 6, and 12 months after surgery (21). PNI was calculated using the following formula: 10 × serum albumin value (g/dl) + 0.005 × lymphocyte count in peripheral blood (22). On the CT images, the cross-sectional area of the psoas muscle was measured at the level of the third lumbar vertebra (L3). Psoas muscle index (PMI) = (area of the psoas muscle at L3 cm2)/(height m2). The 1-year change rate was calculated as follows: (nutrition-related indicators at one year after surgery − preoperative)/(preoperative × 100) (23, 24). Considering the relationship between nutritional status and quality of life, the patient-generated subjective global assessment (PG-SGA) score was used to assess the association with quality of life.
Statistical analysis
All data were processed using SPSS 26.0 and R software (version 4.0.2). To eliminate the potential deviation caused by the lack of equal distribution between the two groups, a logistic regression model with the following covariates was used to calculate the propensity score: age, sex, body mass index, American Society of Anesthesiologists score, tumor location, tumor size, pathological type, cT, cN, and cTNM. Matching was performed at a ratio of 1:2, and a caliper width of 0.01 standard deviation was specified (25). Categorical variables are expressed as examples (%), and the chi-square or Fisher exact test was used. Continuous variables conforming to a normal distribution are expressed as X ± s, and a paired t-test was used for comparisons between groups. Nutrition-related indices were compared before the operation and at 1, 3, and 6 months after the operation, and repeated-measures ANOVA was used. P < 0.05 indicates that the difference is statistically significant.
