- Department of Critical Care Medicine, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Purpose: This study examined whether alterations in Doppler parameters of superior mesenteric artery (SMA) are associated with prolonged mechanical ventilation (PMV) in patients who underwent cardiac valve surgery.
Methods: Hemodynamic and SMA Doppler parameters were collected at intensive care unit(ICU) admission. The duration of mechanical ventilation was monitored. PMV was defined as mechanical ventilation ≥96 h.
Results: A total of 132 patients admitted to ICU after cardiac valve surgery were evaluated for enrollment, of whom 105 were included. Patients were assigned to the control (n = 63) and PMV (n = 42) groups according to the mechanical ventilation duration. The pulsatility index(SMA-PI) and resistive index of SMA (SMA-RI) were 3.97 ± 0.77 and 0.88 (0.84–0.90) in the PMV group after cardiac valve surgery, which was lower than the SMA-PI (2.95 ± 0.71, p < 0.0001) and SMA-RI of controls (0.8, 0.77–0.88, p < 0.0001). SMA-PI at admission had favorable prognostic significance for PMV (AUC = 0.837, p < 0.0001).
Conclusions: An elevated SMA-PI is common in patients after cardiac valve surgery with PMV. Increased SMA-PI could help predict PMV after cardiac valve surgery. Using point-of-care ultrasound to measure SMA-PI at ICU admission is an acceptable and reproducible method for identifying patients with PMV.
Introduction
Cardiac surgery causes tissue hypoperfusion and an excessive inflammatory response, leading multiple organ failure, including that of the gastrointestinal tract. Despite advances in surgical methods and perioperative intensive care management, there is no consensus on the role of gastrointestinal perfusion in the resuscitation of patients who underwent cardiac surgery.
The intestinal blood flow self-regulation function is weak, which was different from brain, kidney and skeletal muscle (1). Cardiopulmonary bypass induced severe stress, cardiogenic shock, and use of vasopressor could worsen the intestinal perfusion after cardiac surgery. However, it should be noticed that the intestine is not only a digestive organ but also an immune organ. Intestine is considered to be the important bacterial barrier and “the initiator of sepsis in critical care status (2). Intestinal bacterial translocation and cytokine release caused by intestinal ischemia/reperfusion might cause the intestine original ARDS. Then, these may lead to prolonged mechanical ventilation (PMV).
Therefore, monitoring arterial blood flow in the intestine may be a potential method for assessing intestinal resuscitation status. The small intestine receives the most blood through the superior mesenteric artery (SMA). The SMA resistance index (SMA-RI) calculated by Doppler ultrasound can indicate the resistance of the whole distal intestinal circulation (3, 4), since splanchnic hypoperfusion alters organ microcirculation by increasing the flow resistance (5, 6). In patients who underwent cardiac surgery, elevated SMA-RI is related to lactate levels and kinetics (7).
PMV is related to a high hospital mortality rate (13%–50%), a longer intensive care unit (ICU) stay, and significantly poorer quality of life in patients after cardiac surgery (8, 9). Because this matches the International Classification of Diseases (ICD) code 96.72 used in many large-scale studies (10), PMV is usually recognized as a definition of ≥96 h. This study aimed to verify whether the high-resistance state of the intestinal perfusion arteries predicts PMV. This may help physicians screen out the shock state with hypoperfusion of visceral organs, which may lead to PMV.
Material and methods
Patient enrolment
This prospective observational study was approved by the Institutional Research and Ethics Committee of Peking Union Medical College Hospital for human subjects. Before enrollment, written informed consent was obtained from all patients or their next of kin.
The trial was performed in a 15-bed adult critical care unit from January 2021 to March 2022.
The inclusion criteria: (1) patients admitted to the ICU immediately after cardiac valve surgery, (2) age 18–80 years, (3) sinus rhythm, (4) no active bleeding or pneumothorax.
The exclusion criteria: (1) history of peripheral vascular disease; (2) severe stenosis defined as SMA peak systolic velocity (SMA-PSV) / abdominal aorta peak velocity >3 or SMA-PSV >275 cm/s (11); (3) end-stage renal disease or advanced cancer; (4) liver cirrhosis, portal hypertension or hepatic dysfunction; and (5) poor quality of abdominal ultrasound images.
