
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 06 January 2023
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1038219
 Guanyu Jiang†
Guanyu Jiang† Chenghu Song†
Chenghu Song† Yongrui Xu
Yongrui Xu Shengfei Wang
Shengfei Wang Huixing Li
Huixing Li Rongguo Lu
Rongguo Lu Xiaokun Wang
Xiaokun Wang Ruo Chen
Ruo Chen Wenjun Mao*
Wenjun Mao* Mingfeng Zheng*
Mingfeng Zheng*
Lung cancer has become the leading cause of cancer death all over the world. Nowadays, there is a consensus that the treatment of non-small cell lung cancer (NSCLC) prefers a combination of multidisciplinary comprehensive treatment and individualized treatment, which can significantly improve the prognosis of patients. Here, we report a female patient with recurrence-prone NSCLC. She had a decade-long disease course, during which the lesion recurred twice and finally cured with Multi-Disciplinary Treatment (MDT). An elderly female patient was admitted to the hospital after diagnosis of lung cancer, and treated with surgery and postoperative adjuvant chemotherapy. Five years later, suspicious lesions were found by computed tomography (CT) reexamination, and then confirmed tumor recurrence by puncture biopsy. Based on the genetic test results, gefitinib was used for subsequent targeted therapy, and the lesion gradually shrunk to disappear. However, the lesion appeared again two years later, after consultation the microwave ablation was adopted and the curative effect was excellent. At last, regular reexamination showed no abnormality, the patient has survived so far. The case proves the great benefit of multidisciplinary comprehensive treatment, especially microwave ablation for patient with recurrence-prone NSCLC. And the effect of systemic anti-tumor immune response induced by microwave ablation on lung cancer also needs to be further explored.
Based on reports, lung cancer has become the second common cancer in the world, and also the leading cause of cancer death (1). With the advancement of medical technology and in-depth research on lung cancer at the genetic level, various treatment modalities such as interventional therapy, targeted therapy and immunotherapy have been introduced (2). Nowadays, after evaluating the patient's body condition, histopathological type and molecular classification of the tumor, the scope of invasion and the trend of development, with a planned and rational application of surgery, radiotherapy, chemotherapy, interventional therapy, molecular targeted therapy and immunotherapy, it has become a consensus to adopt the mode of multidisciplinary comprehensive therapy combined with individualized therapy, which can maximize the survival time of patients. Here, we report a female patient with recurrence-prone NSCLC, whose disease course has lasted for almost ten years since first diagnosed. During this period, she experienced lesion recurrences twice and benefited from the multidisciplinary comprehensive treatment.
The patient, a 61-year-old female, was found to have a right lower lung occupancy of 2.0 cm in size by chest computed tomography (CT) on April 13, 2013 (Figure 1A), and then hospitalized. Tumor indicators: Alpha-fetoprotein (AFP): 3.39 ng/ml; Carcinoembryonic antigen (CEA): 1.30 ng/ml; Carbohydrate antigen (CA) 125: 3.30 U/ml; CA 19-9: 3.80 U/ml; Cytokeratin 19 fragments (CYFRA21-1): 2.0 ng/ml; Neuron-specific enolase (NSE): 14.10 ng/ml; CA 15-3: 5.1 U/ml (Figure 2). The video-assisted lobectomy of lung was performed under general anesthesia on April 17, 2013. During the operation, a mass in the outer basal segment of the right lower lobe was found, which was about 3 cm in diameter, medium texture and with pleural shrinkage on the surface. The postoperative pathology showed that the infiltrative adenocarcinoma of the right lower lung was grade II-III (alveolar predominant type), invading the dirty pleura (Figures 3A,B). “Group 10 lymph nodes” (0/1) and “Group 11 lymph nodes” (0/2). Immunohistochemistry: CK7+, CK5/6-, P63+, CK51+, TTF1+, spa+, P16+, ER+ (20%), PR+ (10%), DOG1−. Specimen genetic test results: EGFR Exon19 Deletion mutant; KRAS Exon2, Exon3 wild type; high expression in ERCC1, high expression in TS, low expression in RRM1, low expression in TUBB3. Pathological stage: PT2aN0M0, stage IB. The patient recovered well after surgery, and received six cycles of adjuvant chemotherapy with “paclitaxel 240 mg/d1 + DDP 30 mg/d1-3” from May 11, 2013. No obvious abnormality was found in the follow-up 5 years after operation.
