- 1Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Operation Room, The Affiliated Hospital of Qingdao University, Qingdao, China
- 3Department of Interventional Radiology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 4Medical Department, Yidu Cloud (Beijing) Technology Co., Ltd., Beijing, China
- 5Shandong Key Laboratory of Digital Medicine and Computer Assisted Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
Objective: Macrovascular invasion (MVI) is an important factor leading to poor prognosis in hepatocellular carcinoma (HCC). Liver resection may offer favorable prognosis for selected patients with HCC. This study aimed to analyze the prognostic factors of HCC with MVI after liver resection as well as demonstrate a case of conversion therapy in an HCC patient with portal vein tumor thrombus (PVTT).
Methods: A total of 168 HCC patients with MVI who underwent primary liver resection at the Affiliated Hospital of Qingdao University between January 2013 and October 2021 were enrolled in the study. Clinicopathological data were collected retrospectively. Univariate and multivariate regression analyses were used to investigate the risk factors influencing recurrence and overall survival. Additionally, conversion therapy with drug-eluting bead transarterial chemoembolization (D-TACE), and sorafenib plus sintilimab treatment was performed in an HCC patient with PVTT.
Results: Among the 168 patients with HCC, 11 were diagnosed with hepatic vein tumor thrombosis, and the rest were diagnosed with PVTT. The 1-year disease-free survival rate was 37.5%, and the 3-year overall survival rate was 52.7%. Univariate and multivariate regression analyses revealed that HBsAg positivity, alpha-fetoprotein (AFP) level ≥400 ng/ml, liver capsule invasion, and tumor number ≥2 were independent prognostic factors for tumor recurrence, whereas HBsAg positivity was an independent risk factor for overall survival. Postoperative prophylactic medication did not significantly prolong the recurrence time. The median survival time (MST) after tumor recurrence was 13.4 months. In the patient treated with conversion therapy, the tumor gradually shrank and was eventually surgically resected.
Conclusions: This study identified the independent prognostic and risk factors associated with recurrence and overall survival in HCC patients with MVI. Additionally, we successfully performed conversion therapy in an HCC patient with PVTT. The findings would help identify patients at high risk of recurrence and indicate that combined therapy may prolong the survival of HCC patients with PVTT.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancy in China (1). More than 50% of patients are diagnosed with advanced HCC, of which macrovascular invasion (MVI) is one of the most common signs (2). The incidence of portal vein tumor thrombus (PVTT), which is the main type of MVI, in patients with HCC is 44%–62.2% (3). PVTT formation promotes distant metastasis and induces portal hypertension, leading to a poor prognosis (4). Patients with untreated HCC with PVTT have a 2.7–4.0-month median survival time (5, 6). Although targeted and immune therapies have greatly improved the prognosis of advanced HCC, the current approach for treating HCC patients with PVTT remains controversial.
Most guidelines recommend non-surgical treatments, including transarterial chemoembolization (TACE) or sorafenib, as the first-line treatment for HCC patients with PVTT (7). However, some patients with HCC and MVI can undergo surgical resection. A meta-analysis comparing the prognosis of surgical resection with non-surgical resection indicated that TACE or other non-surgical treatments were inferior to surgical resection in terms of overall survival (OS) (8). Thus, the latest guidelines in China recommend that surgical resection be considered if the tumor thrombus can be completely removed during the operation, followed by TACE, portal vein chemotherapy, or other systemic treatments to prevent recurrence (8, 9). Neoadjuvant three-dimensional radiotherapy can also be performed preoperatively (9, 10). With the progress in targeted therapy and immunotherapy, more options are available for the treatment of HCC (11). The combination of different therapies has been shown to be secure and more useful in patients with HCC with MVI (12). The strategy of combining different treatments should be arranged according to the risk of recurrence or prolonged survival; however, such strategies have not yet been established.
In this study, we retrospectively analyzed patients who underwent hepatectomy at our hospital and explored the independent risk factors for disease-free survival (DFS) and OS. We report a successful case of conversion therapy with a combination of D-TACE, sorafenib plus sintilimab (PD-1) treatment and surgical resection.
Materials and methods
Study population
This retrospective study included 168 HCC patients with MVI who underwent liver resection at the Affiliated Hospital of Qingdao University between January 2013 and October 2021. The criteria for selecting patients for hepatectomy were as follows: (1) type I or II PVTT, (2) resectable hepatic vein tumor thrombosis, (3) PVTT after liver resection with Child-Pugh class A or B liver function (score ≤7), and (4) absence of extrahepatic metastases or other associated malignancies. We collected clinicopathological data and information on survival outcomes of all patients. The exclusion criteria were as follows: (1) incomplete follow-up information and (2) incomplete tumor resection.
