- Department of Gastrointestinal Surgery, Affiliated Hospital of Qingdao University, QingDao, China
Background: Proximal gastrectomy with gastric tubular reconstruction is a surgical procedure that can preserve function in patients with proximal gastric cancer. However, whether gastric tubular reconstruction with proximal gastrectomy has certain advantage in some aspects over total gastrectomy is controversial. To evaluate the benefit of gastric tubular reconstruction after proximal gastrectomy, we compared gastric tubular reconstruction with total gastrectomy for proximal gastric cancer.
Method: A total of 351 patients were enrolled. Concurrent total gastrectomy patients matched with the Proximal gastrectomy group in age, sex, body mass index, clinical stage, and ASA score were selected by propensity score matching. Preoperative basic information, perioperative indicators, histopathological features, postoperative complications and nutritional status, reflux were compared between the two groups.
Results: There was no significant difference in the incidence of reflux between two groups (14.8% and 6.5% respectively, P = 0.085). There were significant differences between the two groups in bowel function recovery (2.29 ± 1.16 vs. 3.01 ± 1.22; P = 0.039) and start of soft diet (4.06 ± 1.81 vs. 4.76 ± 1.69; P = 0.047). There were no significant differences between the two groups in nutritional status one year after surgery. However, the decrease in serum hemoglobin in the TG group at 3 and 6 months after surgery was significantly higher than that in the PG group (P = 0.032 and 0.046, respectively). One month after surgery, %BW loss in TG group was significantly lower than that in the PG group (P = 0.024).
Conclusion: The Proximal gastrectomy group has better clinical outcome and gastric tubular reconstruction is simple, similar complications and reflux rates, gastric tubular reconstruction may be more suitable for proximal gastric cancer.
Introduction
The incidence of proximal gastric cancer is increasing worldwide (1–3). Proximal gastric cancer can occur in the upper 1/3 of the stomach or in the middle part of stomach; it constitutes more than 30% of gastric cancer cases. According to the Japan Gastric Cancer Association, the incidence of proximal gastric cancer increased by 0.8% from 2002 to 2011 (4). According to the 5th edition of the Japanese Guidelines for the Treatment of Gastric Cancer, total gastrectomy is the standard surgical treatment for upper 1/3 gastric cancer or AEG (5). The advantage of this surgical approach lies in the thorough dissection of lymph nodes that may metastasize and the avoidance of esophagogastric reflux complications. However, after total gastrectomy, patients will inevitably have nutritional metabolic disorders, especially patients with early proximal gastric cancer, which are more prominent (6–8). After total gastrectomy, the storage, mechanical grinding, secretion and other functions of the stomach are permanently lost, resulting in postoperative malnutrition, including decreased postoperative body mass, anemia, diarrhea, and dumping syndrome (8, 9).
Proximal gastrectomy of antireflux anatomical structures at the esophagogastric junction, including the cardia and His Angle, with simultaneous separation of the vagus nerve, resulted in an increased incidence of pyloric spasm and obstruction of residual stomach emptying (10). Some patients developed reflux esophagitis after surgery, which seriously affected the quality of life. Studies have shown that the incidence of postoperative reflux esophagitis is approximately 50% when traditional esophagogastrostomy is used in proximal gastrectomy (11, 12). To prevent RE, several reconstructive procedures after PG have been reported, such as double-flap (13), double-tract (14, 15), and jejunal interposition (16). However, these techniques are complicated, time-consuming and sometimes unsatisfactory.
Esophagogastrostomy was described by Shiraishi et al. in 1998 for the treatment of early proximal gastric cancer (17). This method excises part of the gastric antrum, reduces gastrin and gastric acid secretion, and objectively reduces reflux substances. After anastomosis, the tube stomach can make food pass through quickly and avoid food retention. The top of the residual stomach is similar to the fundus of the stomach, which can buffer the upward reflux of gastric juice and temporarily store the reflux of gastric juice. Therefore, the operation method has a good antireflux effect. Some research results show that compared with traditional residual gastroesophageal anastomosis, esophageal gastric tube anastomosis has a better quality of life for patients (18).
The purpose of this study was to determine whether gastric tubular reconstruction is a viable option after PG in terms of postoperative reflux and some nutritional indicators. We conducted a retrospective matched cohort study comparing the effects of gastric tubular reconstruction and total gastrectomy on patients with proximal gastric cancer.
Methods
All patients with upper one-third gastric cancer consecutively received surgical treatment in the Gastrointestinal Surgery Department of Qingdao University Affiliated Hospital from January 2017 to February 2021. Upper third gastric cancer is defined as adenocarcinoma of the upper third of the stomach, with or without esophagogastric junction adenocarcinoma, according to the Classification of the Japan Gastric Cancer Association (JGCA) (19). The location of the primary carcinoma was determined by esophagogastroscopy. Inclusion criteria were that all patients had tumors located in the upper third of the stomach, clinical stage (CT1N0-1M0/CT2-3N0M0), R0 resection, and age 20–80. Exclusion criteria were neoadjuvant therapy, any malformation or ulcerative scarring of the distal stomach or duodenum, severe heart, lung, liver, kidney disease or mental abnormalities, and double primary carcinoma.
Patients were divided into two groups based on whether they underwent total or proximal gastrectomy. All patients underwent R0 resection. Patients underwent propensity score matching analysis, which adjusted for five factors, namely, age, sex, body mass index (BMI), pathological stage, and American Society of Anesthesiologists (ASA) body condition score, to offset selection bias. The matched whole stomach group was compared with the proximal stomach group based on demographic, clinical, surgical, and pathological features, postoperative outcomes (including early and late complications), postoperative nutritional status, and reflux esophagitis and reflux symptoms at endoscopic examination 1 year after surgery.
Preoperative tumor staging was assessed by computed tomography and gastroscopy. T stage and N stage were determined using the latest AJCC/UICC TNM staging system (20), and histological types were consistent with the Japanese classification of gastric cancer (19).
Surgical procedure for PG with gastric tubular reconstruction and TG
All surgeries were performed by three upper gastrointestinal specialists using the same procedure (Figures 1, 2).
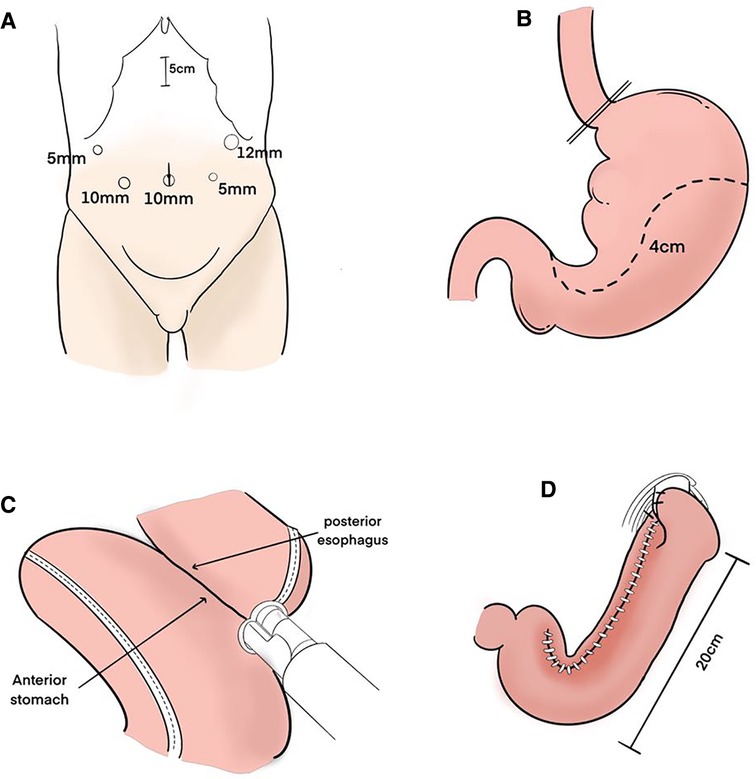
Figure 1. Surgical procedure. (A) Trocar sites. (B) After the esophagus was transected, the upper part of the stomach was excised along a dashed line to create a gastric tube. Its width is about 4 cm. (C) The anterior wall of the gastric tube and the posterior wall of the esophagus were anastomosed with linear stapler. (D) After anastomosis was performed, the entry hole was closed with barbed suture. The gastric tube was anchored with the right and left crus of the diaphragm by one stitch each to prevent hiatus hernia. The length of the whole gastric tube on the greater curvature side is about 20 cm.