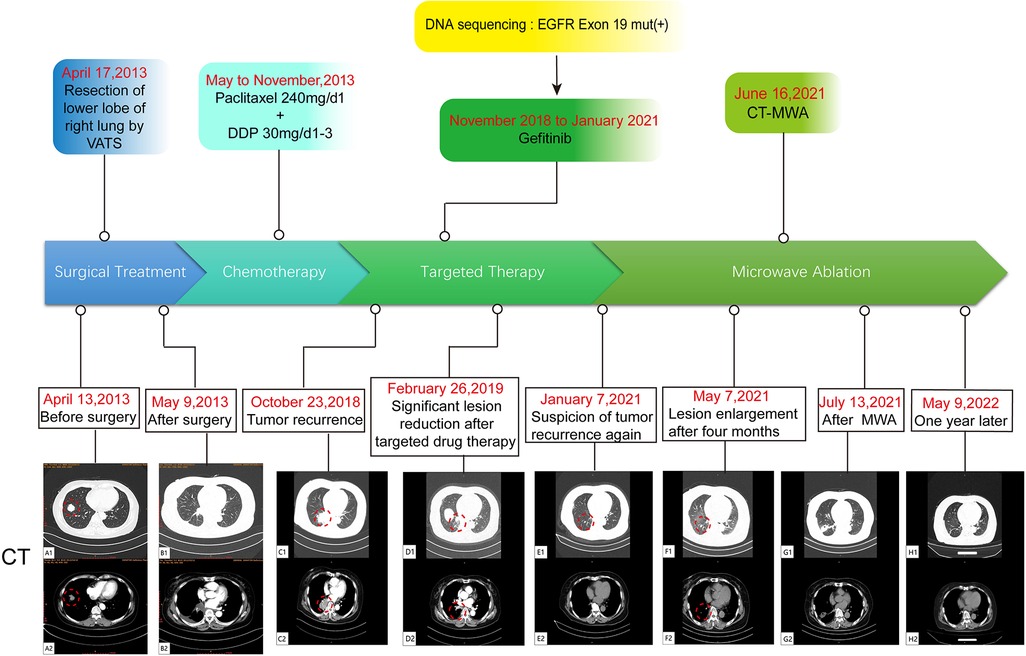
Figure 1. A flow chart of the entire treatment course. Red circles indicate the target lesion. VATS, video-assisted thoracic surgery; DDP, cis-diamminedichloroplatinum; CT-MWA, CT Guided Microwave Ablation.
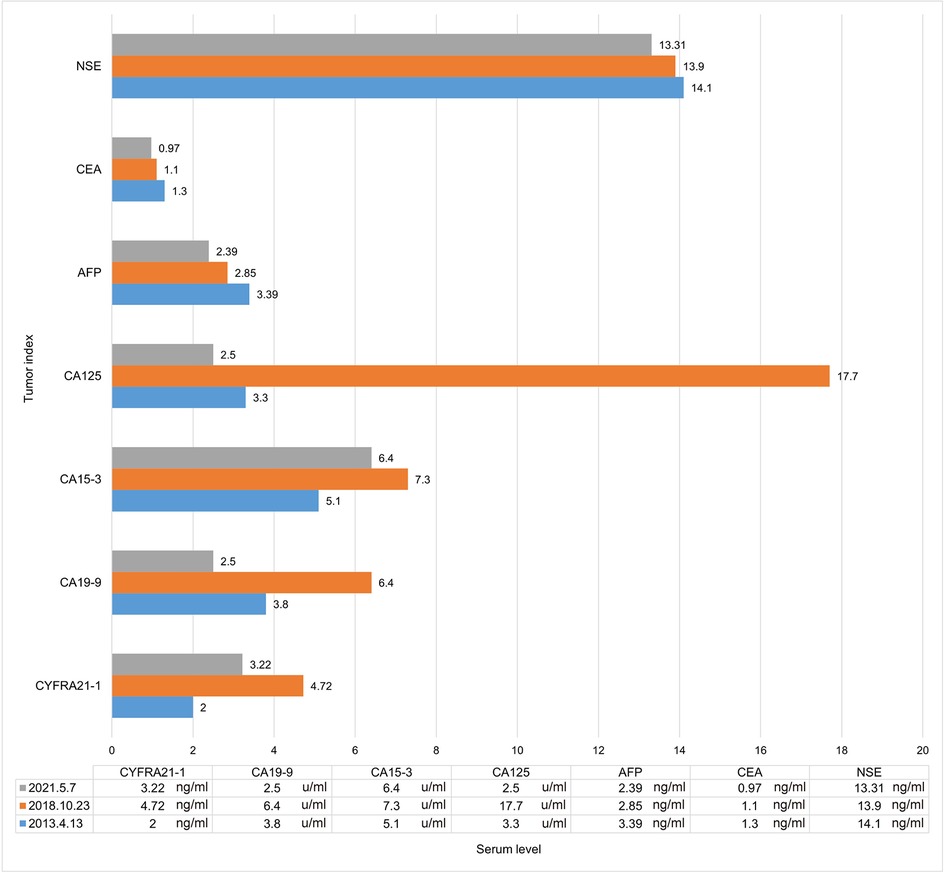
Figure 2. Tumor indicator levels when discovering lesions. AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; CYFRA21-1, cytokeratin 19 fragment; CA125, carbohydrate