Ethics statement
The data for this study were collected in the course of general clinical practice, so informed consent signed by each patient was obtained for any surgical and clinical procedure. This protocol was in line with the ethical guidelines of the World Medical Association Declaration of Helsinki adopted by the 18th World Medical Association Congress held in Helsinki, Finland, in June 1964. Institutional Review Board approval was not needed. Since this study was retrospective, patients’ consent was not required for inclusion in the study.
Results
Patient characteristics
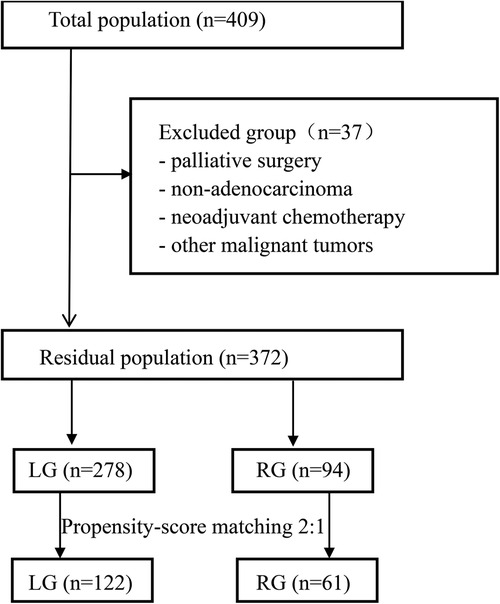
A total of 409 upper third gastric cancer patients underwent surgery (Figure 1). Among these patients, 37 patients were excluded from the study due to palliative surgery (n = 7), non-adenocarcinoma (n = 4), complications with other malignant tumors (n = 6), neoadjuvant chemotherapy (n = 9), and loss to follow-up (n = 11). Finally, a total of 372 patients who underwent laparoscopic-assisted surgery (278 patients) or robotic-assisted surgery (94 patients) were enrolled in this study. After 1:2 matching between the RG group and LG group, 61 patients were included in the RG group and 122 patients were included in the LG group.
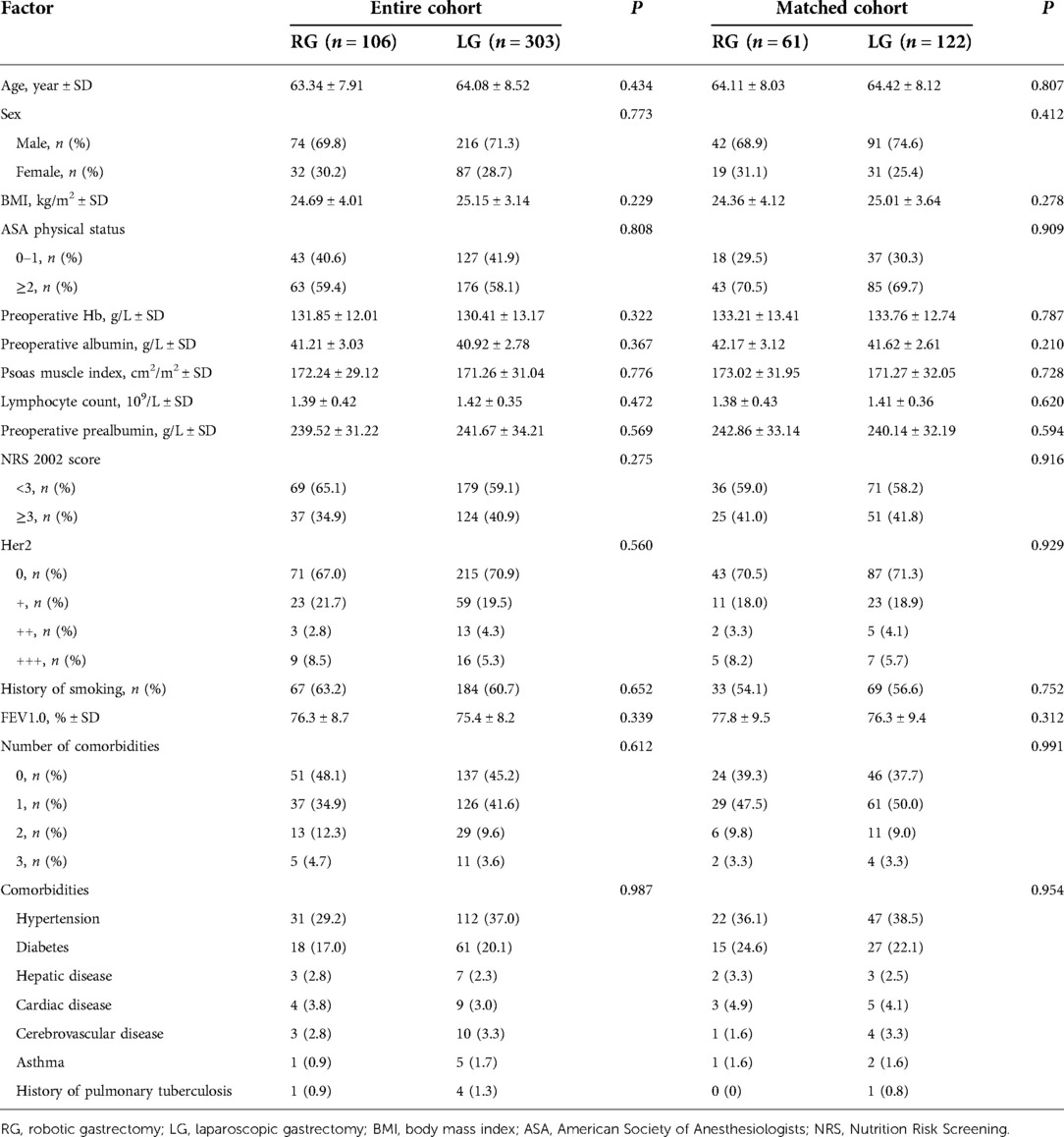
