- 1Department of Urology, The First Affiliated Hospital of Nanchang University, Nanchang City, China
- 2Department of Urology, Shangrao Municipal Hospital, Shangrao City, China
Background: Metastatic bladder cancer (MBC) is an incurable malignancy, which is prone to early death. We aimed to establish models to evaluating the risk of early death in patients with metastatic bladder cancer
Methods: The data of 1,264 patients with MBC registered from 2010 to 2015 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. We utilized X-tile software to determine the optimal cut-off points of age and tumor size in diagnosis. Univariate and multivariate logistic regression analyses were used to identify significant independent risk factors for total early death and cancer-specific early death, then we construct two practical nomograms. In order to validate our prediction models, we performed calibration plots, receiver operating characteristics (ROCs) curves, decision curve analysis (DCA) and clinical impact curve (CIC).
Result: A total of 1,216 patients with MBC were included in this study. 463 patients died prematurely (≤3 months), and among them 424 patients died of cancer-specific early death. The nomogram of total premature death was created by surgery, chemotherapy, tumor size, histological type, liver metastases, and nomogram of cancer-specific early death was based on surgery, race, tumor size, histological type, chemotherapy, and metastases (liver, brain). Through the verify of calibration plots, receiver operating characteristics (ROCs) curves, decision curve analysis (DCA) and clinical impact curve (CIC), we concluded that nomogram were a valid tool with excellent clinical utility to help clinicians predict premature death in MBC patients.
Conclusions: The nomograms derived from the analysis of patients with MBC, which can provide refined prediction of premature death and furnish clinicians with useful ideas for patient-specific treatment options and follow-up scheduling.
Introduction
Bladder cancer is a malignancy disease that occurs widely throughout the world. Most of all diagnosed patients with this disease is non-muscle invasive. 30% have an initial diagnosis of muscle-invasive bladder cancer and 5% a metastatic bladder carcinoma (1). Approximately 550,000 people suffer from this disease each year, nearly 200,000 people died from bladder tumors just in 2018 alone (2). MBC is often accompanied with a high cancer-specific-death rate, and the prognosis of MBC patients tends to be poor (3). In addition, patients with MBC are still at high risk of early death for a variety of reasons. Exploring these risk factors associated with premature death can help clinicians to predict premature death, develop individualized treatment plans, reduce the incidence of premature death and develop follow-up programmes.
To date, studies on premature death in patients with MBC has not been reported. Therefore, there is a great demand to develop a simple-to-use and practical tool to identify the risk factors leading to early death in MBC patients. Nomogram is a useful statistical model of integrate relevant factors to predict individual tumor prognosis (4), which have been widely used to assist physicians in developing treatment plans and assessing the prognosis of various cancers. The National Comprehensive Cancer Network guidelines have already introduced many nomograms with excellent performance (5).
The data for our study were obtained from the Surveillance, Epidemiology and End Results (SEER) database, the definitive cancer population registry in the US, which collects incidence and survival data for approximately 34.6% of all cancers in the US cancer registry. We obtained data of 1,264 patients with metastatic bladder cancer registered between 2010 and 2015 in the SEER, and used these to create two practical nomograms to assess its premature death, with the aim of helping clinicians to better develop their clinical work.