Ethics approval for this study was provided by the ethics committee of the Affiliated Hospital of Qingdao University.
Classification of PVTT
The diagnostic criteria for PVTT were based on typical preoperative imaging studies and confirmed by postoperative histopathological examination (13). According to Cheng's classification (14), there are four different types of PVTT: type I, which invades the segmental branches of the portal vein or higher; type II, which invades the right or left portal vein; type III, which invades the main portal vein; and type IV, which invades the main portal and superior mesenteric veins.
Patient follow-up
All patients who received treatment for HCC at our hospital underwent outpatient follow-up after liver resection once a month for the first three months and then every two–three months until death or withdrawal from the follow-up. Serum alpha-fetoprotein (AFP) level analysis, liver function tests, whole blood counts, abdominal ultrasound, and enhanced CT were used for follow-up examinations.
Statistical analysis
The primary endpoint, which was OS, was determined as the time from the procedure until death or the last follow-up; DFS was calculated as the time from the procedure to recurrence. The chi-square test or Fisher's exact test was used to compare categorical variables, which are represented as rates or constituent ratios. Univariate and multivariate logistic regression analyses were used to explore variables that showed an independent relationship with recurrence. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was set at P < 0.05. Statistical analysis was performed using IBM SPSS Statistics version 20 (SPSS, Chicago, IL, United States).
Results
Patient characteristics
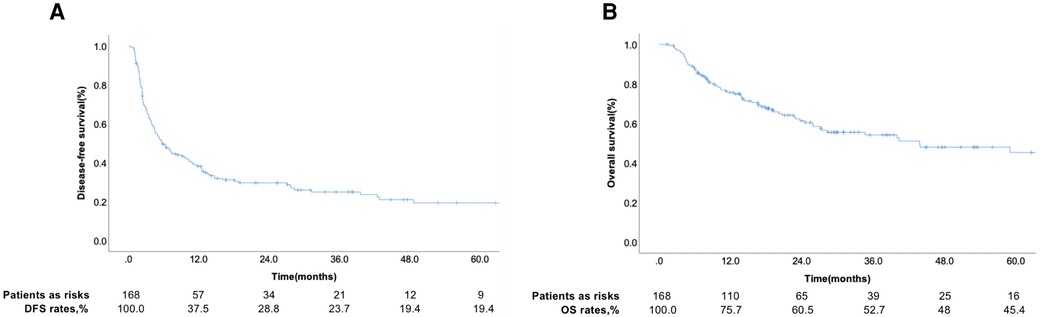
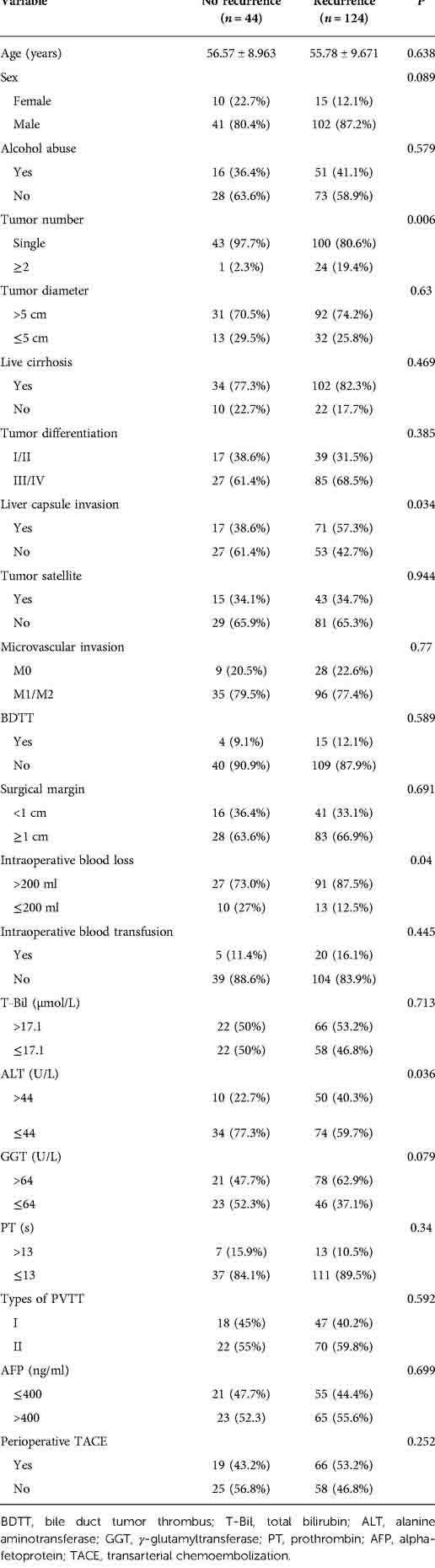
Among the 168 patients, 11 were diagnosed with HCC with hepatic vein tumor thrombosis and the rest with HCC with PVTT. The curves for DFS and OS are shown in Figures 1A, B. Among the patients, 124 patients had recurrence and 44 patients did not show recurrence during the follow-up. The 1-year DFS rate of the entire cohort was 37.5%. We seperated the patients into two groups according to whether patients occured tumor recurrence during follow-up and comprared the risk factor related with tumor recurrence. Table 1 showed that the tumor number ≥ 2 was related with tumor recurrence.. The 1-year and 3-year accumulating OS rates were approximately 75.7% and 52.7%, respectively.