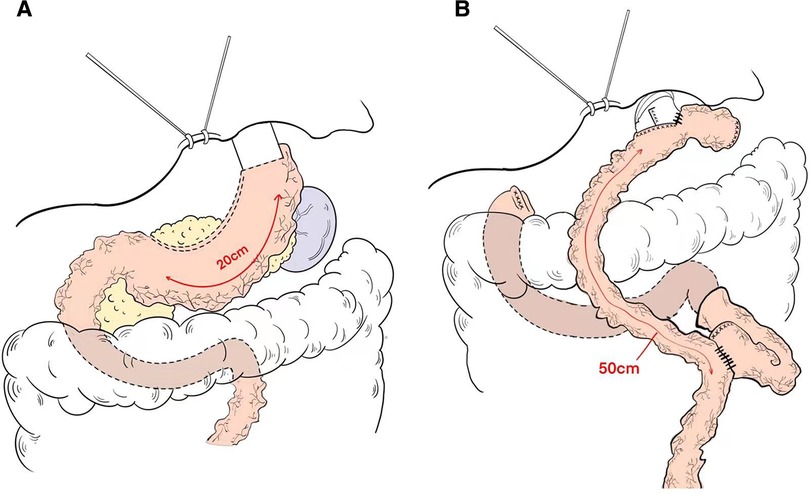
Figure 2. Schema of reconstruction after (A) proximal gastrectomy with tube gastric and (B) total gastrectomy with roux-en-Y esophagojejunostomy reconstruction.
Five ports were introduced, as shown in Figure 1A. Before reconstruction, lymph node dissection was completed according to the Japanese guidelines (5). Open the diaphragm angle, and bare the lower end of the esophagus by 2–3 cm. The esophagus was transected about the cardia with an endo GIA stapler. A 5 cm incision was made in the middle of the upper abdomen, and the stomach was extruded through it. The stomach was marked with a dotted line using a sterile marker and ruler. Approximately 5 cm below the tumor, the upper part of the stomach was excised along a dashed line to make a gastric tube (Figure 1B). The pneumoperitoneum was re-established. An entry hole was made on the posterior side of the esophagus stump and on the anterior wall of the gastric tube 40 mm distal from the proximal stump laparoscopically. Then, the linear stapler was applied between the anterior wall of the gastric tube and the posterior wall of the esophagus laparoscopically (Figure 1C). After anastomosis was performed, the entrance hole was enclosed with stitches. The gastric tube was anchored with the right and left crus of the diaphragm by one stitch each to prevent hiatus hernia (Figure 1D). The vagus nerve was not preserved.
The length of the gastric tube is generally approximately 20 cm and the width is approximately 4 cm, which indicates: (1) The size of the residual stomach was correlated with gastric emptying; the larger the stomach, the worse the emptying; (2) Residual gastric emptying is related to gravity, intragastric pressure and residual gastric compliance; (3) The smaller the gastric tube, the worse its compliance is and the less likely it is to have reflux. The gastric tube after anastomosis with the esophagus is shown in (Figure 2A).
Reconstruction technique of esophagojejunostomy, the jejunum was transected at a distance of 20 cm from the Treus ligament, and a tube stapler was placed at a distance of 50 cm from the distal end by purse-string suture. The jejunum was anastomosed laterally through the proximal jejunum, and the stump was closed by GIA stapler, and the absorbable suture was embedded. The pneumoperitoneum was re-established, and the esophagus and the distal jejunum were punctured. The lateral jejunum anastomosis of the posterior esophageal wall was completed by GIA stapler, and the common opening was closed by continuous suture of the barb line. Esophagojejunostomy (Roux-en-Y) was performed after total gastrectomy (Figure 2B).
Data collection and evaluation of outcomes
Patients are enrolled in this study, three data management staff members will be assigned to collect relevant data. The basic characteristics of patients collected before surgery were age, sex, body mass index, ASA score, hematologic indices, serum tumor markers, FEV1 and Her2 expression. The clinicopathological features were TNM stage, tumor size and location, Lauren classification, and tumor cell differentiation. The operation was characterized by operative time, estimated blood loss, pathological proximal and distal margins, and number of lymph nodes removed. Postoperative outcomes were mean maximum body temperature during the first 3 days, analgesic use 1–5 days after surgery, days of bowel function recovery, time to start soft diet, postoperative hospital stay, and early complications (within 30 days after surgery). Patients were evaluated 1, 3, 6 and 12 months after operation, and their characteristics and results were obtained by viewing electronic medical records and picture archiving and communication systems. Postoperative morbidity was described based on the Clavien-Dindo classification of JOCG criteria for postoperative complications and according to the General Terminology criteria for Adverse Events (CTCAE 5.0) (21–23). Clinical features and nutritional status 1 year after operation were evaluated by PG-SGA (24).
Follow-up results 1 year postoperatively were based on the Visick classification (Grade i: asymptomatic; Grade ii: mild symptoms without medication; Grade iii: mild symptoms that are easily controlled by medication; Grade iiii: severe symptoms, the duration or surgery) assessment of reflux symptoms and endoscopic findings were scored in terms of the Los Angeles Classification of reflux esophagitis (25). The Visick score and endoscopy results were obtained in the clinic. All endoscopy results were graded by the same surgeon according to the Los Angeles Classification System.
Nutritional parameters after gastrectomy were assessed on the basis of changes in serum prealbumin, albumin, hemoglobin, prognostic nutritional index (PNI) and the percentage of BW loss (%BW loss) at 1, 3, 6, and 12 months after surgery (26). The percentage of BW loss (%BW loss) was calculated as follows: %BW loss = (BW at 1/3/6/12 months after surgery−preoperative BW)/(preoperative BW × 100). PNI was calculated using the following formula: 10 × serum albumin value (g/dl) + 0.005 × lymphocyte count in peripheral blood (27). On the CT images, the cross-sectional area of the psoas muscle was measured at the level of the third lumbar vertebra (L3). Psoas muscle index (PMI) = (Area of the psoas muscle at L3 [cm]2)/(height[m]2). %PMI loss was all defined in the same way as %BW loss (28, 29).
Statistical analysis
Propensity score matching was based on gender, age, body mass index, clinical stage, and ASA score. Continuous variables of normal distribution were expressed by (X ± S), and comparison between two groups was compared by T test. Non-normally distributed continuous variables were represented by median (range), and comparisons between the two pairs were performed using Wilcoxon signed rank test. The categorical variables were expressed in terms of number of cases and percentage, and comparisons between the two groups were performed by Chi-square test or Fisher precise test. All analyses were performed using SPSS 26.0. (P < 0.05) was considered statistically significant.
Ethics statement
The data for this study were collected in the course of general clinical practice, so informed consent signed by each patient was obtained for any surgical and clinical procedure. This protocol is in line with the ethical guidelines of the World Medical Association Declaration of Helsinki adopted by the 18th World Medical Association Congress held in Helsinki, Finland in June 1964. Institutional Review board approval is not required. Since this study was retrospective, patients’ consent was not required for inclusion in the study.