The basic clinical characteristics of the patients in the two groups are shown in Table 1. Age, sex, BMI, preoperative nutritional indicators, or comorbidities did not significantly differ between groups in the entire cohort. The matched baseline features were well balanced.
Operative procedures and surgical outcomes
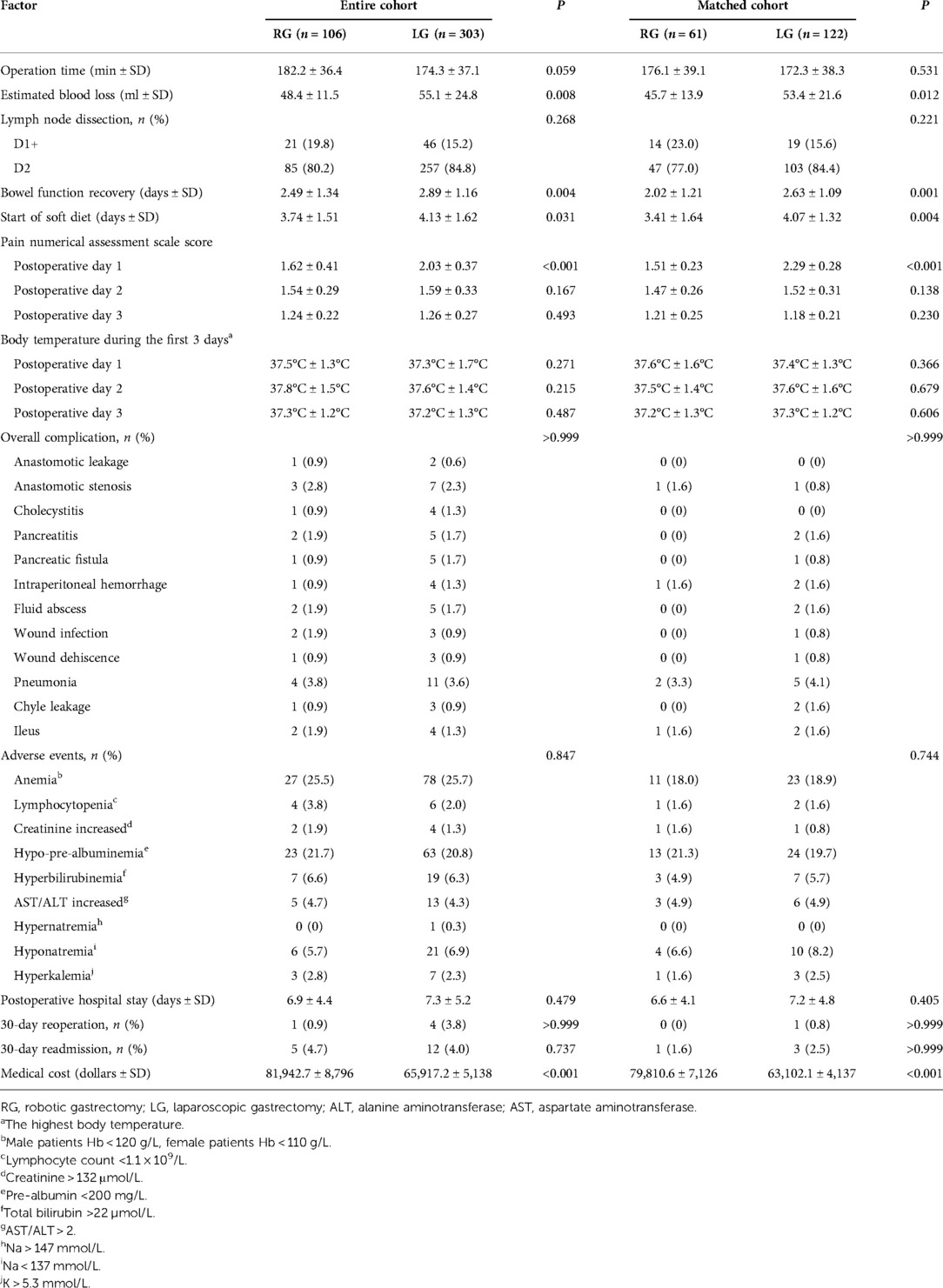
The operation time did not significantly differ between the two groups (P = 0.531). Compared with that in the laparoscopic group, the intraoperative blood loss in the robotic group was lower (45.7 ± 13.9 vs. 53.4 ± 21.6 ml, P = 0.012). In addition, the time to bowel function recovery (2.02 ± 1.21 vs. 2.63 ± 1.09, P = 0.001) and start of a soft diet (3.41 ± 1.64 vs. 4.07 ± 1.32, P = 0.004) in the robotic group were better than those in the laparoscopic group. The pain NAS score on the first postoperative day in the robotic group (1.51 ± 0.23 vs. 2.29 ± 0.28, P < 0.001) was lower than that in the laparoscopic group. No significant difference was detected in the highest temperature or complications. However, cost was higher in the robotic group than in the laparoscopic group (79,810.6 ± 7,126 vs. 63,102.1 ± 4,137, P < 0.001) (Table 2).
Oncologic and pathologic outcomes
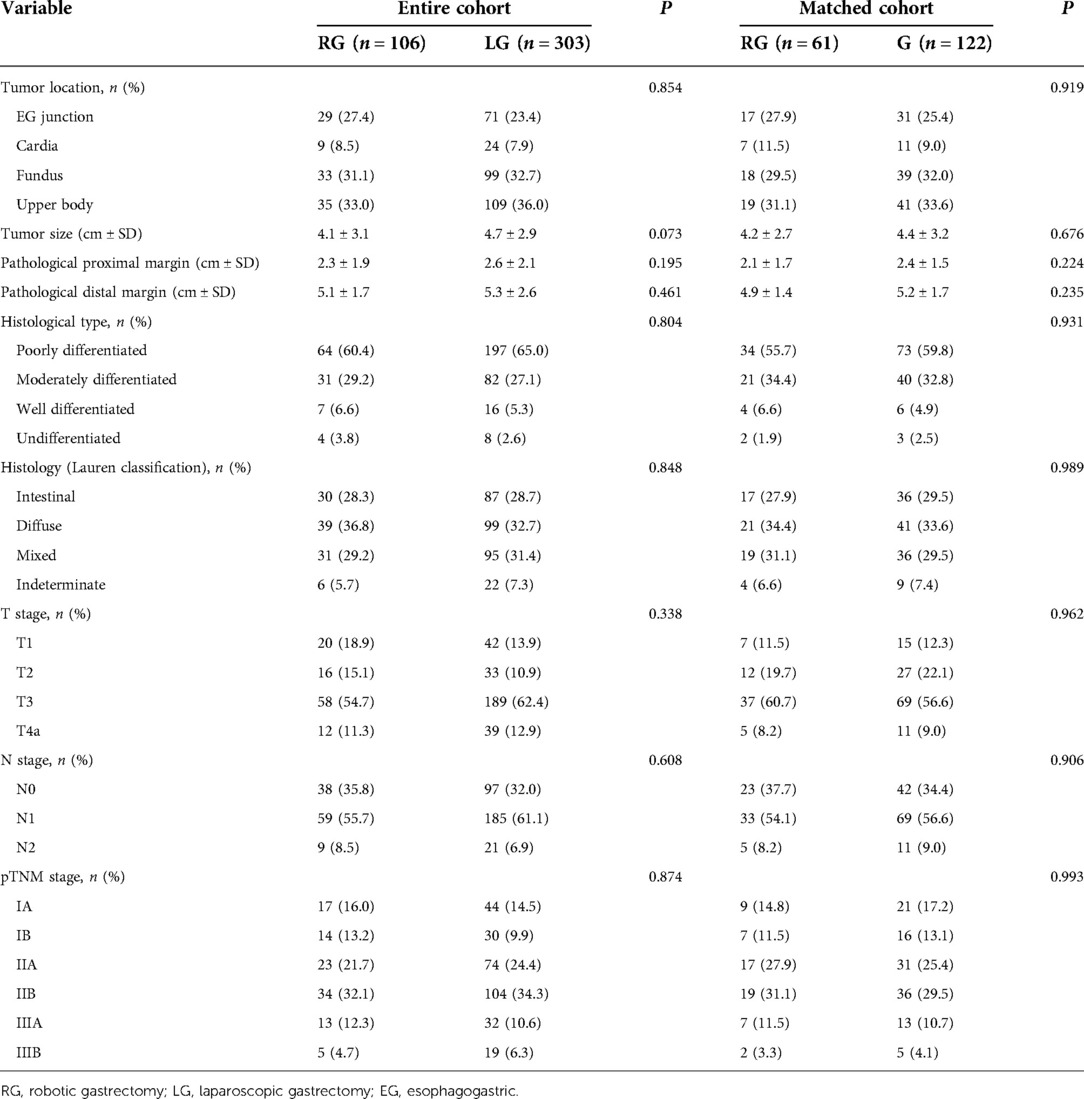
Tumor location and size, proximal and distal resection margins, histological type, or Lauren classification did not significantly differ between the two groups (P > 0.05). After propensity matching, no significant difference in pTNM staging was detected between the two groups (P > 0.05) (Table 3).