Materials and methods
Patients
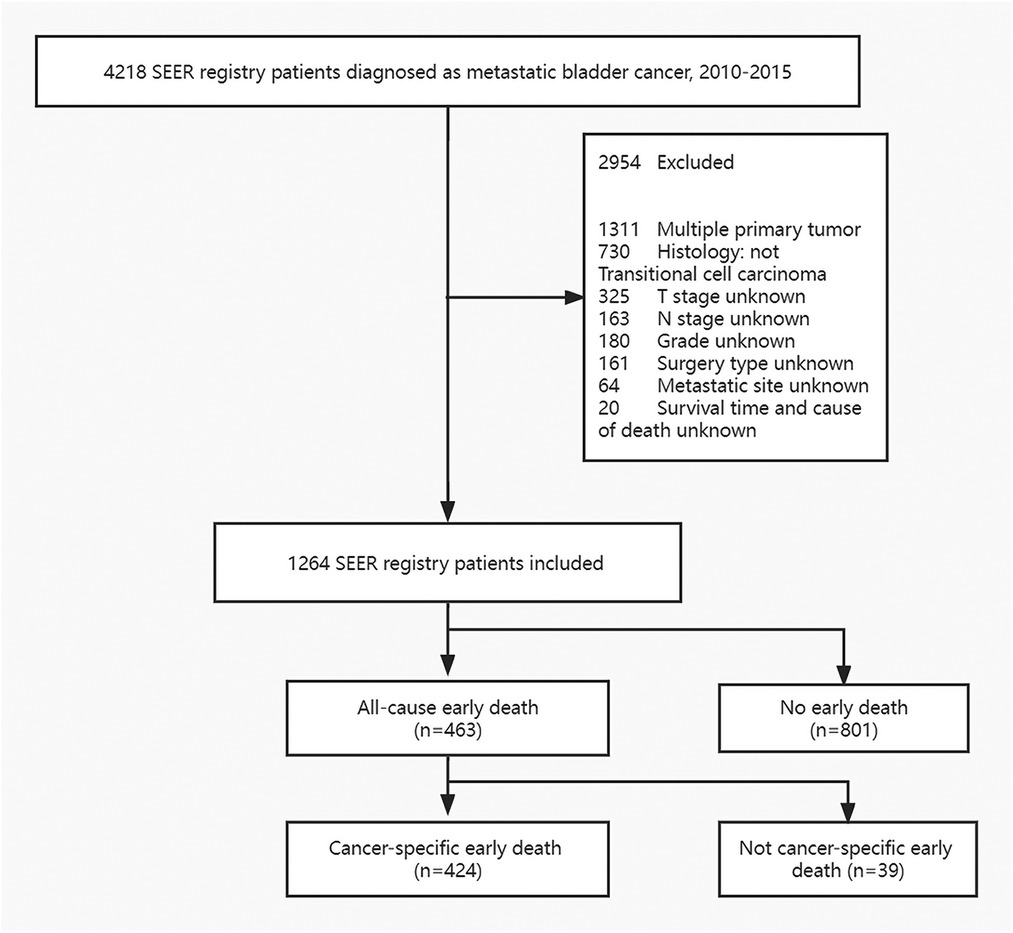
The data were extracted using SEER*Stat version 8.3.6.1. In the SEER database, patients with metastatic bladder cancer were registered from 2010 to 2015 according to the International Classification of Tumor Diseases Third Edition. The inclusion criteria were: (1) bladder cancer is the only one primary carcinoma and (2) Histology type is transitional cell carcinoma. Exclusion principles were: (1) unknown cause of death and survival time, (2) lack of metastatic site information, (3) unknown tumor size, (4) undetermined T and N stage and Grade, (5) unknown race and marital status, 6) lack of surgical information. The concrete screening process was shown in Figure 1.
Ultimately, we included the following variables: Age at diagnosis, gender, race, histological type, tumor size, grade, T-stage (AJCC 7th edition), N-stage (AJCC 7th edition), bone, lung, liver and brain metastases, surgery, radiotherapy, chemotherapy. Based on previous experience, we defined patients who dies within three months of diagnosis as early death (6, 7).
Nomogram construction and statistical analysis
For age and tumor size at diagnosis, we utilized the X-tile software to calculate the optimal cut-off points (Figure 2). The total cohort was randomly divided training and validation cohort in the ratio of 7 : 3, Univariate and multivariate logistic regression analyses were utilized to calculate the odds ratios (OR) and 95% confidence intervals (CI). Then, the identified risk factors were used to construct practical nomograms that can predict early death of MBC. Nomograms were evaluated by bootstrapping (1,000 resamples) to generate calibration plot (8) and the area under the curve (AUC) of the receiver operating characteristic curve (ROC) (9). The clinical benefit of nomograms was assessed by means of clinical decision curves (DCA) and clinical impact curve (CIC) (10). All statistical analyses were performed using SPSS (version 24.0; SPSS, Inc.), X-tile software, and software packages (rms, pROC, and rmda) in R software version 4.1.2. We measured two-tailed p values ≤ 0.05 to be statistically significant.