Doppler ultrasound monitoring
Doppler ultrasound was performed with an ultrasound system consisting of a C60xp probe with 2–5 MHz (X-Porte Ultrasound System, SONOSITE, INC., United States). Doppler parameters were measured by two physicians; each physician performed two assessments and averaged the results of the two measurements. Two ICU physicians had more than 4 years of experience in critical ultrasound and obtained certification from the Chinese Critical Ultrasound Study Group. Two skilled physicians conducted 40 measurements on ten patients after cardiac valve surgery to evaluate inter-observer reproducibility.
SMA Doppler parameters were measured 10 mm proximal to the abdominal aorta. The angle of insonation was <60°. The time-velocity waveform readings were used to calculate the SMA end-diastolic velocity (EDV), peak systolic velocity (PSV), resistance index (RI), time-averaged mean velocity (TAMV), and pulsatility index (PI), according to the calculation software carried by the ultrasound. PI = (PSV - EDV)/TAMV, and RI = (PSV - EDV)/PSV.
Data collection
Demographic characteristics were determined for further analyses (e.g., sex, age, EuroSCORE-II, body mass index (BMI), sequential organ failure assessment (SOFA) score, hemodynamic parameters, and blood gas analysis). In addition, the types of operations and operation times were collected.
The vasopressor–inotrope score (VIS) was used to evaluate the vasopressor and inotrope dosages. The VIS was calculated as follows: VIS = dobutamine dose (µg/kg/min) + dopamine dose (µg/kg/min) + [10,000 * vasopressin dose (U/kg/min)] + [10 * milrinone dose (µg/kg/min)] + [100 * epinephrine dose(µg/kg/min)] + [100 * norepinephrine dose (µg/kg/min)]. Moreover, prognostic indicators (e.g., duration of MV, hospital mortality, and length of ICU stay) were recorded.
Statistical analysis
The mean ± standard deviation or median and interquartile range (IQR) were used to show demographic information. The Shapiro-Wilk test was used to assess the normality of continuous numerical data. Student's t-test or Mann-Whitney U-test were used to compare the differences between-groups for continuous variables. Categorical variables were compared between the patient groups using the Chi-square test. Correlations between PMV and variables were calculated via logistic regression analysis. Variables that showed a statistically