The number of dissected lymph nodes (37.3 ± 13.5 vs. 32.8 ± 11.8, P = 0.022) was significantly different between the two groups. The number of lower mediastinal and subphrenic lymph nodes in the robotic group was greater than that in the laparoscopic group, and the difference was statistically significant (P < 0.001) (Table 4). The total number of abdominal lymph nodes and the number of abdominal lymph nodes at each station between the two groups were not statistically significant (P > 0.05) (Figure 2).
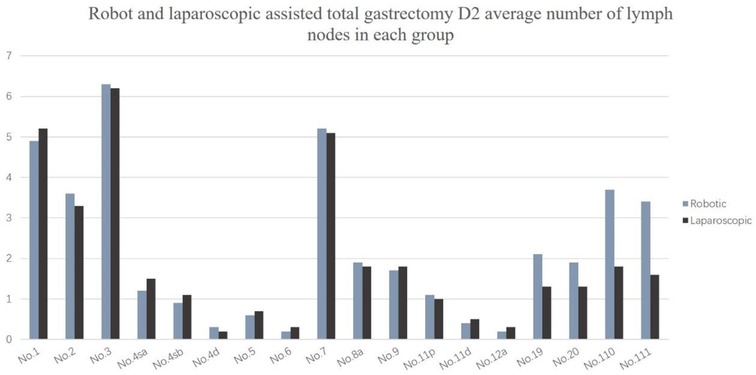
Figure 2. Robot and laparoscopic assisted total gastrectomy D2 average number of lymph nodes in each group.
The lymph node metastasis rates of No. 1, No. 2, No. 3, and No. 7 were the highest, all approximately 20%, followed by No. 8a, No. 9, No. 11p, and No. 110. The lymph node metastasis rate was close to 5%, and the lymph node metastasis probability of other stations was less than 5% (Figure 3).
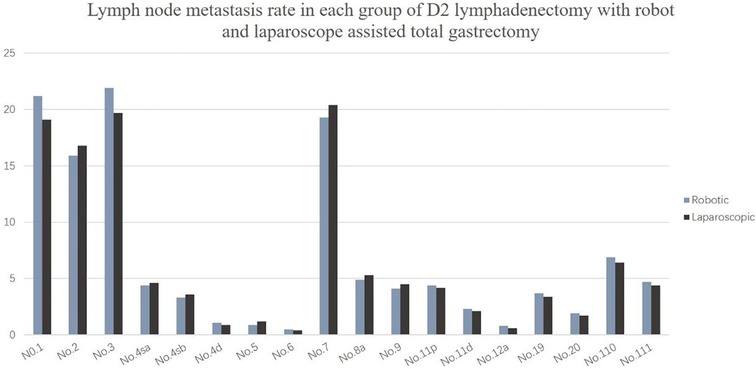
Figure 3. Lymph node metastasis rate in each group of D2 lymphadenectomy with robot and laparoscope assisted total gastrectomy.
Quality of life assessment
In the robotic group, the total scores of physical symptoms before surgery and 3, 6, and 12 months after surgery were 7.9 ± 5.1, 16.4 ± 7.3, 9.6 ± 5.3, and 6.7 ± 1.9, respectively. Compared with the laparoscopic group, the total score of physical symptoms in the robotic group was significantly lower at 6 and 12 months after surgery (P = 0.03 and P = 0.001, respectively). In the robotic group, the total social function scores were 94.7 ± 7.3, 71.6 ± 12.7, 81.6 ± 8.4, and 90.3 ± 7.8 before surgery and 3, 6, and 12 months after surgery, respectively. In the laparoscopic group, the scores were 93.9 ± 7.1, 69.2 ± 9.6, 77.4 ± 10.2, and 87.1 ± 9.3, respectively. Compared with the laparoscopic group, the total score at 6 and 12 months after surgery was higher in the robotic group (P = 0.006 and P = 0.022). The quality of life scores of the robotic group were 95.2 ± 8.1, 78.0 ± 13.1, 85.7 ± 9.4, and 92.9 ± 7.8 before surgery and 3, 6, and 12 months after surgery, respectively. The scores of the laparoscopic group were 94.9 ± 8.9, 74.4 ± 10.6, 83.4 ± 10.5, and 90.7 ± 8.6, respectively. The difference between the two groups at each time point was statistically significant only at 3 months after the operation (P = 0.047) (Table 5).