Clinicopathological factors related to DFS and OS
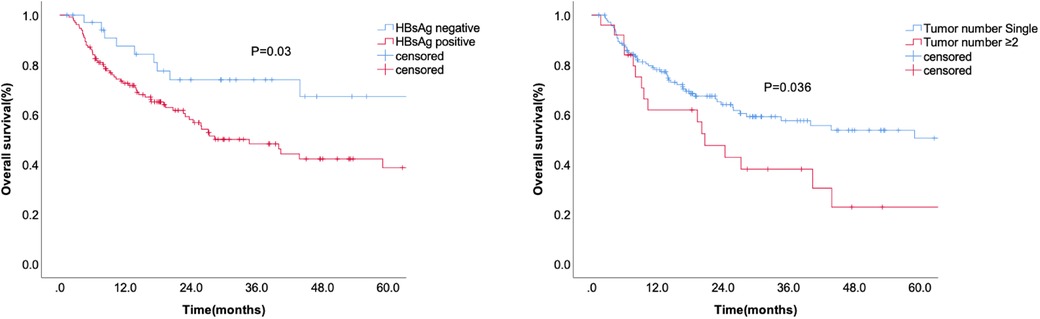
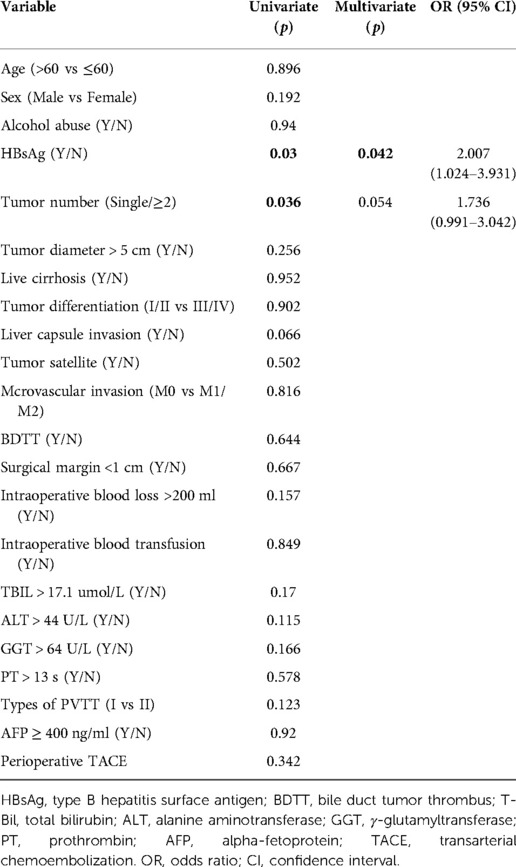
Kaplan-Meier analysis identified significant factors related to DFS (Figure 2). Univariate analysis showed that HBsAg positivity, AFP level ≥400 ng/ml, liver capsule invasion, and tumor number ≥2 were risk factors for tumor recurrence after hepatectomy. Multivariate analysis showed that HBsAg positivity (OR = 1.667, 95% CI: 1.055–2.634, P = 0.029), AFP level ≥400 ng/ml (OR = 1.606, 95% CI: 1.121–2.300, P = 0.01), liver capsule invasion (OR = 1.496, 95% CI: 1.045–2.143, P = 0.028), and tumor number ≥2 (OR = 2.101, 95% CI: 1.337–3.302, P = 0.001) were independent risk factors for tumor recurrence (Table 2). A non-significant difference was observed between type I and type II PVTT (P = 0.263).