Results
Patient characteristics
A total of 1,631 gastric cancer patients underwent surgery (Figure 1). Of those, 1,178 were excluded because the tumor was in the middle or lower part of the stomach. Of the remaining 453 patients, patients with advanced cancer (n = 51), neoadjuvant chemotherapy (n = 48) and dual primary cancer (n = 3). 59 patients received proximal gastrectomy and 292 patients received total gastrectomy. After 1:2 matching between the PG group and TG group, there were 54 patients in the PG group and 108 patients in the TG group (Figure 3 and Table 1).
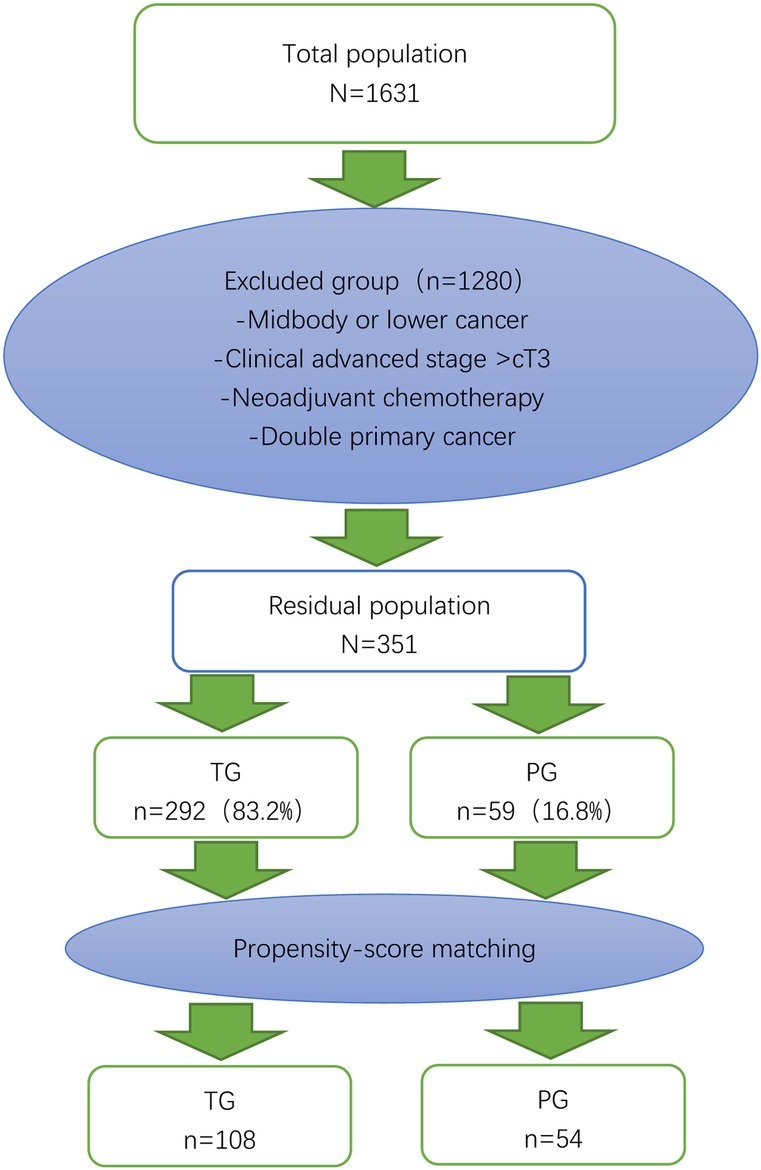
Figure 3. Flow chart of patient selection and propensity score matching. The 54 patients who underwent proximal gastrectomy (PG) were matched to 108 patients who underwent total gastrectomy (TG) in terms of age, sex, body mass index, clinical stage, and American Society of Anesthesiologists (ASA) score.
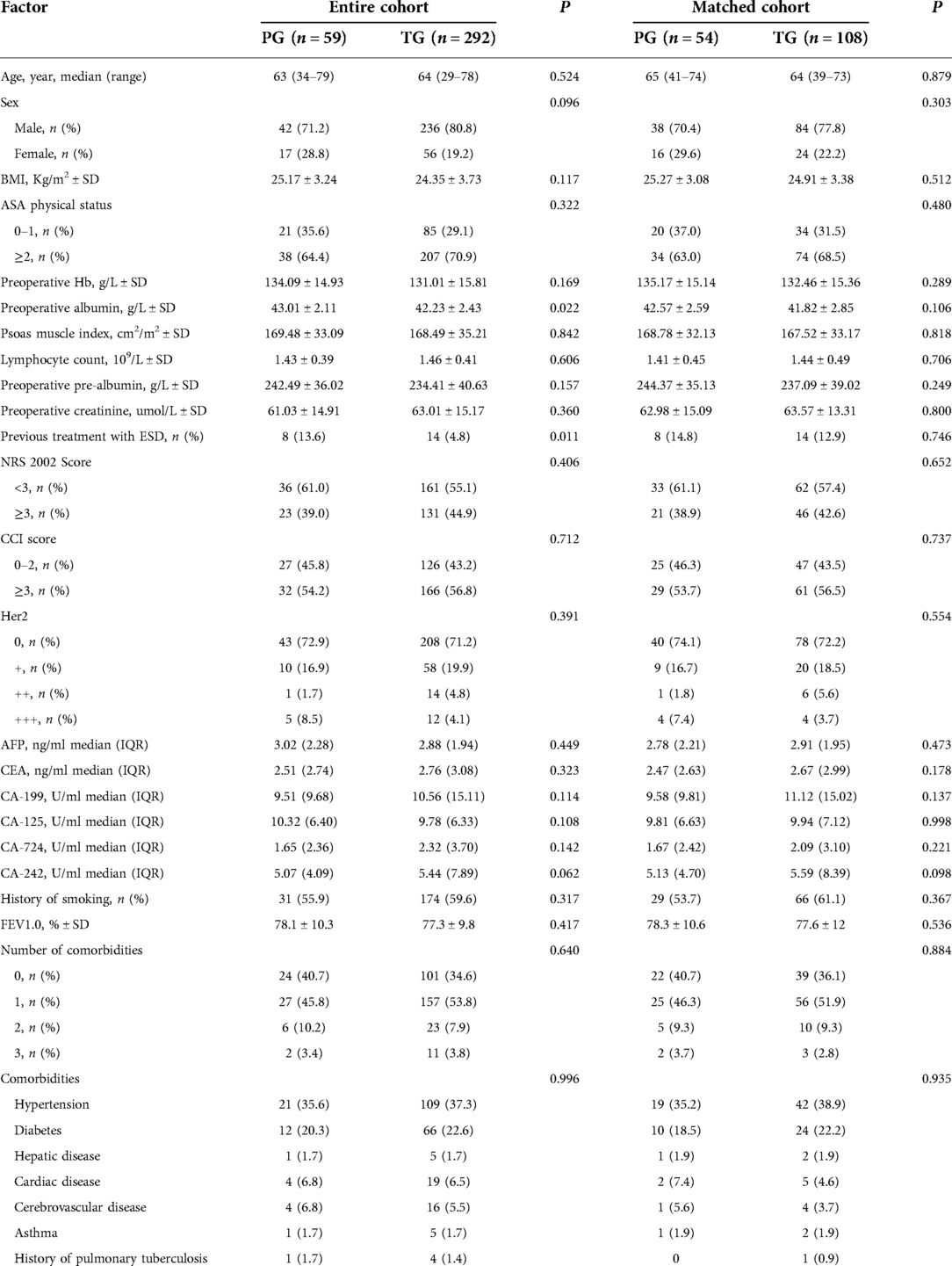
The basic clinical characteristics of patients in the two groups were shown in Table 1. In the whole cohort, the number of patients who underwent ESD before surgery in the PG group was significantly greater than that in the TG group (P = 0.011), and there was no significant difference in preoperative hematological nutritional indicators, tumor markers or complications. The matched baseline features are well balanced.