antigen 125; CA19-9, carbohydrate antigen 19-9; CA15-3, carbohydrate antigen 15-3.
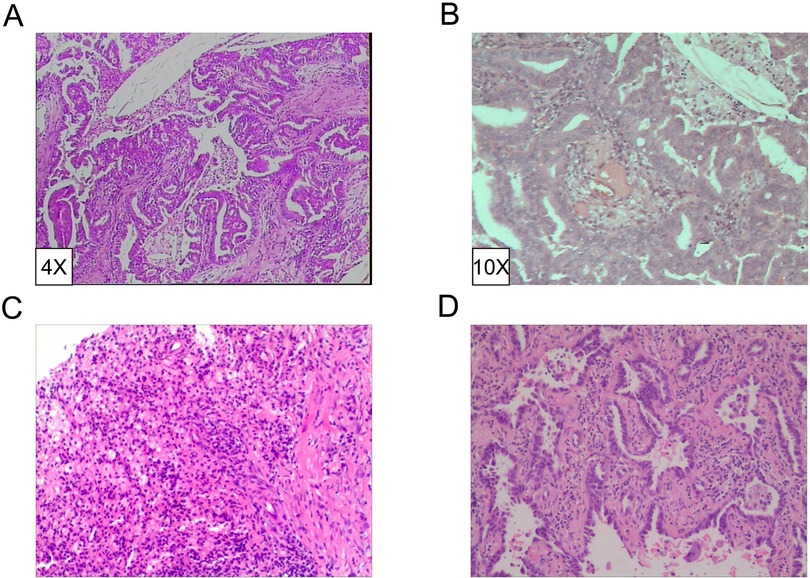
Figure 3. Pathological diagnosis of lesions. (A,B) Invasive adenocarcinoma grade II-III (alveolar dominant type). IHC revealed CK7+, CK5/6-, P63+, CK51+, TTF1+, SPA+, P16+, ER+ (20%), PR+ (10%), DOG1-. (HE4X/10X). (C) Chronic granulomatous inflammation with mechanization. (D) Invasive non-mucinous adenocarcinoma.