significant effect in the univariate logistic regression were selected and included in the multivariate logistic regression. Furthermore, receiver operating characteristic (ROC) curves were constructed to evaluate the predictability of these variables for PMV. Statistical significance was set at p < 0.05. All analyzed p-values were two-sided. Statistical analyses were performed using SPSS software (version 25.0; SPSS, Chicago). Bland–Altman plots with the mean difference and 95 percent limit of agreement (LOA) were performed using MedCalc Statistical software (version 19.7; Ostend, Belgium) and used to assess inter-observer agreement, and statistical analyses.
Results
General characteristics
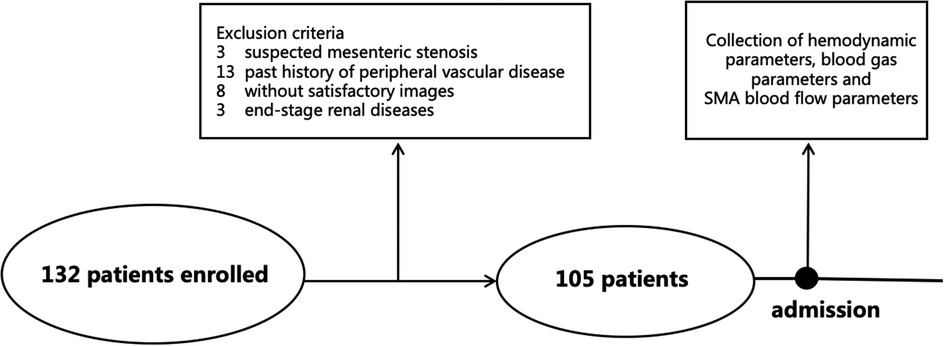
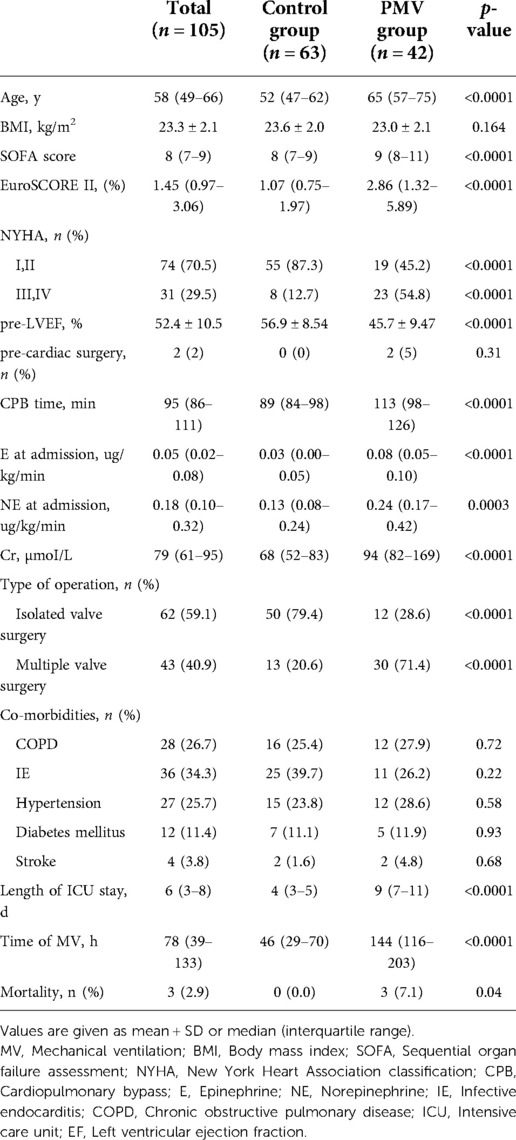
In total, 132 patients were enrolled after cardiac valve surgery, and 105 cases were included in the final analysis (Figure 1). Of the 27 ineligible patients, 13 were excluded because of history of peripheral vascular disease, eight because of unsatisfactory images, three because of suspected mesenteric stenosis, and three because of end-stage renal disease. Of the 105 patients included, 63 had less than 96 h of mechanical ventilation (control group) and 42 had 96 h or more (PMV group). The demographic characteristics are shown in Table 1.
Consistency of measurement
Figure 2 presents the Bland–Altman analysis plots of the inter-observer agreement for the SMA-PI measurements of 20 patients after cardiac valve surgery. The mean bias of the two operators was 0.02 (LOA: −0.20, 0.23), and there was no significant difference between the measurements of the two skilled physicians (p = 0.55).
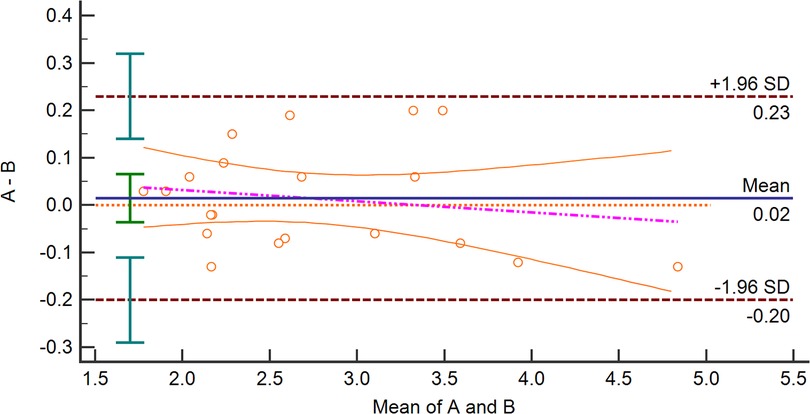
Figure 2. Bland–Altman analysis plots about Inter-Observers agreement for measuring SMA-PI. SMA-PI, the pulsatility index of the superior mesenteric artery.
Parameters at admission to the intensive care unit
The hemodynamic and Doppler parameters of the patients upon admission to the ICU after cardiac valve surgery are listed in Table 2. There were no statistically significant differences between the heart rate (HR), mean arterial pressure, central venous pressure (CVP), or other basic hemodynamic parameters in the control and PMV groups. In the PMV group, the SMA-PI, SMA-RI, and lactate values were significantly higher than in the control group (p < 0.0001).