Perioperative clinical outcomes
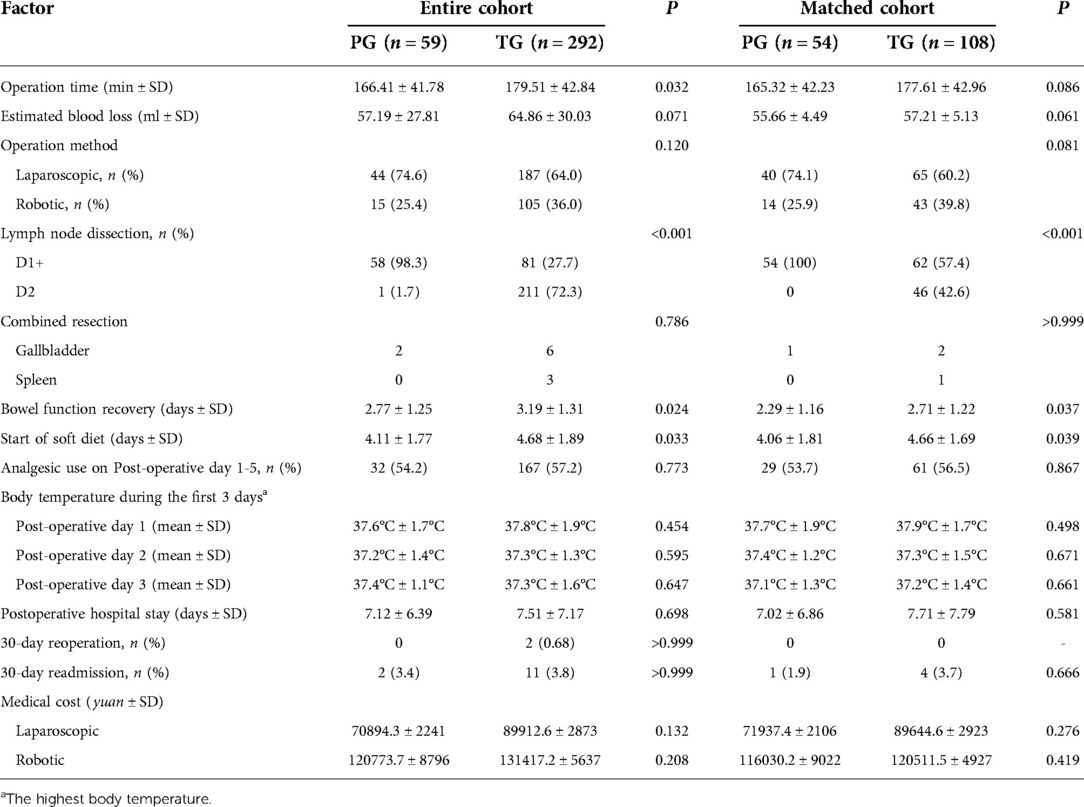
In the entire cohort, the two groups were on a liquid diet (4.11 ± 1.77 in PG vs. 4.91 ± 1.89; P = 0.032) and showed bowel function recovery (2.77 ± 1.25 in PG vs. 3.44 ± 1.62 in TG; P = 0.024). The blood loss and operation time in the PG group were lower than those in TG group, but there were no significant differences (P > 0.05). There was no statistically significant difference in medical cost or 30-day readmission between the two groups (P > 0.05). After propensity score matching, the start of a soft diet after the operation in the PG group was 0.7 days sooner than in the TG group (4.06 ± 1.81 vs. 4.76 ± 1.69; P = 0.047), and the recovery time of bowel function in the PG group was 0.72 days shorter than in the TG group (2.29 ± 1.16 vs. 3.01 ± 1.22; P = 0.039). In the matched cohort, there were no statistically significant differences in the length of postoperative hospital stay, the average maximum body temperature in the first three days after surgery, and the number of patients using analgesics 1–5 days after surgery (P > 0.05) (Table 2).
Histopathologic characteristic
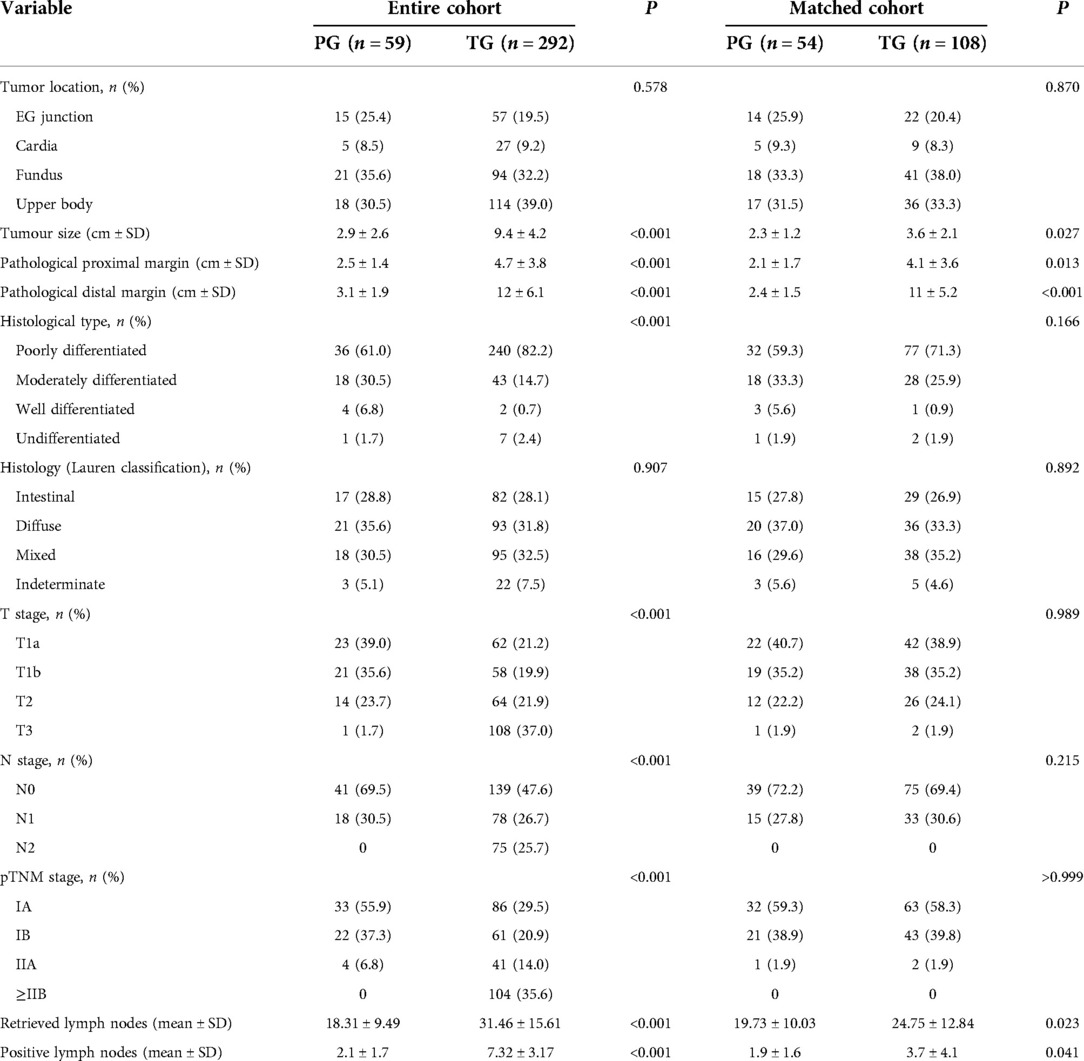
Tumor size (2.9 ± 2.6 vs. 9.4 ± 4.2), proximal resection margin (2.5 ± 1.4 vs. 4.7 ± 3.8), distal resection margin (3.1 ± 1.9 vs. 12 ± 6.1) and number of dissected lymph nodes (18.31 ± 9.49 vs. 31.46 ± 15.61) were measured in the two groups, and the number of positive lymph nodes (2.1 ± 1.7 vs. 7.32 ± 3.17), pTNM stage and histological type were significantly different (P < 0.001). Notably, the TG group accounted for 82.2% of the histological type of poorly differentiated adenocarcinoma. There were no significant differences in tumor location or Lauren classification between the two groups (P > 0.05). After propensity matching, there was no significant difference in pTNM staging between the two groups (P > 0.05), and tumors in the PG group were significantly smaller than those in the TG group (2.3 ± 1.2 vs. 3.6 ± 2.1; P = 0.027). The proximal margin was smaller (2.1 ± 1.7 vs. 4.1 ± 3.6; P = 0.013), lymph nodes were removed (19.73 ± 10.03 vs. 24.75 ± 12.84; P = 0.023) and fewer were positive (1.9 ± 1.6 vs. 3.7 ± 4.1; P = 0.041), and all differences were statistically significant (all P < 0.05) (Table 3).