On October 23, 2018, a soft tissue density occupancy in surgery area with pleural invasion was found by chest CT (Figure 1C), and the possibility of lung cancer recurrence was considered. Tumor indices: AFP: 2.85 ng/ml; CEA: 1.10 ng/ml; CA 125: 17.70 U/ml; CA 19-9: 6.40 U/ml; CYFRA21-1: 4.72 ng/ml; NSE: 13.90 ng/ml; CA 15-3: 7.30 U/ml (Figure 2). On October 30, 2018, the percutaneous aspiration biopsy of lung (PABL) was performed, and the pathology manifested chronic granulomatous inflammation with mechanization (Figure 3C). On November 6, 2018, positron emission tomography (PET-CT) showed a mass-like hyperdense shadow in the lower lobe of the right lung with abnormal increased glucose metabolism, considering the high possibility of lung cancer recurrence. On November 15, 2018, the second PABL was performed and the pathology of the right lower lung puncture tissue showed invasive non-mucinous adenocarcinoma (Figure 3D). In conjunction with the second molecular testing report: EGFR Exon19 Deletion mutant, the patient received gefitinib (Iressa) as the first-line treatment, and the right lung lesion was found rapidly shrunk on February 26, 2019 (Figure 1D), which proved that the treatment was effective. The subsequent two-year follow-up showed progressive disappearance of lesion.
On January 7, 2021, a lesion was found in the middle lobe of right lung during reexamination, about 8 mm (Figure 1E). On May 7, 2021, the lesion was found to be progressively enlarged, approximately 10 mm in size (Figure 1F). Radiographic examination suggested a high probability of tumor (Supplementary Figure S1). Tumor indicators: AFP: 2.39 ng/ml; CEA: 0.97 ng/ml; CA 125: 2.50 U/ml; CA 19-9: 2.50 U/ml; CYFRA21-1: 3.22 ng/ml; NSE: 13.31 ng/ml; CA 15-3: 6.40 U/ml (Figure 2). Whole-body examination was applied to exclude the presence of metastasis, including head-thorax-abdomen CT, bone scan and so on. Considering the higher risk of surgical bleeding and the relatively less benefit of reoperation after recurrence, CT Guided Microwave Ablation was performed on June 16, 2021, and the pathological biopsy during microwave therapy was confirmed to be adenocarcinoma (Supplementary Figure S2). On July 13, 2021, the chest CT showed that the right middle lung mass with peripheral speckles and cords, which was considered as changes after microwave ablation (Figure 1G). With regular follow-up to May 2022 (Figure 1H), no obvious abnormality was found, which proves a good outcome. The whole therapy process of the patient is shown in Figure 1.
From the last century to present, surgery has always been the best option for the treatment of lung cancer. According to the principles of surgery, lobectomy is the best option for NSCLC patients with good tolerance, while hilar and mediastinal lymph node dissection should also be a routine part of the procedure (3). Several reports have indicated that postoperative adjuvant chemotherapy based on cisplatin can result in a better prognosis (4). The patient's condition also remained stable after six cycles of adjuvant chemotherapy. Although early stage of NSCLC can be resected radically, the postoperative recurrence rate can be 30% to 55%. According to previous research, some concealed micrometastatic cancer cells already exist systematically during surgery, and standard staging methods cannot encompass these cancer cells. Secondly, the spread of tumor cell may also occur during surgical management of the tumor (5). Prior studies have also proved that compared with upper lobectomy, the non-recurrence rate (excluding non-specific death) and overall survival after lower lobectomy are significantly worse (6).
Nevertheless, owing to the tumor genetic test, the patient has a mutation in EGFR Exon19, which meets the criteria of targeted therapy. EGFR, as one kind of tyrosine kinase receptor, its mutations and elevated levels of expression are associated with a variety of cancers, particularly in lung cancer (7). Research have shown that about 15% western patients and 35% Asian patients have EGFR mutations, the most common of which is exon 19 deletion (Del19) (8). A significant increase was recorded in the effectiveness of the therapy in NSCLC patient after applying gefitinib (first-generation tyrosine kinase inhibitor), and the lesion decreased rapidly. Nevertheless, almost all NSCLC patients with EGFR mutations will eventually develop drug resistance to TKI therapy (9). The underlying mechanisms include: 1, EGFR-dependent on-target resistance; 2, bypass-activated off-target resistance; 3, histological type transformation (10). Clinical data also showed that the median progression-free survival after gefitinib treatment was about 10 months (11).