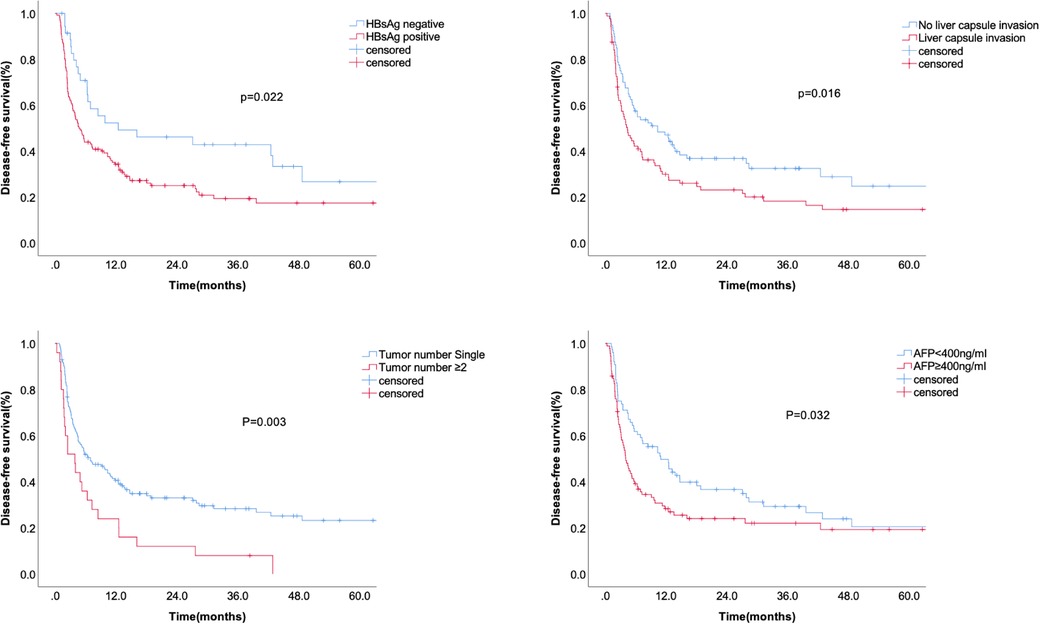
Figure 2. The disease-free survival according to HBsAg, liver capsule invasion, AFP level, and tumor number.
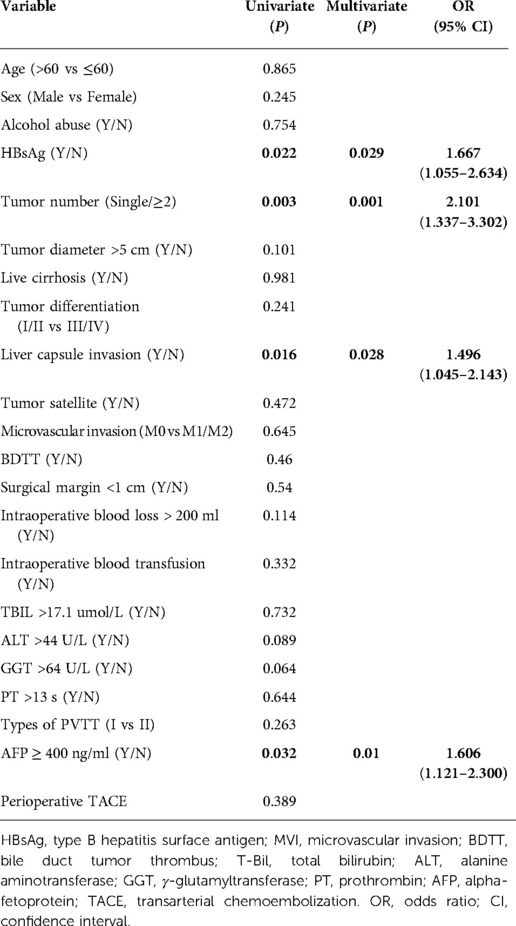
Table 2. Univariate and multivariate analysis of prognostic factors that related with tumor recurrence.