Operative complications and adverse events
Across the cohort, the severity of complications was classified by Clavien-Dindo and CTCAE version 5.0 classification. The number of early complications (i.e., complications occurring in the first 30 days after surgery) in both groups (PG group 12 vs. TG group 64; P = 0.788) and hematological adverse events (36 in PG group vs. 188 in TG group; P = 0.624) were evaluated. Postoperative reflux occurred in 6 patients in the PG group and 13 patients in the TG group (P = 0.076), and severe complications (grade iii or above) occurred in 3 patients in the PG group and 13 patients in the TG group. Among the hematological serious adverse events, there were 2 cases in the PG group and 11 cases in the TG group (grades 3–4). After matching, the number of complications in the two groups was 9 cases and 21 cases respectively (P = 0.668). Among the number of reflux cases, there were 6 cases in the PG group and 4 cases in the TG group (P = 0.133), showing no statistical significance. The number of adverse events was 32 cases and 71 cases (P = 0.419), among which, the number of anemia cases was 10 cases and 26 cases (P = 0.423), respectively, showing no statistically significant differences (Table 4).
Clinical manifestations and nutritional status
There was no significant difference in clinical characteristics between the two groups 1 year after the operation. Overall, 26 patients in the PG group and 43 patients in the TG group (P = 0.312) reported no dietary problems. Reflux was present in 6 patients in the PG group and 3 patients in the TG group (P = 0.069), and there was no significant difference. In terms of nutrition score, although there was no statistical significance in different degrees of malnutrition between the two groups (P = 0.406), the PG group included a large proportion of mild malnutrition patients: 21 (38.9%) in the PG group and 33 (30.6%) in the TG group. Similarly, in the severe malnutrition patients, 2 (3.7%) were in the PG group. There were 9 patients in the TG group (8.3%) and a relatively small number in the PG group (Table 5).
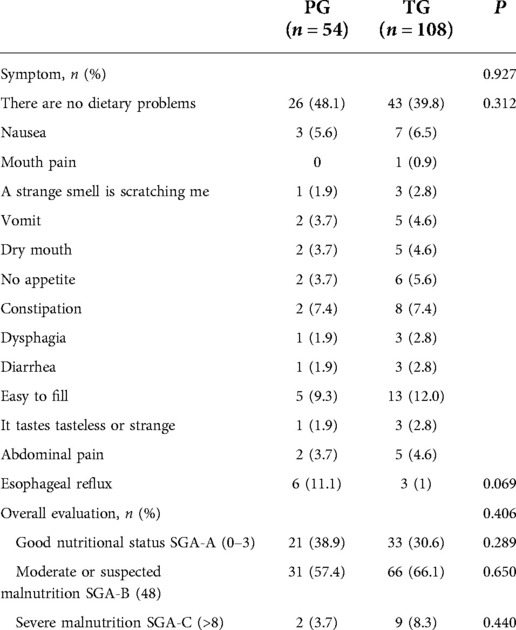
Table 5. Comparison of postoperative clinical manifestations and nutritional score by PG-SGA between PG and TG 1 year after surgery.
We assessed the rate of weight loss, the rate of Psoas muscle index loss, and changes in nutritional parameters in 162 patients followed for at least 1 year (Figure 4). The annual decrease in serum hemoglobin in the TG group was greater than that in the PG group, and the decrease in serum hemoglobin in the TG group at 3 and 6 months after surgery was significantly higher than that in the PG group (P = 0.032 and 0.046, respectively), There was no significant difference between the two groups at 1 and 12 months after surgery (P = 0.131 and P = 0.072, respectively). Regarding PNI, there was no significant difference between the PG group and the TG group (P > 0.05). However, the PNI decline in the TG group was always higher than that in the PG group after surgery. Although the TG group recovered faster from 1 to 3 months, the curves between the two groups have no intersection. Serum prealbumin levels in both groups were not significantly different at any time point (P > 0.05), but the TG group recovered faster at 3 to 6 months after surgery, and the levels in the two groups were almost the same at 12 months. Like albumin, there was no statistical significance at any time point, and the trend of change was not exactly the same as that of prealbumin. One month after surgery, %BW loss in TG group was significantly lower than that in the PG group (P = 0.024), and 6 months after surgery, %BW loss in the TG group was higher than that in the PG group. No significant differences were observed at any time point in %PMI loss between the two groups. No patients in either group died or relapsed during one year of follow-up.
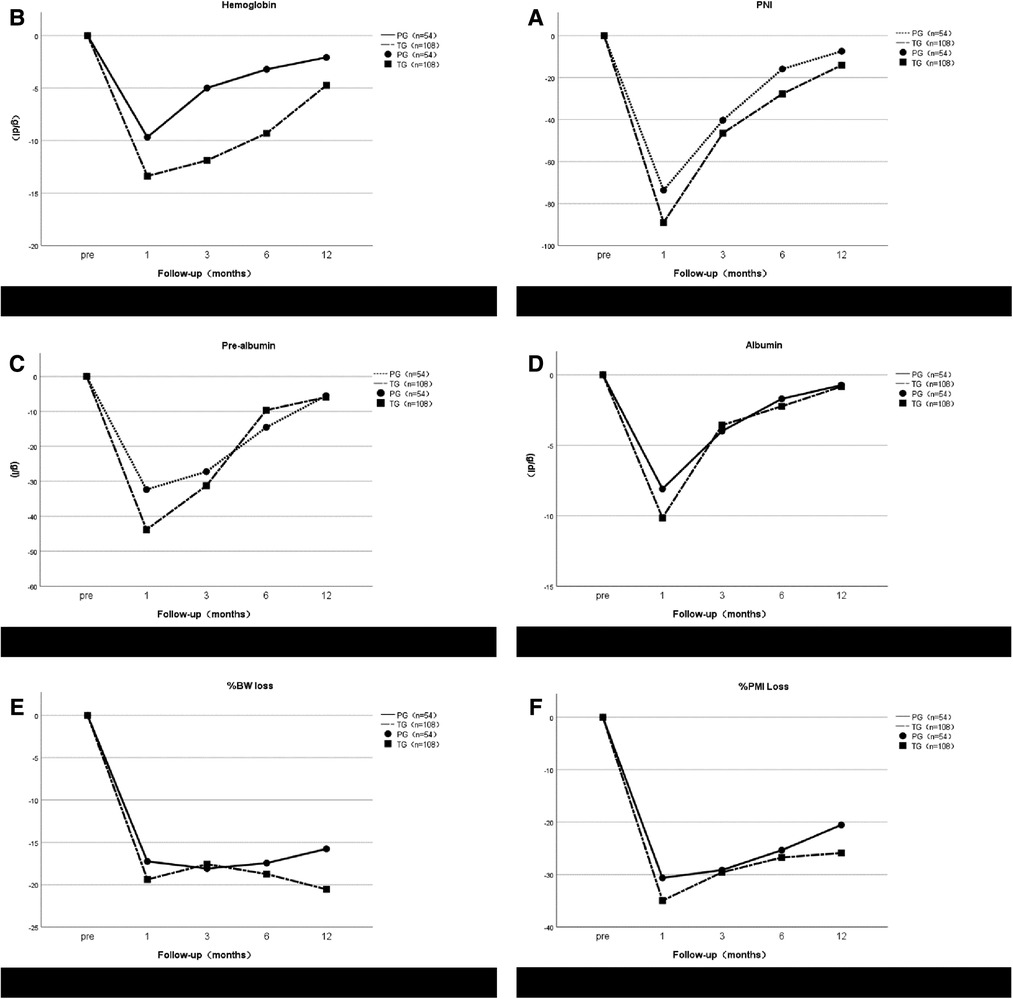
Figure 4. Postoperative changes of prognostic nutritional index (PNI) (A), hemoglobin (B), pre-albumin (C), albumin (D), %BW loss (E) and %PMI loss (F) in the proximal gastrectomy (PG) group and total gastrectomy (TG) group. All postoperative data are represented as values (mean ± standard error) relative to preoperative.