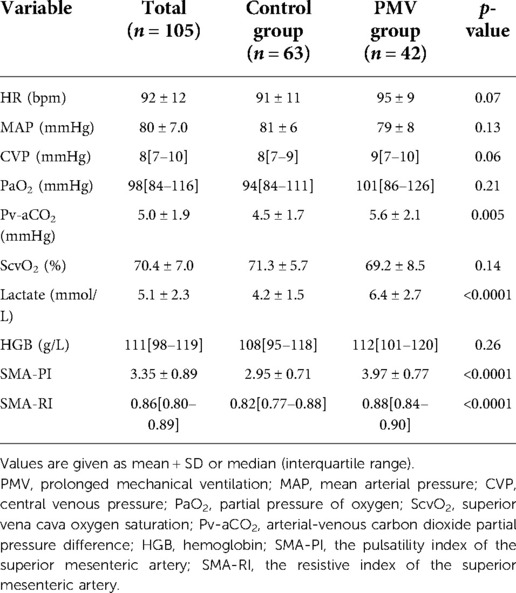
Table 2. The hemodynamic and Doppler variables at admission of the patients after cardiac valve surgery.
Then, the prognostic significance of the relevant variable for PMV was distinguished by logistic regression analysis. Univariate logistic regression indicated the correlations between PMV and SMA-PI (odds Ratio [OR] per 10−1 units: 5.594, 95% confidence interval [CI]: 2.296–13.631, p < 0.0001) and SMA-RI (OR per 10−1 units: 1.188, 95% CI: 1.087–1.299, p < 0.0001).At the same time, PMV was also related to patient age, cardiopulmonary bypass(CPB) time, creatinine and left ventricular ejection fraction(EF) before surgery, lactate value, arterial-venous carbon dioxide partial pressure difference(Pv-aCO2), norepinephrine(NE) dose, CVP, and HR at admission (Table 3).
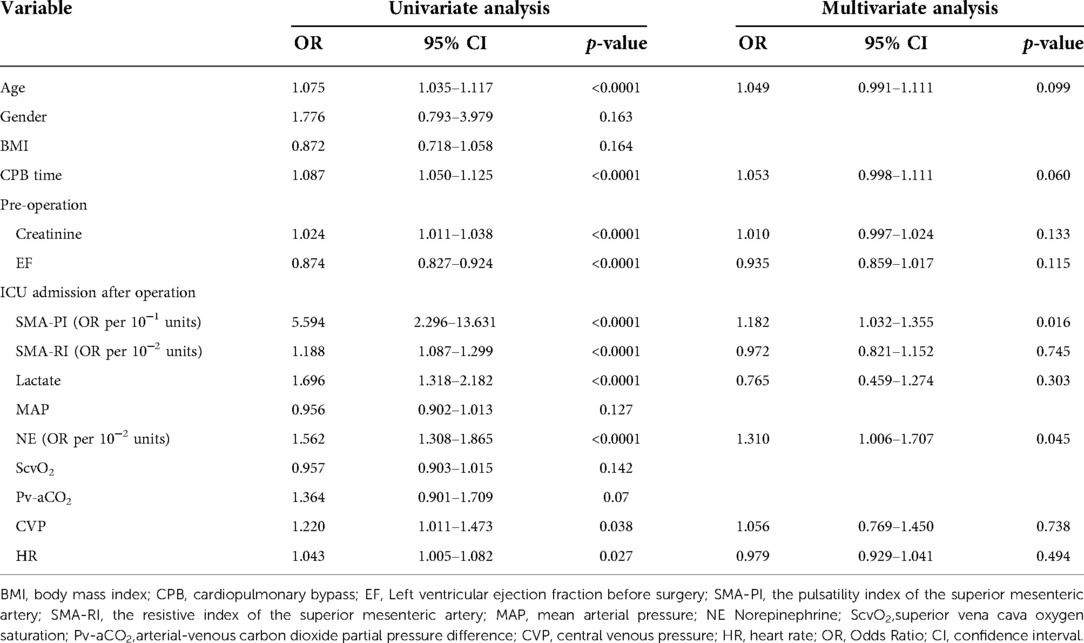
Table 3. Logistic regression for baseline characteristics and variables at admission to predict prolonged mechanical ventilation.
According to a thorough multiple stepwise logistic regression analysis using the factors mentioned above, the significant associations between SMA-PI (OR, 1.182; 95% CI: 1.032–1.355, p = 0.016), NE dose at admission (OR, 1.310; 95% CI: 1.006–1.707, p = 0.045), and PMV were discovered (Table 3). SMA-PI also showed a strong predictive value for PMV, with an area under the ROC curve of 0.84 (95% CI: 0.76–0.91, p < 0.0001, Figure 3). With a cut-off value of 3.31, SMA-PI had a sensitivity of 83.33% and a specificity of 71.43% for PMV, respectively.