Nutritional status
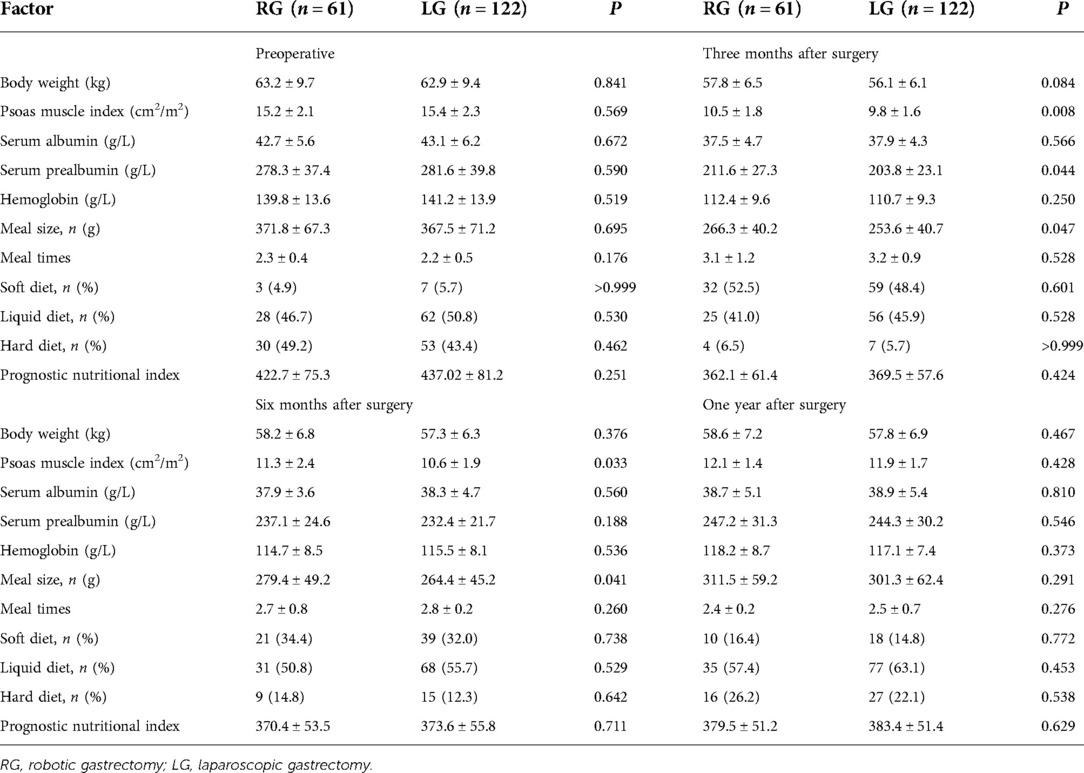
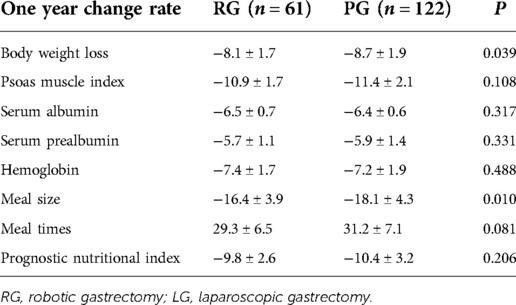
The preoperative baseline data of the two groups were very balanced (Tables 6, 7). Although body weight did not significantly differ between the two groups during the same period after surgery, the 1-year change rate was statistically significant [(−8.1 ± 1.7) vs. (−8.7 ± 1.9), P = 0.039]. PMI significantly differed between the two groups at 3 and 6 months after the operation (P < 0.05), but no significant difference was detected in the 1-year change rate after the operation (P > 0.05). The proportion of meal size change [(−16.4 ± 3.9)% vs. (−18.1 ± 4.3)%, P = 0.001] and the proportion of meal time change [(29.3 ± 6.5)% vs. (31.2 ± 7.1)%, P = 0.081] significantly differed between groups. At 3, 6, and 12 months after the operation, the proportion of solid diet in the robotic group was higher than that in the laparoscopic group, but this difference was not significant (P > 0.05). As seen in Table 8, a higher PG-SGA score significantly correlated (P < 0.001) with a higher related physical symptom score, lower social function score, and lower quality of life score.
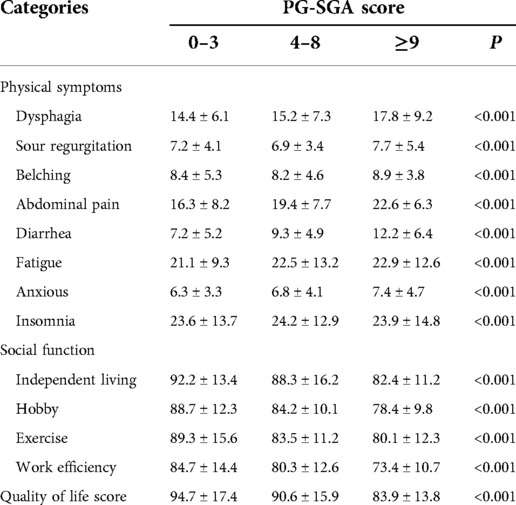
Table 8. Correlation between nutritional status and quality of life in gastric cancer patients 1 year after surgery.