Reflux symptom and endoscopic findings
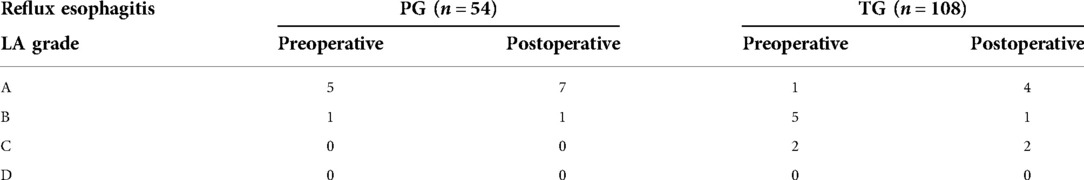
The patients will be graded according to the Visick score 1 year after operation, Visick score of reflux symptoms showed that there were 6 patients (11.1%) in the PG group and 3 patients (2.8%) in the TG group with grade ii or higher reflux symptoms (P = 0.069), which was not statistically significant. All patients underwent endoscopy approximately 1 year after surgery. These endoscopic findings were scored against the Los Angeles classification of reflux esophagitis and the results of preoperative endoscopy. In the PG group, 5 patients had grade A reflux esophagitis and 1 patient had grade B reflux esophagitis before surgery. After surgery, 7 patients developed grade A reflux esophagitis and 1 patient developed grade B reflux. In the TG group, grade A, B, and C reflux esophagitis occurred preoperatively in 1, 5, and 2 patients, respectively. After surgery, 4, 1, and 2 patients had grade A, B, and C reflux esophagitis, respectively (Tables 6, 7). After surgery, 8 patients (14.8%) in the PG group and 7 patients (6.5%) in the TG group had ≥A reflux (P = 0.085), showing no statistical significance.
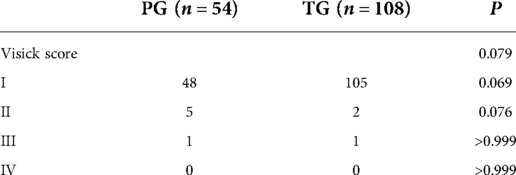
Table 6. Reflux symptom scores 1 year after surgery in the propensity score-matched patients who underwent proximal or total gastrectomy.
Discussion
Proximal gastrectomy was introduced to improve patient performance status by conserving half of the stomach; thus, it is widely believed that proximal gastrectomy reduces postoperative weight loss. In addition, PG in the upper third of the stomach was believed to be appropriate in terms of both its radicality and safety (30, 31).
Our study showed that PG gastric tubular reconstruction had the advantage of less postoperative anemia and less %BW loss than TG. This result is consistent with previous reports (9, 32). Some studies have reported that PG with double-tract reconstruction does not have any advantages for postoperative anemia (28, 33, 34). Our data showed no significant difference in total protein and serum prealbumin. This result was consistent with previous reports (9, 35, 36). Based on its safety and simplicity, we believe that gastric tubular reconstruction can be a viable option after proximal PG. The use of a gastric tube provides a simple and safe anastomosis for PG because it is a single anastomosis. Kitano et al. introduced a reconstruction method using a gastric tube after PG (37). The authors indicated that the technique was simple and less invasive for EGC in the upper third of the stomach and evaluate the effectiveness of gastric tubular reconstruction to prevent reflux after open PG (38).
In 2017, Toyomasu reported that gastric tubular reconstruction has advantages, including being less invasive compared to jejunal interposition, shorter surgical duration, less surgical blood loss, and maintenance of postoperative nutritional status (39). Some previous reports showed different types of complications of PG. RE is common complication after PG. In the present study, RE with symptoms was diagnosed in 6 (11.1%) of 54 patients. This result was almost compatible with previous reports (40–43). But the rate of RE (≥Los Angeles grade A) has been reported to be over 30% (36, 44). Chen et al. (40) reported that only 14.3% of patients showed reflux symptoms after tube gastric anastomosis, and 57% of patients exhibited reflux esophagitis. Compared with traditional esophagogastric anastomosis, this method has obvious antireflux advantages. Aihara et al. (45) showed that 14% of patients had reflux symptoms after tube gastric anastomosis, while the incidence of anastomotic stenosis was 35%. Ronellenfitsch et al. (46) demonstrated that the incidence of reflux symptoms was 21.4% early (1–6 months) after esophagogastrostomy and 33.3% long (>6 months) after esophagogastrostomy. However, the symptoms were mild. Endoscopic examination results revealed that 29% of patients had esophagitis, and only 2 of them had reflux symptoms. Another study reported that after 3 weeks to 1 year follow-up, gastric tube anastomosis in patients with reflux symptoms was lighter than traditional residual stomach esophagus anastomosis; however, after 2–10 years of follow-up, there was no statistically significant difference the rate of reflux symptoms in patients with in gastric tube esophagus anastomosis compared with traditional residual stomach esophagus anastomosis (47).
The reflux symptoms of all patients in this study were graded 1 year after surgery using the Visick score. In total, nine patients had grade II reflux symptoms. Notably, the PG patient with Visick grade II reflux did not exhibit signs of reflux esophagitis on endoscopy 1 year after surgery: the Los Angeles scores were both grade 0. In contrast, the fifteen patients who exhibited reflux esophagitis on endoscopy 1 year after surgery (their grades ranged from A to C) all had Visick grade I scores. Thus, reflux symptoms did not correlate well with endoscopic findings. Several other studies have also reported this, both in patients who underwent gastrectomy and in patients with gastroesophageal reflux disease (46, 48). This may reflect differences between individuals in terms of sensitivity to subjective symptoms. Further prospective studies on the relationship between reflux symptoms and reflux esophagitis on endoscopy are needed.
Recently, several useful assessment scales and questionnaires have been developed to measure the subjective reflux symptoms of patients. They include Post gastrectomy Syndrome Assessment Scale (PGSAS-45), Functional Assessment of Cancer Therapy-Gastric (FACT-Ga), and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Gastric Cancer (EORTC QLQ-STO22 and EORTC QLQ-C30). Future studies comparing surgical modalities for EGC should use these tools to assess the postoperative functional benefits of each modality (8, 49, 50).