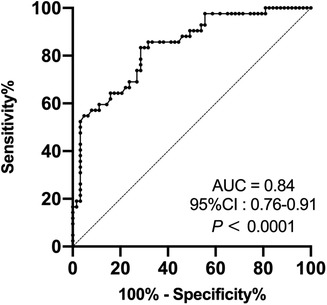
Figure 3. Receiver operating characteristic curve of SMA-PI's ability to predict PMV in patient after cardiac valve surgery. SMA-PI, the pulsatility index of the superior mesenteric artery; PMV, prolonged mechanical ventilation; AUC, area under curve; CI, confidence interval.
Discussion
To our knowledge, this is the first study that explored the relationship between gastrointestinal perfusion blood flow and PMV in patient post-operation of cardiac valve surgery.
This study had two main findings. First, we found that patients with PMV after cardiac valve surgery had a markedly elevated SMA-PI at ICU admission. Second, SMA-PI effectively predicted PMV in patients after cardiac valve surgery.
After cardiac surgery, patients are usually weaned from mechanical ventilation once they recover from anesthesia. However, some patients experience cardiogenic shock or severe stress caused by cardiopulmonary bypass. These factors could reduce perfusion to the intestine, and lead to intestinal ischemia. At the same time, these factors are also the main reasons for prolonging the duration of mechanical ventilation. Intestinal ischemia might weaken its barrier function and lead to sepsis of intestinal origin1, ultimately worsening patient prognosis. Therefore, monitoring blood flow in the intestine is relevant in critically ill patients (12, 13). PI and RI are indicators that can respond to blood flow and microcirculatory resistance in the distal part of the vessel (3, 4). The correlation between the SMA-RI and lactate levels and kinetics in post-cardiac surgery patients was identified in our previous study.
In the present study, SMA-PI and RI values in the PMV group were significantly higher than those in the control group. This suggests that decreased blood flow and impaired microcirculation of the intestine are associated with PMV in patients after cardiac valve surgery.
Univariate regression analysis results were similar to previous studies which examined the risk factors for PMV after cardiac surgery, i.e., age, CPB time (14), preoperative creatinine, preoperative EF (15), lactate at admission and a high dose of vasopressors (16).
We also acknowledge that many factors besides shock and severe stress can affect the PI and RI values of the SMA, such as age, sex, chronic diseases such as hypertension and diabetes mellitus, and the use of vasopressors. Therefore, we chose non-coronary artery bypass grafting to reduce the impact of underlying peripheral vascular disease. A subgroup comparison was also conducted, and the results showed that SMA-PI could predict PMV independent of age and sex (Figure 4). Moreover, many factors that could affect the duration of mechanical ventilation were analyzed by subgroup comparisons (Figure 4). The results showed that the SMA-PI could not predict PMV in patients with ARDS after cardiac valve surgery. ARDS has an independent and important effect on weaning from mechanical ventilation.
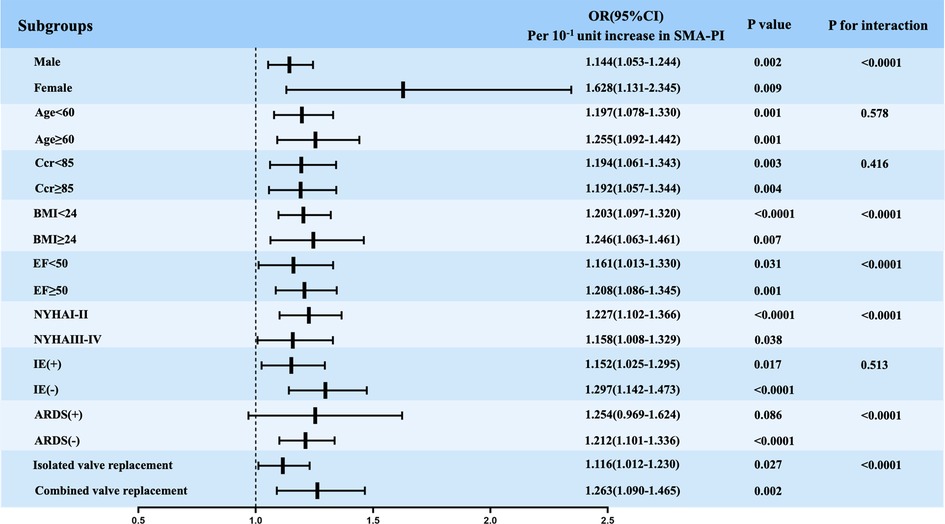
Figure 4. Logistic regression analysis evaluating the implication of SMA-PI in predicting PMV in subgroups. SMA-PI, the pulsatility index of the superior mesenteric artery; PMV, prolonged mechanical ventilation; Ccr, endogenous creatinine clearance rate; BMI, body mass index; EF, left ventricular ejection fraction before surgery; NYHA, New York heart association; IE, infective endocarditis; ARDS, acute respiratory distress syndrome; OR, Odds ratio; CI, confidence interval.