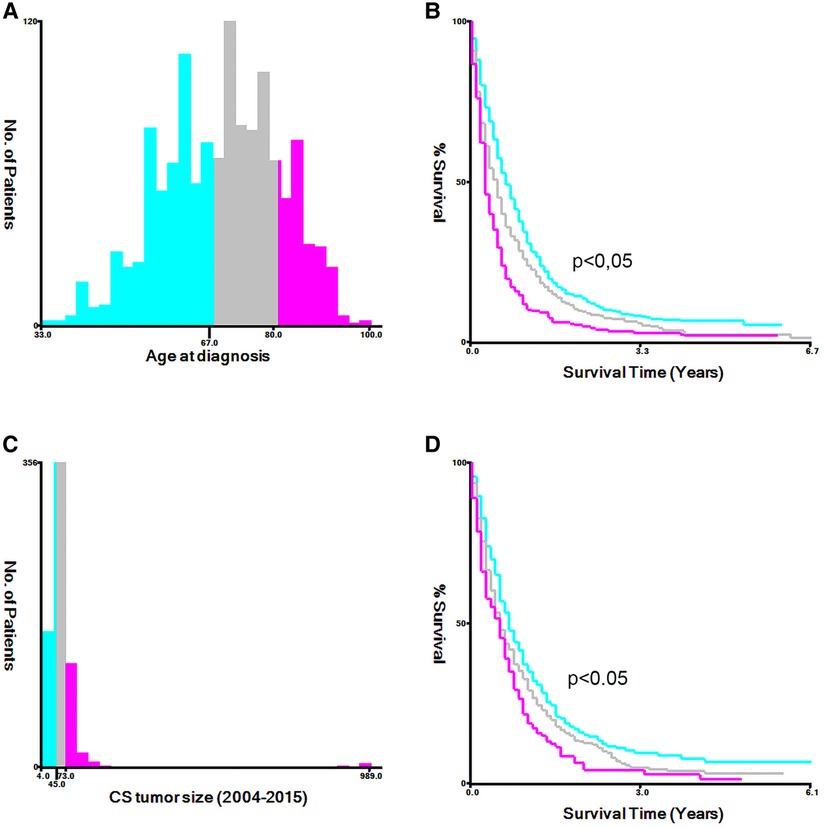
Figure 2. The appropriate cutoff values of age and tumor size was assessed by X-tile analysis. (A,B) The appropriate cutoff values of age were 67 and 80 years; (C,D) The appropriate cutoff values of tumor size were 45 and 73 mm.
Result
Patient characteristics
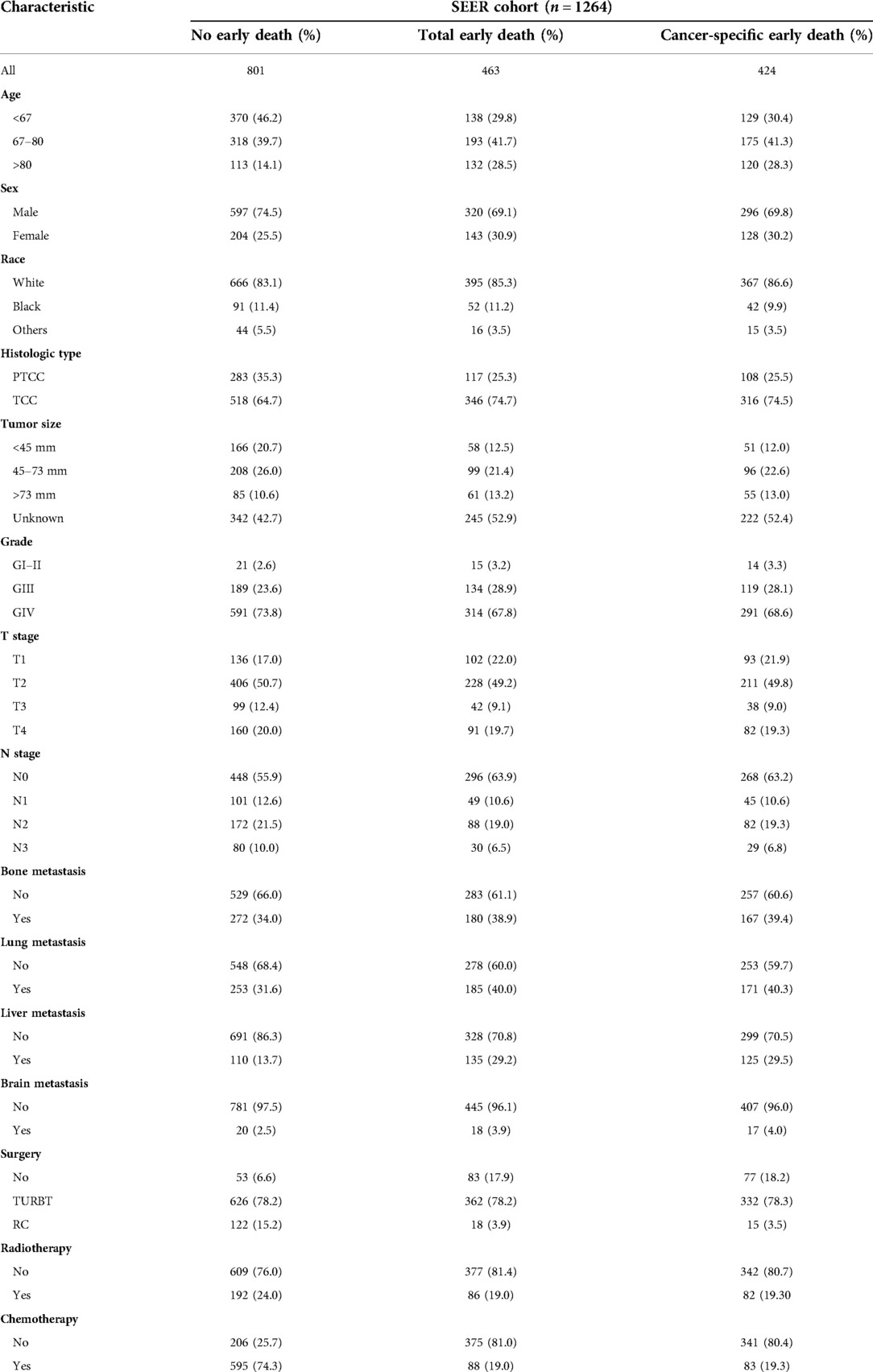
A total of 1,264 patients with metastatic bladder cancer were included in the SEER database according to the inclusion and exclusion criteria. Among them, 463 people died prematurely, and 424 people died of tumor specificity. The majority of those who died prematurely were white (86.6%) and between the ages of 67 and 80 (41.3%). The most common pathological type was transitional cell carcinoma (74.5%). And the more common sites of tumor metastasis are bone (38.9%) and lung (40.0%). Most patients with early death received transurethral resection of bladder tumor (78.3%), without radiotherapy (80.7%) and chemotherapy (80.4%). The concrete characteristics of patients with MBC are shown in Table 1.
Analysis of independent risk factors
Risk factors associated with early death of MBC patients were identified by univariate and multivariate logistic regression analysis. Five common factors were considered to be strongly associated with total and cancer-specific early death, including histological type, tumor size, liver metastasis, surgery, and chemotherapy. Differently, race and brain metastasis were independent risk factors for cancer-specific early death. Tables 2, 3 demonstrate the result of univariate and multivariate logistic regression analysis.