Discussion
A large number of studies have confirmed that robotic radical gastrectomy has the advantages of fewer complications, less bleeding, faster postoperative recovery, and shorter hospital stays compared with laparoscopic surgery (26–28). Moreover, robotic surgery can ensure that the surgical field is clean and clear, is easier to use for the surgeon and team, minimize the occurrence of vascular and organ side injuries, and increase the number of lymph nodes obtained. Lymph node dissection is the most complex and challenging part of radical gastrectomy. Some studies have shown that robots are more suitable for complex operations and lymph node dissection than laparoscopic radical gastrectomy for gastric cancer (29). This study shows that the average number of lymph nodes cleaned by robot surgery is 37.3, which is significantly higher than that of laparoscopic surgery is 32.8. This difference suggests that robot lymph node cleaning is superior to laparoscopic surgery, which may bring patients potential advantages in terms of better tumor treatment. This improvement may be related to the advantages of robot surgery, such as 10–15 times enlarged vision, distortion-free 3D display, and 7 degrees of freedom of surgical instruments, which make lymph node cleaning more accurate. Lee et al. (27) reported that patients with a high body mass index who underwent robotic-assisted distal gastrectomy plus D2 lymph node dissection had less blood loss and higher lymph node dissection quality. At the same time, since the operation time in this study does not include installation time, the operation time did not significantly differ between the two groups.
This study showed that the No. 1, No. 2, No. 3, and No. 7 lymph node metastasis rates were higher, followed by the metastasis rates of the No. 8, No. 9, No. 11, No. 19, No. 20, No. 110, and No. 111 lymph nodes; the lymph node metastasis rate of No. 4, No. 5, No. 6, and No. 12 were the lowest, which was similar to findings reported in the Japanese literature (26). These results all suggest that upper third gastric cancer is characterized by a unique pattern of lymph node metastasis, which can flow to the lower mediastinum and abdominal lymph nodes. Furthermore, the abdominal lymph nodes are the main lymph nodes, while the lower mediastinum still experiences a certain proportion of lymph node metastasis. Therefore, lymph node dissection for upper third gastric cancer should consider these two regions. The number of abdominal lymph nodes cleaned did not significantly differ between the robotic and laparoscopic groups, but the main difference lies in the subphrenic lymph nodes (No. 19 and No. 20) and the lower mediastinal lymph nodes (No. 110 and No. 111), and robot surgery is superior to laparoscopy.
In addition to the quality of radical surgery, the postoperative quality of life of gastric cancer patients is also the focus of surgeons (30). A number of clinical studies have confirmed the minimally invasive advantages of laparoscopic radical gastrectomy and its exact oncological efficacy. This study integrated the scales of the European Cancer Research and Treatment Assistance Organization (EORTC QLQ—C30 questionnaire and EORTC QLQ—STO22 questionnaire) to evaluate the quality of life recovery of patients in the robotic and laparoscopic groups after surgery. The results showed that dysphagia, abdominal pain, fatigue, diarrhea, and other physical symptoms after surgery were significantly better in the robotic group than in the laparoscopic group, which may be due to the more sophisticated operation of the robotic group (31). In addition, the amount of intraoperative bleeding was reduced in the robotic group, which may help reduce the formation of intra-abdominal adhesions and the resulting abdominal discomfort. These factors are conducive to the recovery of intestinal function after surgery.
The results of this study also showed that the social function scores of independent living, hobbies, fitness exercise, and work efficiency were better in the robotic group than in the laparoscopic group at 3–12 months after surgery, and the overall quality of life scores of the robotic group were better than those of the laparoscopic group. The reason may be that the robotic group has less intraoperative trauma, earlier bowel function recovery, an earlier return to a soft diet, a faster reduction of physical symptoms, less mental burden, and earlier physiological function recovery to the preoperative level (32). The results of this study also showed that the physical symptoms score of patients in the robotic group returned to the preoperative level at 6 months after surgery, and the improvement was more obvious at 12 months after surgery, while the physical symptoms score of patients in the laparoscopic group did not return to the preoperative level until 12 months after surgery. Therefore, compared with the laparoscopic group, patients in the robotic group were able to achieve self-care earlier, were more willing to resume leisure activities and fitness exercises, had higher work efficiency, and were better able to recover their social roles.
Due to the changes in the anatomical and physiological structure of the digestive tract and the impairment of gastrointestinal function after operation, patients undergoing radical gastrectomy are recommended to eat smaller and more frequents meals, mainly a liquid diet (33). However, this study found that more patients in the robotic group ate a solid diet and soft food than those in the laparoscopic group at 3, 6, and 12 months after surgery and had a greater tendency to recover to the proportion of preoperative dietary components. This finding indicated that patients in the robotic group were more able to tolerate a solid diet after surgery. Furthermore, the robotic group had a lower score of dysphagia, diarrhea, and other physical symptoms than the laparoscopic group, which further indicated that gastrointestinal symptoms in the robotic group recovered quickly in the early postoperative period.
The recovery of postoperative nutritional indicators is also an important standard to consider the quality of life of gastric cancer patients after surgery. Lower changes in nutritional indicators and faster nutritional recovery after surgery tend to promote a better prognosis, indicating better postoperative quality of life (34). The results of this study showed that the proportion of changes in body weight and meal size were significantly lower in the robotic group than in the laparoscopic group 1 year after surgery. This difference may be related to the fact that the patients in the robotic group have a higher tolerance to diet than those in the laparoscopic group and can quickly recover their preoperative eating habits after surgery. In addition, the patients in the robotic group consumed a solid diet as early as possible after the operation, which provided them with more energy and nutrients needed by the human body, thus promoting the maintenance of body weight after the operation.
We found that as the PG-SGA score increased, values from the functional category and for the overall health status of patients with a lower mean field rank and the symptoms category rank mean increased. Specifically, as functional abilities and quality of life worsened, symptoms or problems, such as fatigue, nausea, belching, diarrhea, and insomnia, worsened and added to the poor quality of life.