After the second recurrence of lesion, the patient opted for minimally invasive interventional therapy. Percutaneous image-guided thermal ablation (IGTA), including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, is a minimally invasive treatment for NSCLC and thoracic metastatic tumors (12). Several researches have stated that compared with drug therapy, carefully selected local ablation for lung cancer can also improve overall survival and progression-free survival (13). A number of recent studies have noted that in addition to thermal injury, local thermal ablation can induce abscopal effect—one kind of systemic anti-tumor immune response, which may be another mechanism of tumor cell destruction and death. According to previous studies, in MWA-induced immune response, B cells were significantly activated, whose antigen presentation capacity was significantly enhanced. And then tumor antigens were presented to CD4 + T cells, which activated CD4 + T cells and produced more IFN-γ through inducible costimulatory molecule (ICOS-ICOSL) pathway, driving the balance of Th1/Th2 to Th1, and further aiding CD8 + T cell activation to generate CTL, ultimately leading to an enhanced anti-tumor capacity of the systemic immune system (14–17). In addition, macrophages are activated by tumor-associated antigens and produce IL-15, which activates NK cells, generally enhance functional activity of NK cells, and then facilitates the destruction of tumor cells (18). The specific immune mechanisms are shown in Figure 4. The patient then continued maintenance treatment with targeted drugs and is now in stable condition. If the patient relapses in future, we will perform puncture biopsy again for pathological typing and genetic testing (tissue and blood) to determine whether drug-resistant genes are developed, and then consider other treatment options such as stereotactic body radiation therapy (SBRT).
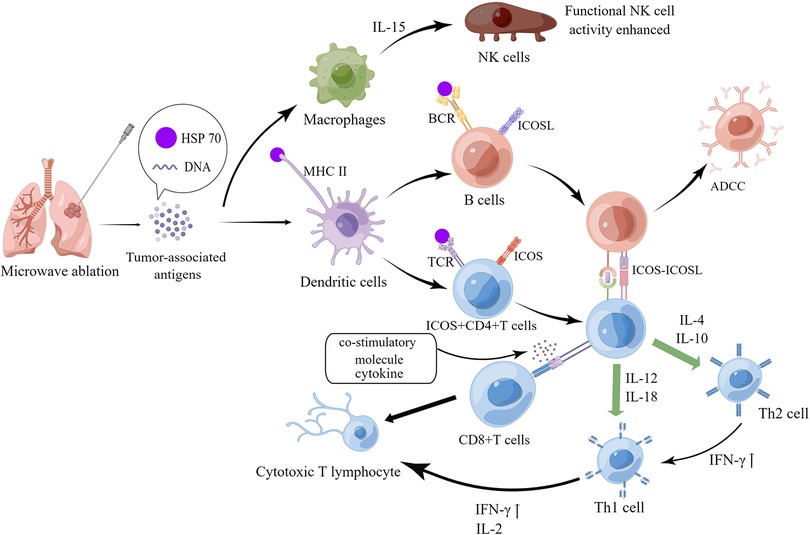
Figure 4. Mechanism of systemic anti-tumor immune response induced by microwave ablation. The figure was drawn by Figdraw.
The entire course of this patient's disease lasted 9 years and 4 months, during which two relapses occurred and treated with different methods. The whole process was rather complicated, while the final prognosis was well.
In conclusion, multidisciplinary comprehensive diagnosis and treatment of recurrence-prone lung cancer has tremendous value in improving the survival of lung cancer patients by developing precise, personalized and appropriate treatment plans. And the effect of systemic anti-tumor immune response induced by microwave ablation on lung cancer also needs to be further explored.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee, The Affiliated Wuxi People's Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WM and MZ: designed the study and participated in coordination and project control. GJ, SW and HL: collected the information of patient. GJ, CS and YX: wrote the draft. RL, XW, RC: revised the manuscript. WM and MZ: got financial support. All authors reviewed and approved the final edition. All authors contributed to the article and approved the submitted version.