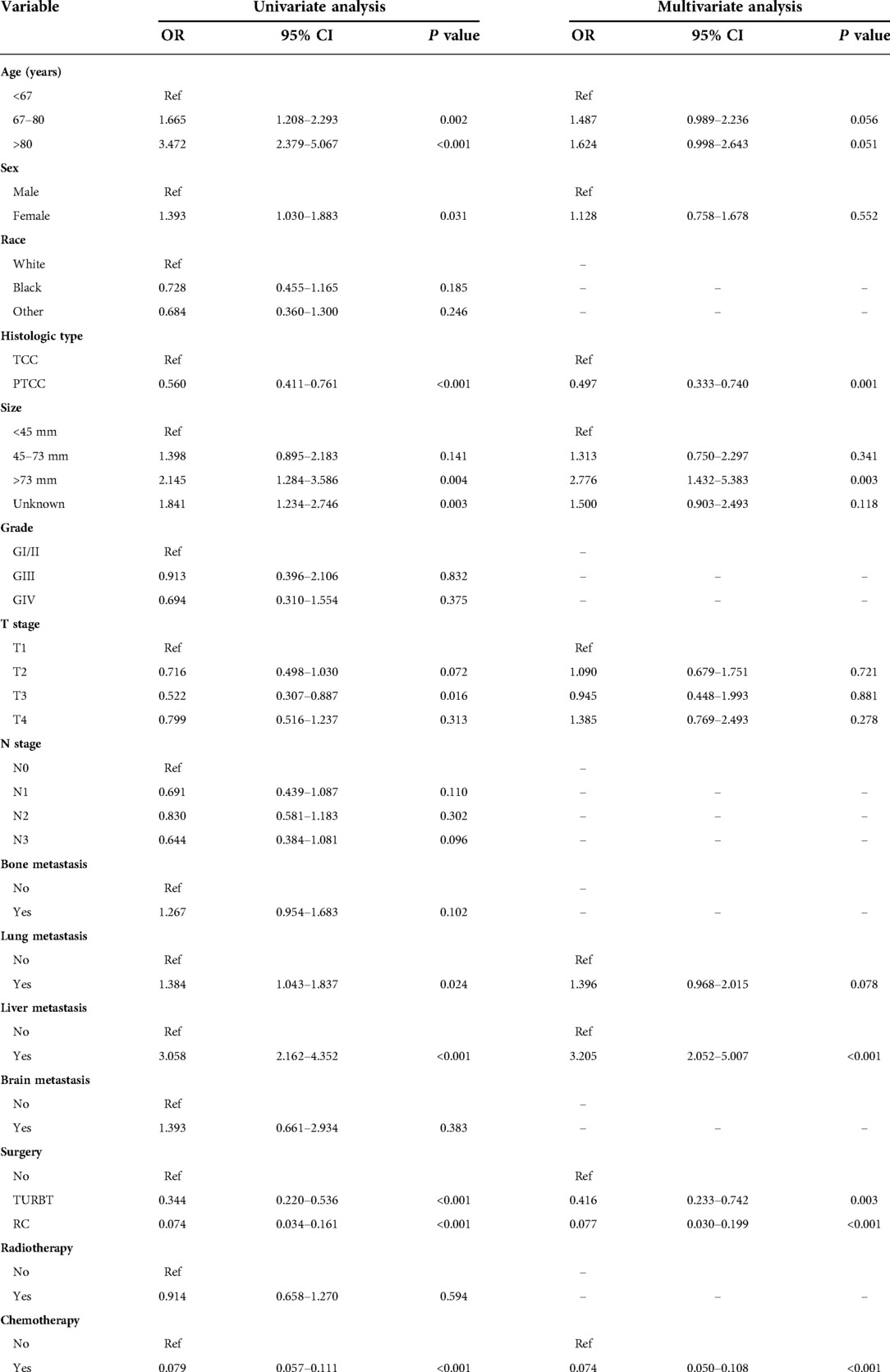
Table 2. The univariable and multivariate logistic regression analysis of total early death in the training cohort.