Learning curve
Although studies regarding the learning curve of robotic gastrectomy are scarce, all reported that the robotic system is more adaptable than the laparoscopic environment (35–37). Moreover, in contrast to the longer operation time, the robotic system makes surgeons rapidly overcome the learning curve for robotic gastrectomy, which may help less-experienced surgeons. The actual impact of the robotic system on the learning curve of robotic gastrectomy is difficult to evaluate without considering the experience of laparoscopic gastrectomy because the robotic gastrectomy procedure is identical to laparoscopic gastrectomy. Thus, the exact assessment of the learning curve effect would be difficult.
Cost
Studies have consistently reported that the costs of robotic gastrectomy are higher than those of laparoscopic gastrectomy. Robotic gastrectomy consistently costs more than laparoscopic gastrectomy. The high cost of robotic gastrectomy is mainly associated with the cost of robotic system installation and disposable drapes and instruments (38, 39). Moreover, since a longer operation time itself means another source of extra expense of robotic surgery, balancing the cost of robotic surgery with that of laparoscopic surgery is difficult. Thus, further studies to determine whether the benefits of robotic surgery would reduce the other costs related to postoperative care or readmission are necessary.
This research is subject to limitations. The malnourished patients did not receive further nutritional intervention, and we hope to clarify in future research whether an improvement in the nutritional status in gastric cancer patients will improve clinical outcome. In addition, the effect of nutritional status on the final clinical outcome after nutritional therapy was not followed up.
Conclusion
In summary, robotic-assisted radical total gastrectomy for upper third gastric cancer is safe and feasible. Compared with laparoscopic surgery, it is more sophisticated, has less bleeding, and has a higher quality of lymph node dissection, especially for subphrenic and lower mediastinal lymph nodes. At the same time, patients in the robotic group also had better quality of life and faster postoperative nutritional recovery than patients in the laparoscopic group.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JF contributed to this study. ZN made substantial contributions to the conception and design for this study, YL and XJ acquired and analyzed data and JF drafted the article. HQ and YW gave many important suggestions for this study. ZN and XL participated in critical interpretation of important intellectual results. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the Department of gastrointestinal surgery, Affiliated Hospital of Qingdao University, China. We thank ZN and XL for their critical reading and informative advice during the process of study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chevallay M, Bollschweiler E, Chandramohan S, Schmidt T, Koch O, Demanzoni , et al. Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann N Y Acad Sci. (2018) 1434(1):132–8. doi: 10.1111/nyas.13954
2. Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on gastric cancer in 2014. J Gastric Cancer. (2016) 16(3):131–40. doi: 10.5230/jgc.2016.16.3.131
3. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. (2011) 14(2):97–100. doi: 10.1007/s10120-011-0040-6
4. Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. (2002) 16(8):1187–91. doi: 10.1007/s004640080154
5. Tsai SH, Liu CA, Huang KH, Lan YT, Chen MH, Chao Y, et al. Advances in laparoscopic and robotic gastrectomy for gastric cancer. Pathol Oncol Res. (2017) 23(1):13–7. doi: 10.1007/s12253-016-0131-0
6. Xiong JJ, Nunes Q, Huang W, Tan CL, Ke NW, Xie SM, et al. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. (2013) 19(44):8114–32. doi: 10.3748/wjg.v19.i44.8114
7. Huang C, Yu F, Zhao G, Xia X. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared with laparoscopy-assisted distal gastrectomy for early gastric cancer. J Gastroenterol Hepatol. (2020) 35(10):1712–9. doi: 10.1111/jgh.14985
8. Brierley JD, Gospodarowicz M, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. NJ, USA: Wiley Blackwell (2017).
9. Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. (2014) 101(10):1209–29. doi: 10.1002/bjs.9582
10. Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz N, Dhatariya K, et al. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. (2020) 39(11):3211–27. doi: 10.1016/j.clnu.2020.03.038
11. Apfelbaum J, Caplan R, Connis R, Epstein B, Nickinovich D, Warner M, et al. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. (2011) 114(3):495–511. doi: 10.1097/ALN.0b013e3181fcbfd9
12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
13. Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. (2016) 46(6):668–85. doi: 10.1007/s00595-015-1236-x
14. Institute NC. Common terminology criteria for adverse events (CTCAE) version 5.0. Bethesda, MD: National Cancer Institute (2017).