In models with multivariate stepwise logistic regression, SMA-PI and NE dose still predicted PMV (Table 3). NE dose is not only related to the severity of shock but also affects intestinal vascular resistance, which in turn affects SMA-PI. This result reinforces the need for SMA blood flow monitoring and the need to pay attention to the negative effects of vasopressors on intestinal blood flow (12). Recent studies on critically ill patients with non-obstructive mesenteric ischemia showed that the vasopressor dose affects the diastolic effect of prostacyclin E1 on the SMA (17).
This is precisely because blood flow in the SMA is the result of the influence of many factors, and blood flow monitoring of the SMA is of greater significance in patients after cardiac surgery. However, we also have multiple therapeutic approaches that can improve blood flow when altered blood flow in the SMA is detected. The key to achieving this purpose might be the real-time and bedside monitoring of SMA blood flow using Doppler.
The intestine is an important immune and digestive organ that is particularly prone to a decrease in blood flow in the critical care state. Monitoring and intervention for intestinal hypoperfusion may be a potential way to reduce the inflammatory response, reduce organ dysfunction due to perioperative shock, and improve prognosis.
This research had some limitations: (1) The SMA-PI in basic state before surgery was not measured, because water fasting measures were required only for 6 h before surgery, and the interference of feeding on SMA blood flow could not be removed. However, patients with coronary heart disease and peripheral vascular disease, which were used to reduce the influence of vascular disease on SMA blood flow, were excluded. (2) SMA-PI can only partially reflect the blood flow status of the SMA. (3) SMA-PI could be influenced by many factors, such as cardiac output, vasopressors, intra-abdominal pressure and vascular elasticity, the non-obstructive intestinal ischemia seen in a few post-cardiac surgery patients is due to the cumulative effect of various factors on the intestinal circulation. Therefore, we evaluated the consequences of these factors. (4) The SMA-PI monitoring was not dynamic.
In conclusion, an elevated SMA-PI is common and is associated with PMV in patients after cardiac valve surgery. Increased SMA-PI could act as a predictor of PMV in these patients. Using point-of-care ultrasound to assess SMA-PI at ICU admission is an acceptable and reproducible method for identifying PMV patients after cardiac valve surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Research and Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, and final approval of the version to be published. HH and NC helped in data collection and data analysis; XW and DL helped to build up the research idea and guided the discussion; YL guided the whole research program and should be considered the correspondence author of this article. All authors contributed to the article and approved the submitted version..