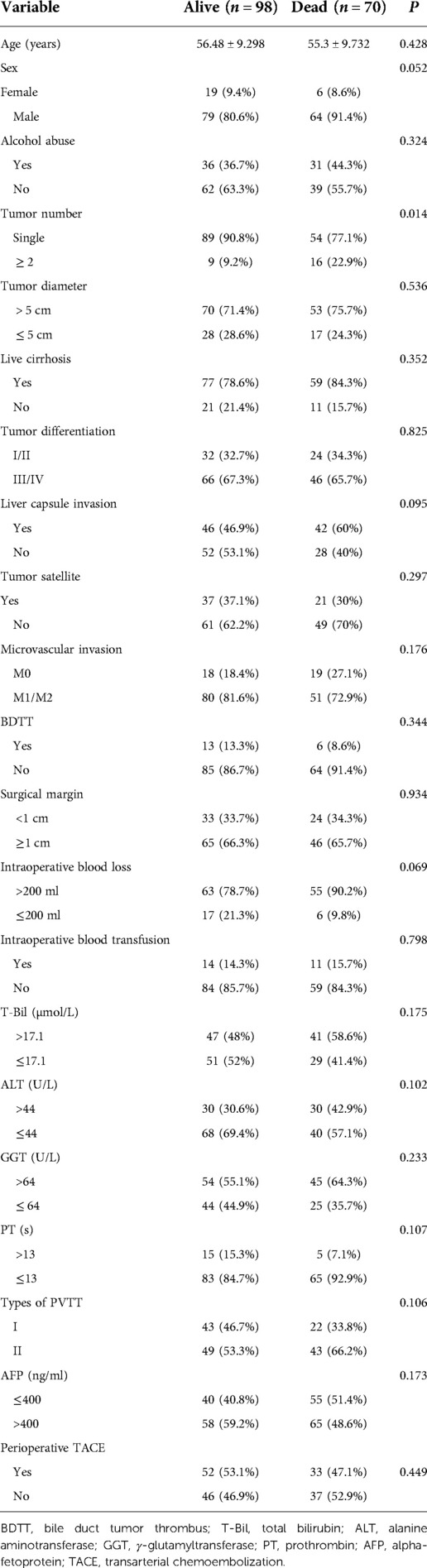
Table 3 showed the correlation analysis of clinicopathological factors with overall survival. Furthermore, Kaplan-Meier analysis identified the significant factors associated with OS (Figure 3). Univariate analysis showed that HBsAg positivity and tumor number ≥2 were significantly correlated with OS (Table 4). Multivariate analysis showed that HBsAg positivity (OR = 2.007, 95% CI: 1.024–3.931, P = 0.042) was an independent risk factor for OS.
Effect of targeted therapy on patient prognosis
We analyzed the effects of targeted therapy on DFS and OS. Postoperative prophylactic medication did not significantly prolong the recurrence time (Figure 4A). The median surveillance time of OS after recurrence was 33 months in the targeted therapy group and 7.3 months in the non-targeted therapy group (Figure 4B).
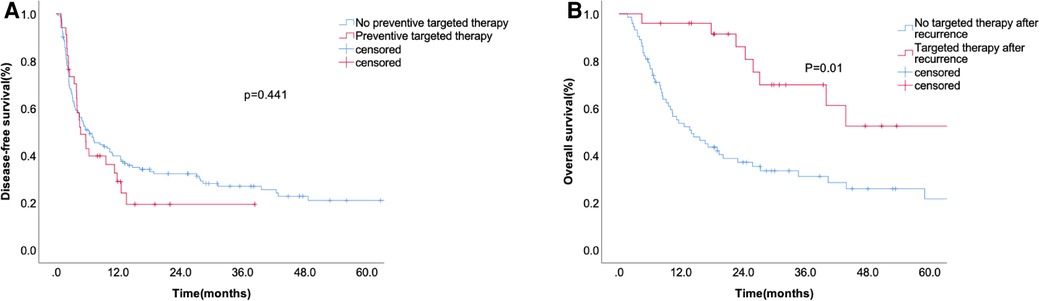
Figure 4. The disease-free survival according to preventive targeted therapy before liver resection (A) and overall survival (B) according to whether accepting targeted therapy after recurrence.
Successful case of conversion therapy
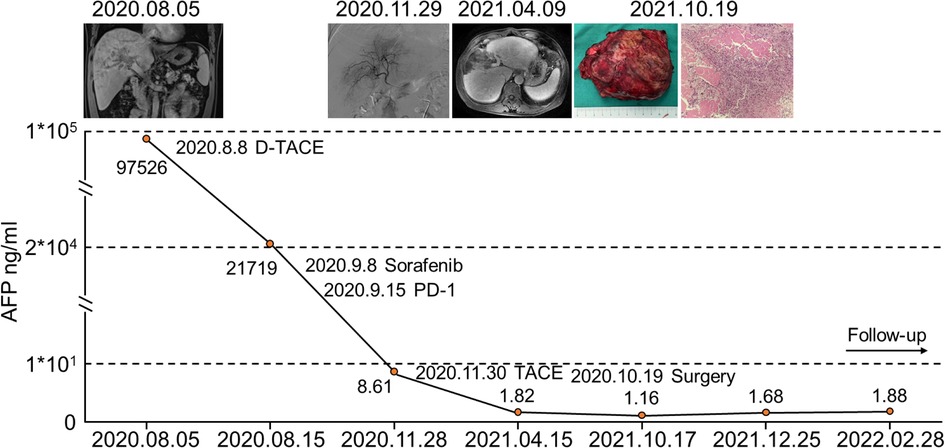
We report a successful case of conversion therapy with drug-eluting bead-TACE (D-TACE), sorafenib plus sintilimab (PD-1) treatment and liver resection. A 49-year-old male was admitted to our institution with a complaint of an intrahepatic mass. Enhanced CT of the upper abdomen indicated HCC with type II PVTT. The pretreatment AFP level was 97,526 ng/ml. First, D-TACE was performed, followed by sorafenib plus sintilimab (PD-1) targeted immunotherapy. The tumor gradually shrank, and the patient eventually underwent surgical resection. The entire treatment process and the changes in AFP levels are shown in Figure 5. After combined conversion therapy, the patient successfully underwent surgery.