15. Xue W, Dong BZ, Zhao YJ, Wang YX, Yang CY, Xie YW, et al. Upregulation of TTYH3 promotes epithelial-to-mesenchymal transition through Wnt/β-catenin signaling and inhibits apoptosis in cholangiocarcinoma. Cell Oncol (Dordr). (2021) 44(6):1351–61. doi: 10.1007/s13402-021-00642-9
16. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez N, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85(5):365–76. doi: 10.1093/jnci/85.5.365
17. Davda J, Kibet H, Achieng E, Komen T. Assessing the acceptability, reliability, and validity of the EORTC Quality of Life Questionnaire (QLQ-C30) in Kenyan cancer patients: a cross-sectional study. J Patient Rep Outcomes. (2021) 5(1):4. doi: 10.1186/s41687-020-00275-w
18. Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. (2004) 40(15):2260–8. doi: 10.1016/j.ejca.2004.05.023
19. Oñate-Ocaña LF, Alcántara-Pilar A, Vilar-Compte D, García-Hubard G, Rojas-Castillo E, Alvarado-Aguilar S, et al. Validation of the Mexican Spanish version of the EORTC C30 and STO22 questionnaires for the evaluation of health-related quality of life in patients with gastric cancer. Ann Surg Oncol. (2009) 16(1):88–95. doi: 10.1245/s10434-008-0175-9
20. Wintner LM, Sztankay M, Aaronson N, Bottomley A, Giesinger J, Groenvold M, et al. The use of EORTC measures in daily clinical practice-A synopsis of a newly developed manual. Eur J Cancer. (2016) 68:73–81. doi: 10.1016/j.ejca.2016.08.024
21. Demirelli B, Babacan NA, Ercelep Ö, Öztürk MA, Kaya S, Tanrikulu E, et al. Modified Glasgow Prognostic Score, Prognostic Nutritional Index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. (2021) 73(2):230–8. doi: 10.1080/01635581.2020.1749290
22. Furukawa H, Kurokawa Y, Takiguchi S, Tanaka K, Miyazaki K, Makino T, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. (2018) 21(3):500–7. doi: 10.1007/s10120-017-0755-0
23. Sugiyama M, Oki E, Ando K, Nakashima Y, Saeki H, Maehara Y, et al. Laparoscopic proximal gastrectomy maintains body weight and skeletal muscle better than total gastrectomy. World J Surg. (2018) 42(10):3270–6. doi: 10.1007/s00268-018-4625-7
24. Asaoka R, Irino T, Makuuchi R, Tanizawa Y, Bando E, Kawamura T, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. (2019) 89(1–2):79–83. doi: 10.1111/ans.15023
25. Benedetto U, Stuart J, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. (2018) 53(6):1112–7. doi: 10.1093/ejcts/ezy167
26. Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. (2017) 20(Suppl 1):69–83. doi: 10.1007/s10120-016-0663-8
27. Lee J, Kim YM, Woo YH, Obama K, Noh SH, Hyung WJ, et al. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg Endosc. (2015) 29(11):3251–60. doi: 10.1007/s00464-015-4069-1
28. Shen W, Xi HQ, Wei B, Cui JX, Bian SB, Zhang KC, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. (2016) 30(2):574–80. doi: 10.1007/s00464-015-4241-7
29. Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. (2009) 249(6):927–32. doi: 10.1097/01.sla.0000351688.64999.73
30. Tanaka C, Kanda M, Murotani K, Yoshikawa T, Cho H, Ito Y, et al. Long-term quality of life and nutrition status of the aboral pouch reconstruction after total gastrectomy for gastric cancer: a prospective multicenter observational study (CCOG1505). Gastric Cancer. (2019) 22(3):607–16. doi: 10.1007/s10120-018-0893-z
31. Tang T, Peng WH, Zhang LY, Zuo ZK, Cao D, Huang JS, et al. Effectiveness and safety of total laparoscopic distal gastrectomy versus laparoscopy-assisted distal gastrectomy for gastric cancer: a retrospective cohort study. Am J Surg. (2018) 216(3):528–33. doi: 10.1016/j.amjsurg.2018.05.005
32. Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R, et al. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. (2011) 25(5):1395–401. doi: 10.1007/s00464-010-1402-6
33. Japanese gastric cancer treatment guidelines 2018 (5th edition). Japanese Gastric Cancer Association. (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
34. Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer. (2020) 28(1):373–80. doi: 10.1007/s00520-019-04803-3
35. Kim HI, ParkbK MS, Songc J, WoodW Y, Hyungae J. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. (2014) 40(10):1346–54. doi: 10.1016/j.ejso.2013.09.011
36. Jaulim A, Srinivasan A, Hori S, Kumar N, Warren AY, Shah NC, et al. A comparison of operative and margin outcomes from surgeon learning curves in robot assisted radical prostatectomy in a changing referral practice. Ann R Coll Surg Engl. (2018) 100(3):226–9. doi: 10.1308/rcsann.2018.0001
37. Wang Y, Wang GH, Li Z, Ling H, Yi B, Zhu SH, et al. Comparison of the operative outcomes and learning curves between laparoscopic and “Micro Hand S” robot-assisted total mesorectal excision for rectal cancer: a retrospective study. BMC Gastroenterol. (2021) 21(1):251. doi: 10.1186/s12876-021-01834-1
38. Suda K, Man-I M, Ishida Y, Kawamura Y, Satoh S, Uyama I, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. (2015) 29(3):673–85. doi: 10.1007/s00464-014-3718-0
Keywords: upper third gastric cancer, robotic surgery, laparoscopic surgery, lymph node dissection, quality of life
Citation: Fu J, Li Y, Liu X, Jiao X, Qu H, Wang Y and Niu Z (2023) Effects of robotic and laparoscopic-assisted surgery on lymph node dissection and quality of life in the upper third of gastric cancer: A retrospective cohort study based on propensity score matching. Front. Surg. 9:1057496. doi: 10.3389/fsurg.2022.1057496
Received: 29 September 2022; Accepted: 21 November 2022;
Published: 4 January 2023.
Edited by:
He Liu, Jilin University, ChinaReviewed by:
Paolo Aurello, Sapienza University of Rome, ItalyWeijie Xue, Kumamoto University Hospital, Japan
Zhiming Ma, Jilin University, China
© 2023 Fu, Li, Liu, Jiao, Qu, Wang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaojian Niu cWRmeW56akBxZHUuZWR1LmNu
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Jingxiao Fu
Jingxiao Fu Zhaojian Niu
Zhaojian Niu