Multiple studies have described the advantages of PG for treating this cancer. However, to date, few studies have assessed the usefulness of PG with gastric tube placement. In particular, the oncological safety of PG with gastric tube placement remains unclear due to the lack of long-term studies. For PG with gastric tube to become the standard surgical option for early proximal gastric cancer, it must be as oncologically safe as TG, offer a functional benefit, and be associated with minimal postoperative complications.
The oncological safety of proximal gastrectomy mainly involves the preservation of the supratropyloric, supratropyloric, distal lesser curvature of the stomach, and lymph nodes along the right perivascular gastroomentum. Studies have shown that there is no statistically significant difference in the overall postoperative survival rate between patients undergoing total gastrectomy and patients undergoing proximal gastrectomy for early upper gastric cancer (51). Therefore, the clinical oncology safety of proximal gastrectomy for early upper gastric cancer is not controversial, but for advanced upper gastric cancer, the oncology safety of proximal gastrectomy is still controversial. Oncology safety depends primarily on the impact of lymph node preservation on patient survival.
Yamashita et al. (52) showed that for esophageal and gastric junction carcinoma with tumor length <4 cm, the lymph node metastasis rates of Groups 4sa, 4sb, 4d, 5 and 6 were extremely low and were independent of tumor location and T stage. The results of a separate study of 202 patients with stage T2 and T3 proximal gastric cancer undergoing proximal gastrectomy demonstrated that the lymph node metastasis rates of Groups 4sa, 4sb, 4d, 5, 6, 8a and 12a were 3.47%, 1.49%, 0.99%, 0.00%, 0.00%, 2.02% and 0.006%, respectively, and the overall 5-year survival rate of the patients was 72.9%. Proximal gastrectomy was recommended for the treatment of T2 and T3 proximal gastric cancer (53). However, no prospective randomized controlled studies have been conducted on the long-term outcomes of total gastrectomy and proximal gastrectomy for locally advanced upper gastric cancer, and the oncological safety of proximal gastrectomy needs further clinical evidence.
There are several limitations to this study. First, the small number of patients in the cohort and the retrospective design of this study made the evidence of retrospective analysis less reliable than that of randomized controlled trials. These limitations were offset using propensity score matching to select a TG group that matched the PG group in terms of important baseline features. Even though propensity score matching was used, there is a selection bias according to the preference of the surgeon. However, surgery performed during the same period and the proficiency of the surgical method are not different. Second, the lack of long-term follow-up of nutritional status, assessment of patients’ long-term quality of life, and oncology outcomes largely limit the true benefits of PG. Third, we did not compare surgical methods in terms of postoperative VitB12 and serum iron, and anemia is a common complication of gastrectomy (54). Future studies comparing PG with tube gastric versus TG should examine postoperative iron panel blood test results. These studies should also determine the nutritional benefit of the surgical modalities by assessing the postoperative lipid profile. Finally, different reflux-scoring tools are needed to determine the effect of the surgeries on reflux symptoms.
Conclusion
In this study, proximal gastrectomy with tubular gastrostomy was superior in clinical outcome to Roux-en-Y reconstruction for gastric cancer. These results suggest that PG combined with gastric tube anastomosis may be an appropriate surgical option for proximal gastric cancer. However, there is no standard procedure for early upper gastric cancer, and only prospective randomized trials in the future will clarify the true benefits of one procedure over another.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZN made substantial contributions to conception and design for this study, YL and XJ acquired and analyzed data and JF drafted the article. HQ and YW gave many important suggestions for this study. ZN and XL participated in interpreting critically for important intellectual results. ZN gave final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank ZN and XL for their critical reading and informative advice during the process of study. Meanwhile, we would like to thank AJE (www.aje.cn) for English language editing. We thank the Department of gastrointestinal surgery, Affiliated Hospital of Qingdao University, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. (2008) 23(11):1662–5. doi: 10.1111/j.1440-1746.2008.05572.x
2. Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. (2013) 23(1):3–9. doi: 10.1016/j.semradonc.2012.09.008
3. Information Committee of Korean Gastric Cancer Association. Korean gastric cancer association nationwide survey on gastric cancer in 2014. J Gastric Cancer. (2016) 16(3):131–40. doi: 10.5230/jgc.2016.16.3.131
4. Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988-2012: a single-institution, high-volume experience in China. Ann Surg. (2016) 263(1):88–95. doi: 10.1097/SLA.0000000000001148
5. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2021) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
6. Fujiya K, Kawamura T, Omae K, Makuuchi R, Irino T, Tokunaga M, et al. Impact of malnutrition after gastrectomy for gastric cancer on long-term survival. Ann Surg Oncol. (2018) 25(4):974–83. doi: 10.1245/s10434-018-6342-8
7. Rosa F, Quero G, Fiorillo C, Bissolati M, Cipollari C, Rausei S, et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (on behalf of the Italian research group for gastric cancer-GIRCG). Gastric Cancer. (2018) 21(5):845–52. doi: 10.1007/s10120-018-0804-3
8. Takiguchi N, Takahashi N, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. (2015) 18(2):407–16. doi: 10.1007/s10120-014-0377-8
9. Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, et al. Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer. (2014) 17(1):141–5. doi: 10.1007/s10120-013-0257-7
10. Shi M, Hu Z, Wu K, Yang D, Fu H, Zhang J, et al. Comparative study of pyloromyotomy and H-M pyloroplasty in proximal gastrectomy for adenocarcinoma of esophageal-gastric junction. J Gastrointest Surg. (2022) 26(8):1585–95. doi: 10.1007/s11605-022-05347-4
11. Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E, et al. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg. (2013) 398(5):697–701. doi: 10.1007/s00423-012-0921-0
12. Jung DH, Ahn SH, Park DJ, Kim HH. Proximal gastrectomy for gastric cancer. J Gastric Cancer. (2015) 15(2):77–86. doi: 10.5230/jgc.2015.15.2.77
13. Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T, et al. Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer. (2019) 22(5):1036–43. doi: 10.1007/s10120-019-00940-0
14. Sato R, Kinoshita T, Akimoto E, Yoshida M, Nishiguchi Y, Harada J, et al. Feasibility and quality of life assessment of laparoscopic proximal gastrectomy using double-tract reconstruction. Langenbecks Arch Surg. (2021) 406(2):479–89. doi: 10.1007/s00423-020-02076-7
15. Aburatani T, Kojima K, Otsuki S, Murase H, Okuno K, Gokita K, et al. Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc. (2017) 31(11):4848–56. doi: 10.1007/s00464-017-5539-4
16. Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T, et al. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc. (2013) 27(1):146–53. doi: 10.1007/s00464-012-2401-6
17. Shiraishi N, Hirose R, Morimoto A, Kawano K, Adachi Y, Kitano S, et al. Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy. Gastric Cancer. (1998) 1(1):78–9. doi: 10.1007/s101200050058
18. Toyomasu Y, Mochiki E, Ishiguro T, Ito T, Suzuki O, Ogata K, et al. Clinical outcomes of gastric tube reconstruction following laparoscopic proximal gastrectomy for early gastric cancer in the upper third of the stomach: experience with 100 consecutive cases. Langenbecks Arch Surg. (2021) 406(3):659–66. doi: 10.1007/s00423-021-02132-w
19. Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. (2011) 14(2):97–100. doi: 10.1007/s10120-011-0040-6
20. Brierley JD GM, Wittekind C. TNM classification of malignant tumours. 8th ed. New Jersey: Wiley Blackwell (2017).
21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
22. Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended clavien-dindo classification of surgical complications: japan clinical oncology group postoperative complications criteria. Surg Today. (2016) 46(6):668–85. doi: 10.1007/s00595-015-1236-x
23. Institute NC. Common terminology criteria for adverse events (CTCAE) version 5.0. Bethesda: National Cancer Institute (2017).