Discussion
One of the most important adverse factors affecting the prognosis of patients with HCC is macrovascular invasion (MVI), particularly PVTT. Although several new treatment methods have been developed for HCC patients with MVI, selecting the best treatment remains controversial. With the progress in surgical technology, surgical resection of HCC with MVI has been identified as safe and effective (15). However, owing to the high recurrence rate of HCC with MVI, the postoperative long-term survival prognosis of these patients is poor (16). This study analyzed the risk factors that affect prognosis after hepatectomy, providing clues for improving patient prognosis.
Surgical resection is an effective treatment for patients with HCC and MVI. A propensity-score matched study in a large North American multi-institutional cohort showed that any surgical management was associated with improved survival (17). In another Japanese cohort including 100 HCC patients with Vp3 or Vp4 PVTT, hepatectomy was an effective treatment, with a median survival time of 14.5 months (18). A nationwide study in China, including 19 hospitals, found that the actual 3-year survival rate for patients with HCC with PVTT after hepatectomy was 11.7% (19). One study investigated the optimal treatment for patients with different types of PVTT, indicating that surgical resection was the best option for type I or II PVTT, TACE was recommended for type III PVTT, and sorafenib was more appropriate for type IV PVTT (20). Moreover, liver transplantation seems to offer a better prognosis for HCC with type I PVTT than liver resection, especially in patients with AFP levels >200 ng/ml (21). Patients underwent living donor liver transplantation after downstaging PVTT using stereotactic body radiotherapy (SBRT) and tumor ablation (with transarterial chemo- or radio-embolization). After successful downstaging, HCC patients with PVTT can have prolonged survival with living donor liver transplantation (22).
As for the prognostic risk factors, various clinicopathological risk factors, including AFP level >400 ng/ml, extent of PVTT, and tumor diameter >5 cm, and almost all risk factors related to HCC patient prognosis, were related to actual long-term survival in a Chinese nationwide study (19). In a single center in China, AFP level, ascites, and total bilirubin level were independent risk factors for poor short-term survival, whereas tumor differentiation and AFP level were associated with long-term survival (23). Another study found that tumor recurrence occurred after liver resection in 82.1% of patients with HCC with PVTT, and the median time for tumor recurrence was 4.1 months. PVTT in the main portal trunk and maximum tumor size ≥5 cm were the major prognostic factors influencing HCC recurrence following liver resection (24). In our study, the median recurrence was 13.4 months. and HBsAg positivity, AFP level ≥400 ng/ml, invasion of the liver capsule, and tumor number ≥2 were independent prognostic factors for recurrence, whereas HBsAg positivity was an independent risk factor of overall survival.
Furthermore, we have reported a successful case of conversion therapy with combined D-TACE, sorafenib plus sintilimab (PD-1) treatment and surgical resection. TACE is an effective and safe treatment for patients with advanced HCC. A meta-analysis examined the role of TACE in the treatment of HCC with PVTT, and the median OS was 8 (range: 5–15) months (25). The combination of TACE and targeted therapy can improve the prognosis of patients with advanced HCC. In patients with advanced HCC and PVTT, TACE plus lenvatinib was safe, well-tolerated, and more effective than TACE plus sorafenib (7). Patients with unresectable HCC may benefit from hepatic arterial infusion of oxaliplatin plus raltitrexed, regardless of whether they have PVTT (26). In patients with HCC and PVTT, cTACE-HAIC was superior to cTACE alone in terms of OS and PFS (27). A randomized phase 3 trial showed a higher response rate and longer median progression-free survival with sorafenib plus HAIC than sorafenib alone (28). Moreover, radiotherapy has been confirmed as an effective method for regressing tumor thrombi in the portal vein (10). Complete regression was achieved following radiotherapy for Vp3/Vp4 HCC in 3.6% of cases, partial regression in 50.2% of cases, and stable illness in 25.6% of cases, with an MST of 10.6 months, demonstrating the effectiveness of radiotherapy (29). A randomized multicenter controlled study showed that neoadjuvant radiotherapy provided considerably better postoperative survival outcomes than surgery alone, wherein 17 patients (20.7%) in the neoadjuvant radiation group experienced partial remission (10). The combination of SBRT and tumor ablation successfully downstaged 63% (27/43) of patients (22). Following partial hepatectomy ± thrombectomy, patients with HCC and PVTT who received postoperative IMRT experienced considerably better overall survival outcomes than those without IMRT (30). A prospective, controlled, multicenter study showed that TACE-125 iodine treatment greatly increased the survival of patients with type II PVTT and HCC, particularly subtype IIa, with few adverse events (31). Imbrave 150 has demonstrated similar effectiveness to T + A (32). In our study, liver resection was successfully performed after treatment with D-TACE and sorafenib plus PD-1.