This work was partially supported by the Natural Science Foundation of Jiangsu Province (grant no. BK20210068), the Top Talent Support Program for Young and Middle-aged People of Wuxi Municipal Health Commission (grant no. HB2020003) and the High-end Medical Expert Team of the 2019 Taihu Talent Plan (grant no. 2019-THRCTD-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1038219/full#supplementary-material.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. Br Med J. (2021) 375:n2363. doi: 10.1136/bmj.n2363
3. Mangiameli G, Cioffi U, Testori A. Lung cancer treatment: from tradition to innovation. Front Oncol. (2022) 12:858242. doi: 10.3389/fonc.2022.858242
4. Hammerschmidt S, Wirtz H. Lung cancer: current diagnosis and treatment. Dtsch Arztebl Int. (2009) 106:809–18. quiz 819–820. doi: 10.3238/arztebl.2009.0809
5. Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. (2014) 3:242–9. doi: 10.3978/j.issn.2218-6751.2013.12.05
6. Ueda K, Murakami J, Tanaka T, Nakamura T, Yoshimine S, Hamano K. Postoperative complications and cancer recurrence: impact on poor prognosis of lower lobe cancer. Ann Thorac Surg. (2020) 109:1750–6. doi: 10.1016/j.athoracsur.2019.12.061
7. Ernani V, Steuer CE, Jahanzeb M. The end of Nihilism: systemic therapy of advanced non-small cell lung cancer. Annu Rev Med. (2017) 68:153–68. doi: 10.1146/annurev-med-042915-102442
8. Pakkala S, Ramalingam SS. Personalized therapy for lung cancer: striking a moving target. JCI Insight. (2018) 3:120858. doi: 10.1172/jci.insight.120858
9. Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med. (2012) 136:504–9. doi: 10.5858/arpa.2011-0618-RA
10. Passaro A, Leighl N, Blackhall F, Popat S, Kerr K, Ahn MJ, et al. ESMO Expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann Oncol. (2022) 33:466–87. doi: 10.1016/j.annonc.2022.02.003
11. Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. (2019) 137:113–22. doi: 10.1016/j.lungcan.2019.09.017
12. Murphy MC, Wrobel MM, Fisher DA, Cahalane AM, Fintelmann FJ. Update on image-guided thermal lung ablation: society guidelines, therapeutic alternatives, and postablation imaging findings. AJR Am J Roentgenol. (2022) 219(3):471–85. doi: 10.2214/AJR.21.27099
13. Abbas G, Danish A, Krasna MJ. Stereotactic body radiotherapy and ablative therapies for lung cancer. Surg Oncol Clin N Am. (2016) 25:553–66. doi: 10.1016/j.soc.2016.02.008
14. Zhou W, Yu M, Mao X, Pan H, Tang X, Wang J, et al. Landscape of the peripheral immune response induced by local microwave ablation in patients with breast cancer. Adv Sci (Weinh). (2022) 9:e2200033. doi: 10.1002/advs.202200033
15. Li L, Wang W, Pan H, Ma G, Shi X, Xie H, et al. Microwave ablation combined with OK-432 induces Th1-type response and specific antitumor immunity in a murine model of breast cancer. J Transl Med. (2017) 15:23. doi: 10.1186/s12967-017-1124-9
16. Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. (2021) 70. doi: 10.1007/s00262-020-02734-1.33006650
17. Zhou W, Yu M, Pan H, Qiu W, Wang H, Qian M, et al. Microwave ablation induces Th1-type immune response with activation of ICOS pathway in early-stage breast cancer. J Immunother Cancer. (2021) 9:e002343. doi: 10.1136/jitc-2021-002343
Keywords: lung cancer, multidisciplinary treatment, target therapy, microwave ablation, immune reaction
Citation: Jiang G, Song C, Xu Y, Wang S, Li H, Lu R, Wang X, Chen R, Mao W and Zheng M (2023) Recurrent lung adenocarcinoma benefits from microwave ablation following multidisciplinary treatments: A case with long-term survival. Front. Surg. 9:1038219. doi: 10.3389/fsurg.2022.1038219
Received: 6 September 2022; Accepted: 21 November 2022;
Published: 6 January 2023.
Edited by:
Calvin Sze Hang Ng, The Chinese University of Hong Kong, ChinaReviewed by:
Leonidas Papastavrou, Athens Medical Center, Greece© 2023 Jiang, Song, Xu, Wang, Li, Lu, Wang, Chen, Mao and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingfeng Zheng emhlbmdtZm1lZGljYWxAMTI2LmNvbQ== Wenjun Mao bWFvd2VuanVuMUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.