24. De Groot LM, Lee G, Ackerie A, van der Meij BS. Malnutrition screening and assessment in the cancer care ambulatory setting: mortality predictability and validity of the patient-generated subjective global assessment short form (PG-SGA SF) and the GLIM criteria. Nutrients. (2020) 12(8):1–18. doi: 10.3390/nu12082287
25. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. (2002) 5(2):83–9. doi: 10.1007/s101200200014
26. Demirelli B, Babacan N, Ercelep Ö, Öztürk M, Kaya S, Tanrıkulu E, et al. Modified Glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. (2021) 73(2):230–8. doi: 10.1080/01635581.2020.1749290
27. Furukawa H, Kurokawa Y, Takiguchi S, Tanaka K, Miyazaki Y, Makino T, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cstage I proximal gastric carcinoma. Gastric Cancer. (2018) 21(3):500–7. doi: 10.1007/s10120-017-0755-0
28. Sugiyama M, Oki E, Ando K, Nakashima Y, Saeki H, Maehara Y, et al. Laparoscopic proximal gastrectomy maintains body weight and skeletal muscle better than total gastrectomy. World J Surg. (2018) 42(10):3270–6. doi: 10.1007/s00268-018-4625-7
29. Asaoka R, Irino T, Makuuchi R, Tanizawa Y, Bando E, Kawamura T, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. (2019) 89(1-2):79–83. doi: 10.1111/ans.15023
30. Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. (2003) 90(7):850–3. doi: 10.1002/bjs.4106
31. Shiraishi N, Adachi Y, Kitano S, Kakisako K, Inomata M, Yasuda K, et al. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. (2002) 26(9):1150–4. doi: 10.1007/s00268-002-6369-6
32. Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol. (2017) 24(6):1635–42. doi: 10.1245/s10434-017-5782-x
33. Cho M, Son T, Kim H, Noh S, Choi S, Seo W, et al. Similar hematologic and nutritional outcomes after proximal gastrectomy with double-tract reconstruction in comparison to total gastrectomy for early upper gastric cancer. Surg Endosc. (2019) 33(6):1757–68. doi: 10.1007/s00464-018-6448-x
34. Park JY, Park KB, Kwon OK, Yu w. Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur J Surg Oncol. (2018) 44(12):1963–70. doi: 10.1016/j.ejso.2018.08.014
35. Ahn SH, Lee JH, Park DJ, Kim HH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer. (2013) 16(3):282–9. doi: 10.1007/s10120-012-0178-x
36. Chen S, Li J, Liu H, Zeng J, Yang G, Wang J, et al. Esophagogastrostomy plus gastrojejunostomy: a novel reconstruction procedure after curative resection for proximal gastric cancer. J Gastrointest Surg. (2014) 18(3):497–504. doi: 10.1007/s11605-013-2391-2
37. Kitano S, Adachi Y, Shiraishi N, Suematsu T, Bando T. Laparoscopic-assisted proximal gastrectomy for early gastric carcinomas. Surg Today. (1999) 29(4):389–91. doi: 10.1007/BF02483072
38. Adachi Y, Inoue T, Hagino Y, Shiraishi N, Shimoda K, Kitano S, et al. Surgical results of proximal gastrectomy for early-stage gastric cancer: jejunal interposition and gastric tube reconstruction. Gastric Cancer. (1999) 2(1):40–5. doi: 10.1007/s101200050019
39. Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, et al. Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg Endosc. (2017) 31(3):1393–401. doi: 10.1007/s00464-016-5127-z
40. Chen XF, Zhang B, Chen ZX, Hu JK, Dai B, Wang F, et al. Gastric tube reconstruction reduces postoperative gastroesophageal reflux in adenocarcinoma of esophagogastric junction. Dig Dis Sci. (2012) 57(3):738–45. doi: 10.1007/s10620-011-1920-7
41. Hosogi H, Yoshimura F, Yamaura T, Satoh S, Uyama I, Kanaya S, et al. Esophagogastric tube reconstruction with stapled pseudo-fornix in laparoscopic proximal gastrectomy: a novel technique proposed for siewert type II tumors. Langenbecks Arch Surg. (2014) 399(4):517–23. doi: 10.1007/s00423-014-1163-0
42. Yasuda A, Yasuda T, Imamoto H, Kato H, Nishiki K, Iwama M, et al. A newly modified esophagogastrostomy with a reliable angle of his by placing a gastric tube in the lower mediastinum in laparoscopy-assisted proximal gastrectomy. Gastric Cancer. (2015) 18(4):850–8. doi: 10.1007/s10120-014-0431-6
43. Ueda Y, Shiraishi N, Toujigamori M, Shiroshita H, Etoh T, Inomata M, et al. Laparoscopic proximal gastrectomy with gastric tube reconstruction. JSLS. (2016) 20(3):1–8. doi: 10.4293/JSLS.2016.00046
44. Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T, et al. Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg. (2008) 32(7):1473–7. doi: 10.1007/s00268-007-9459-7
45. Aihara R, Mochiki E, Ohno T, Yanai M, Toyomasu Y, Ogata K, et al. Laparoscopy-assisted proximal gastrectomy with gastric tube reconstruction for early gastric cancer. Surg Endosc. (2010) 24(9):2343–8. doi: 10.1007/s00464-010-0947-8
46. Ronellenfitsch U, Najmeh S, Andalib A, Perera R, Rousseau M, Mulder D, et al. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. (2015) 22(3):772–9. doi: 10.1245/s10434-014-4078-7
47. Zhang M, Zhang C, Wu QC. Health-related quality of life and survival among 10-year survivors of esophageal cancer surgery: gastric tube reconstruction versus whole stomach reconstruction. J Thorac Dis. (2019) 11(8):3284–91. doi: 10.21037/jtd.2019.08.56
48. Fennerty MB, Johnson DA. Heartburn severity does not predict disease severity in patients with erosive esophagitis. MedGenMed. (2006) 8(2):6. PMID:16926745; PMCID: PMC1785158 16926745
49. Garland SN, Pelletier G, Lawe A, Biagioni B, Easaw J, Eliasziw M, et al. Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-ga) quality-of-life instrument. Cancer. (2011) 117(6):1302–12. doi: 10.1002/cncr.25556
50. Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. (2004) 40(15):2260–8. doi: 10.1016/j.ejca.2004.05.023
51. Xu Y, Tan Y, Wang YB, Xi C, Ye NY, Xu XZ, et al. Proximal versus total gastrectomy for proximal early gastric cancer: a systematic review and meta-analysis. Medicine. (2019) 98(19):e15663. doi: 10.1097/MD.0000000000015663
52. Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M, et al. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. (2017) 20(Suppl 1):69–83. doi: 10.1007/s10120-016-0663-8
53. Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer. (2019) 22(5):1029–35. doi: 10.1007/s10120-019-00938-8
Keywords: proximal gastrectomy, gastric tubular reconstruction, reflux esophagitis, nutritional status, hemoglobin
Citation: Fu J, Li Y, Liu X, Jiao X, Wang Y, Qu H and Niu Z (2023) Clinical outcomes of proximal gastrectomy with gastric tubular reconstruction and total gastrectomy for proximal gastric cancer: A matched cohort study. Front. Surg. 9:1052643. doi: 10.3389/fsurg.2022.1052643
Received: 24 September 2022; Accepted: 26 October 2022;
Published: 12 January 2023.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Tevfiktolga Sahin, İnönü University, TurkeyWeidong Xiao, Army Medical University, China
© 2023 Fu, Li, Liu, Jiao, Wang, Qu and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaojian Niu cWRmeW56akBxZHUuZWR1LmNu
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Jingxiao Fu
Jingxiao Fu Zhaojian Niu
Zhaojian Niu