Our study had several limitations. First, this was a retrospective study conducted at a single center and we included patients with long-time follow up. Second, as a single-center study, targeted therapy may be affected by the bias of subjects and the experience and preferences of surgeons. Multicenter and prospective studies should be designed to further explore the strategies for treating HCC with MVI.
In conclusion, we analyzed the prognostic factors for patients with HCC and MVI and demonstrated that the use of targeted therapy after recurrence can improve the overall survival of patients. We anticipate that the findings of this study will provide clues for identifying patients at high risk of recurrence and provide evidence of successful targeted treatment in patients with recurrence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
CS and CZ designed the study, provided strategic guidance. MJ and HZ analyzed the results, wrote the manuscript. GL and BZ performed the conversion therapy. BS, ZX, FP, MC and YS collected the data. TD is a staff of Yidu Cloud Technology, helping the data extraction. All authors commented on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Taishan Scholars Program of Shandong Province (grant number 2019010668), the Shandong Higher Education Young Science and Technology Support Program (grant number 2020KJL005), and Natural Science Foundation of Shandong Province (grant number ZR2021MH171).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Buonaguro L. Human hepatocellular carcinoma (HCC). Cancers (Basel). (2020) 12(12):3739. doi: 10.3390/cancers12123739
2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. (2015) 35(9):2155–66. doi: 10.1111/liv.12818
3. Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. (2019) 4(9):721–30. doi: 10.1016/S2468-1253(19)30178-5
4. Lim J, Kim HI, Kim E, Kim J, An J, Chang S, et al. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: a matched nested case-control study. BMC Cancer. (2021) 21(1):11. doi: 10.1186/s12885-020-07708-1
5. Bruix J, Reig M, Sherman M. Evidence-Based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterol. (2016) 150(4):835–53. doi: 10.1053/j.gastro.2015.12.041
6. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. (2012) 379(9822):1245–55. doi: 10.1016/S0140-6736(11)61347-0
7. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. (2021) 127(20):3782–93. doi: 10.1002/cncr.33677
8. Liang L, Chen TH, Li C, Xing H, Han J, Wang MD, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB (Oxford). (2018) 20(12):1119–29. doi: 10.1016/j.hpb.2018.06.1804
9. Bureau of Medical Administration NHCotPsRoC. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30(4):367-88. doi: 10.3760/cma.j.cn501113-20220413-00193
10. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. (2019) 37(24):2141–51. doi: 10.1200/JCO.18.02184
11. Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional therapy approaches for hepatocellular carcinoma: recent advances and management strategies. Cancers (Basel). (2020) 12(7):1914. doi: 10.3390/cancers12071914
12. Zhao Y, Zhu X, Wang H, Dong D, Gao S, Zhu X, et al. Safety and efficacy of transcatheter arterial chemoembolization plus radiotherapy combined with sorafenib in hepatocellular carcinoma showing macrovascular invasion. Front Oncol. (2019) 9:1065. doi: 10.3389/fonc.2019.01065
13. Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatol. (2018) 68(3):977–93. doi: 10.1002/hep.29883
14. Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. (2011) 18(1):74–80. doi: 10.1007/s00534-010-0314-0
15. Yang J, Kim JM, Rhu J, Choi GS, David Kwon CH, Joh JW. Surgical resection is preferred in selected solitary hepatocellular carcinoma with portal vein tumor thrombosis. Dig Surg. (2022) 39(1):42–50. doi: 10.1159/000521827
16. Rungsakulkij N, Suragul W, Mingphruedhi S, Tangtawee P, Muangkaew P, Aeesoa S. Prognostic role of alpha-fetoprotein response after hepatocellular carcinoma resection. World J Clin Cases. (2018) 6(6):110–20. doi: 10.12998/wjcc.v6.i6.110