Funding
This work was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-216), the Excellence Program of Key Clinical Specialty of Beijing of critical care medicine in 2020, the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-115).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meng L, Wang Y, Zhang L, McDonagh DL. Heterogeneity and variability in pressure autoregulation of organ blood flow: lessons learned over 100+ years. Crit Care Med. (2019) 47(3):436–48. doi: 10.1097/ccm.0000000000003569
2. Bauer M. The liver-gut-axis: initiator and responder to sepsis. Curr Opin Crit Care. (2022) 28(2):216–20. doi: 10.1097/mcc.0000000000000921
3. Gladman G, Sims DG, Chiswick ML. Gastrointestinal blood flow velocity after the first feed. Arch Dis Child. (1991) 66(1 Spec No):17–20. doi: 10.1136/adc.66.1_spec_no.17
4. Wicklein S, Mühlberg W, Richter B, Sieber CC. Increased splanchnic arterial vascular resistance in oldest old patients—possible relevance for postprandial hypotension. Z Gerontol Geriatr. (2007) 40(1):37–42. doi: 10.1007/s00391-007-0431-9
5. Corradi F, Via G, Tavazzi G. What's new in ultrasound-based assessment of organ perfusion in the critically ill: expanding the bedside clinical monitoring window for hypoperfusion in shock. Intensive Care Med. (2020) 46(4):775–9. doi: 10.1007/s00134-019-05791-y
6. Legrand M, Zarbock A. Ten tips to optimize vasopressors use in the critically ill patient with hypotension. Intensive Care Med. (2022) 48(6):736–9. doi: 10.1007/s00134-022-06708-y
7. Zhou Y, He H, Wang X, Cui N, Zhou X, Long Y, et al. Resistance index of the superior mesenteric artery: correlation with lactate concentration and kinetics prediction after cardiac surgery. Front Med (Lausanne). (2021) 8:762376. doi: 10.3389/fmed.2021.762376
8. Fernandez-Zamora MD, Gordillo-Brenes A, Banderas-Bravo E, Arboleda-Sánchez JA, Hinojosa-Pérez R, Aguilar-Alonso E, et al. Prolonged mechanical ventilation as a predictor of mortality after cardiac surgery. Respir Care. (2018) 63(5):550–7. doi: 10.4187/respcare.04915
9. Sharma V, Rao V, Manlhiot C, Boruvka A, Fremes S, Wąsowicz M. A derived and validated score to predict prolonged mechanical ventilation in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2017) 153(1):108–15. doi: 10.1016/j.jtcvs.2016.08.020
10. Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. (2015) 3(7):544–53. doi: 10.1016/s2213-2600(15)00150-2
11. Sharafuddin MJ, Olson CH, Sun S, Kresowik TF, Corson JD. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. (2003) 38(4):692–8. doi: 10.1016/s0741-5214(03)01030-9
12. Piton G, Le Gouge A, Boisramé-Helms J, Anguel N, Argaud L, Asfar P, et al. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: a post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. (2022) 48(4):458–66. doi: 10.1007/s00134-022-06637-w
13. Wang X, Liu D. Hemodynamic influences on mesenteric blood flow in shock conditions. Am J Med Sci. (2021) 362(3):243–51. doi: 10.1016/j.amjms.2021.04.014
14. Aksoy R, Karakoc AZ, Cevirme D, Elibol A, Yigit F, Yilmaz Ü, et al. Predictive factors of prolonged ventilation following cardiac surgery with cardiopulmonary bypass. Braz J Cardiovasc Surg. (2021) 36(6):780–7. doi: 10.21470/1678-9741-2020-0164
15. Bauer A, Korten I, Juchem G, Kiesewetter I, Kilger E, Heyn J. Euroscore and IL-6 predict the course in ICU after cardiac surgery. Eur J Med Res. (2021) 26(1):29. doi: 10.1186/s40001-021-00501-1
16. Zante B, Erdoes G. Risk of prolonged mechanical ventilation after cardiac surgery: predicting the unpredictable? J Cardiothorac Vasc Anesth. (2019) 33(10):2717–8. doi: 10.1053/j.jvca.2019.04.008
17. Rittgerodt N, Pape T, Busch M, Becker LS, Schneider A, Wedemeyer H, et al. Predictors of response to intra-arterial vasodilatory therapy of non-occlusive mesenteric ischemia in patients with severe shock: results from a prospective observational study. Crit Care. (2022) 26(1):92. doi: 10.1186/s13054-022-03962-w
Keywords: pulsatility index of the superior mesenteric artery, prolonged mechanical ventilation, cardiac valve surgery, intestinal perfusion, Doppler ultrasound (DUS)
Citation: Zhou Y, He H, Cui N, Wang X, Long Y and Liu D (2023) Elevated pulsatility index of the superior mesenteric artery indicated prolonged mechanical ventilation in patients after cardiac valve surgery. Front. Surg. 9:1049753. doi: 10.3389/fsurg.2022.1049753
Received: 21 September 2022; Accepted: 11 November 2022;
Published: 6 January 2023.
Edited by:
Carolien Bulte, VU Medical Center, NetherlandsReviewed by:
Anne Beukers, VU Medical Center, NetherlandsWeiping Cheng, Capital Medical University, China
© 2023 Zhou, He, Cui, Wang, Long and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Long bHlfaWN1QGFsaXl1bi5jb20=
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Yuankai Zhou
Yuankai Zhou Huaiwu He
Huaiwu He Yun Long
Yun Long