17. Ryon EL, Kronenfeld JP, Lee RM, Yopp A, Wang A, Lee AY, et al. Surgical management of hepatocellular carcinoma patients with portal vein thrombosis: the United States safety net and academic center collaborative analysis. J Surg Oncol. (2021) 123(2):407–15. doi: 10.1002/jso.26282
18. Komatsu S, Kido M, Kuramitsu K, Tsugawa D, Gon H, Fukushima K, et al. Impact of hepatectomy for advanced hepatocellular carcinoma with Major portal vein tumor thrombus. J Gastrointest Surg. (2022) 26(4):822–30. doi: 10.1007/s11605-021-05181-0
19. Chen ZH, Zhang XP, Lu YG, Li LQ, Chen MS, Wen TF, et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int. (2020) 14(5):754–64. doi: 10.1007/s12072-020-10032-2
20. Zhang Y, Wu JL, Li LQ. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol. (2022) 27(1):100552. doi: 10.1016/j.aohep.2021.100552
21. Lv JY, Zhang NN, Du YW, Wu Y, Song TQ, Zhang YM, et al. Comparison of liver transplantation and liver resection for hepatocellular carcinoma patients with portal vein tumor thrombus type I and type II. Yonsei Med J. (2021) 62(1):29–40. doi: 10.3349/ymj.2021.62.1.29
22. Soin AS, Bhangui P, Kataria T, Baijal SS, Piplani T, Gautam D, et al. Experience with LDLT in patients with hepatocellular carcinoma and portal vein tumor thrombosis postdownstaging. Transplantation. (2020) 104(11):2334–45. doi: 10.1097/TP.0000000000003162
23. Huo L, Wei W, Yan Z, Lei Z, Xie Y, Gong R, et al. Short-term and long-term outcomes of liver resection for HCC patients with portal vein tumor thrombus. Cell Biosci. (2019) 9:23. doi: 10.1186/s13578-019-0285-z
24. Wang YC, Lee JC, Wu TH, Cheng CH, Lee CF, Wu TJ, et al. Improving outcomes of liver resection for hepatocellular carcinoma associated with portal vein tumor thrombosis over the evolving eras of treatment. World J Surg Oncol. (2021) 19(1):313. doi: 10.1186/s12957-021-02425-w
25. Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). (2017) 19(8):659–66. doi: 10.1016/j.hpb.2017.04.016
26. Chen S, Yu W, Zhang K, Liu W, Wang X, Chen C. Hepatic arterial infusion of oxaliplatin plus raltitrexed in unresectable hepatocellular carcinoma with or without portal vein tumour thrombosis. Gastroenterol Rep (Oxf). (2022) 10:goac016 doi: 10.1093/gastro/goac016
27. Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, et al. Combination therapy of chemoembolization and hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis compared with chemoembolization alone: a propensity score-matched analysis. Biomed Res Int. (2021) 2021:6670367. doi: 10.1155/2021/6670367
28. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
29. Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, et al. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. (2011) 26(8):1014–22. doi: 10.3346/jkms.2011.26.8.1014
30. Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z, et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: an open-label randomized controlled trial. Radiother Oncol. (2019) 140:20–5. doi: 10.1016/j.radonc.2019.05.006
31. Hu HT, Luo JP, Cao GS, Li Z, Jiang M, Guo CY, et al. Hepatocellular carcinoma with portal vein tumor thrombus treated with transarterial chemoembolization and sorafenib vs. (125)Iodine implantation. Front Oncol. (2021) 11:806907. doi: 10.3389/fonc.2021.806907
Keywords: hepatocellular carcinoma, macrovascular invasion, liver resection, prognosis, conversion therapy
Citation: Ji M, Zou H, Shu B, Liu G, Zhang B, Xu Z, Pang F, Cheng M, Sun Y, Du T, Sun C and Zhu C (2022) Prognostic analysis of hepatocellular carcinoma with macrovascular invasion after liver resection and a successful case of conversion therapy. Front. Surg. 9:1042431. doi: 10.3389/fsurg.2022.1042431
Received: 12 September 2022; Accepted: 10 October 2022;
Published: 7 November 2022.
Edited by:
Mingyu Chen, Sir Run Run Shaw Hospital, ChinaReviewed by:
Win Topatana, Zhejiang University, ChinaMatas Jakubauskas, Vilnius University, Lithuania
© 2022 Ji, Zou, Shu, Liu, Zhang, Xu, Pang, Cheng, Sun, Du, Sun and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan-dong Sun c3VuY2RAcWR1aG9zcGl0YWwuY24= Cheng-zhan Zhu emh1Y2hlbmd6QHFkdWhvc3BpdGFsLmNu
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Mengling Ji
Mengling Ji Hao Zou
Hao Zou Baojun Shu2
Baojun Shu2 Chengzhan Zhu
Chengzhan Zhu