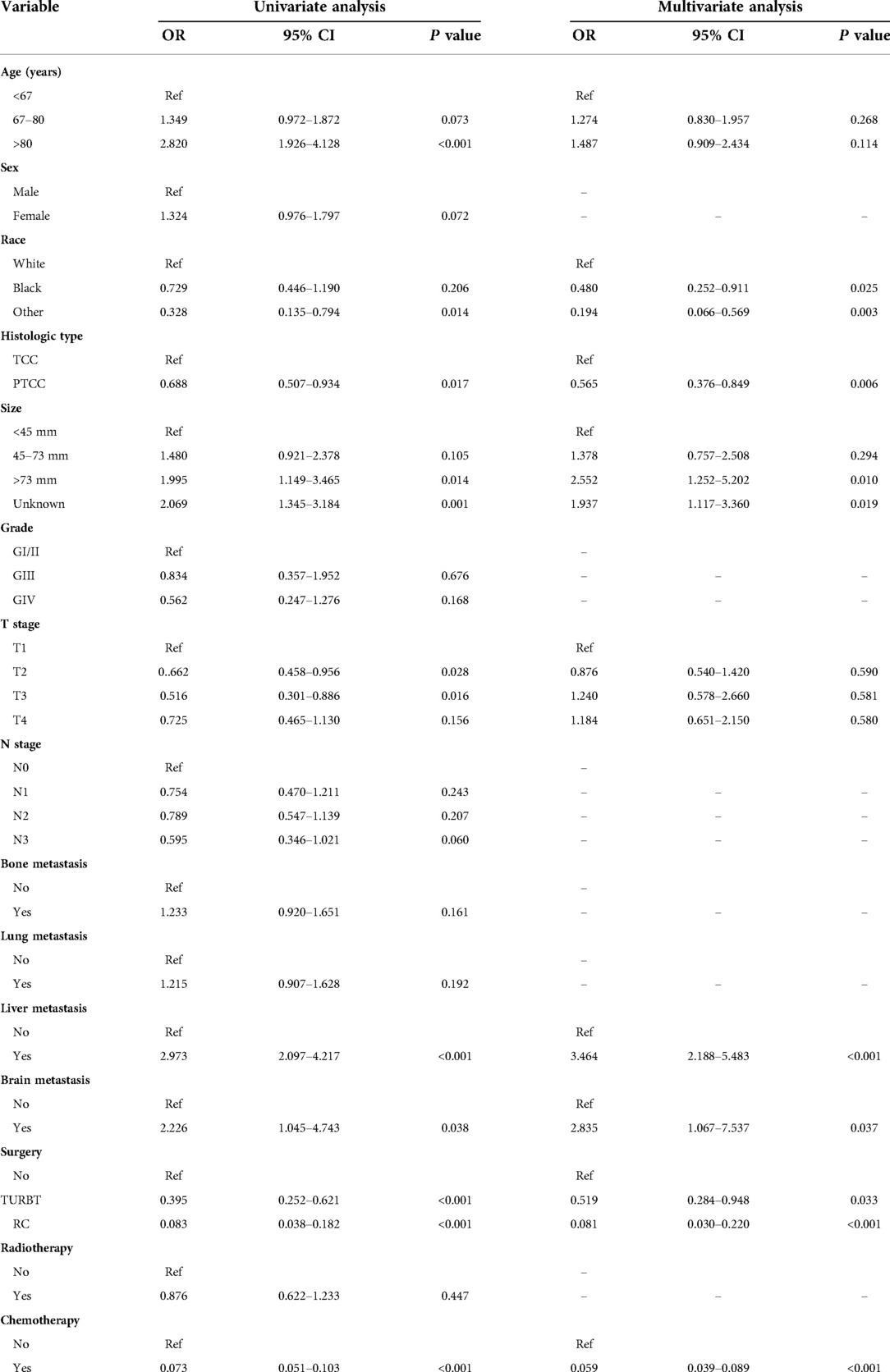
Table 3. The univariable and multivariate logistic regression analysis of cancer-specific early death in the training cohort.
Nomogram construction
After identifying relevant variables by univariate regression analysis and multivariate regression analysis, nomograms for total early death and cancer-specific early death were constructed. The incidence of early death in MBC patients can be predicted by calculating a sum of the scores for each risk factor (Figure 3).
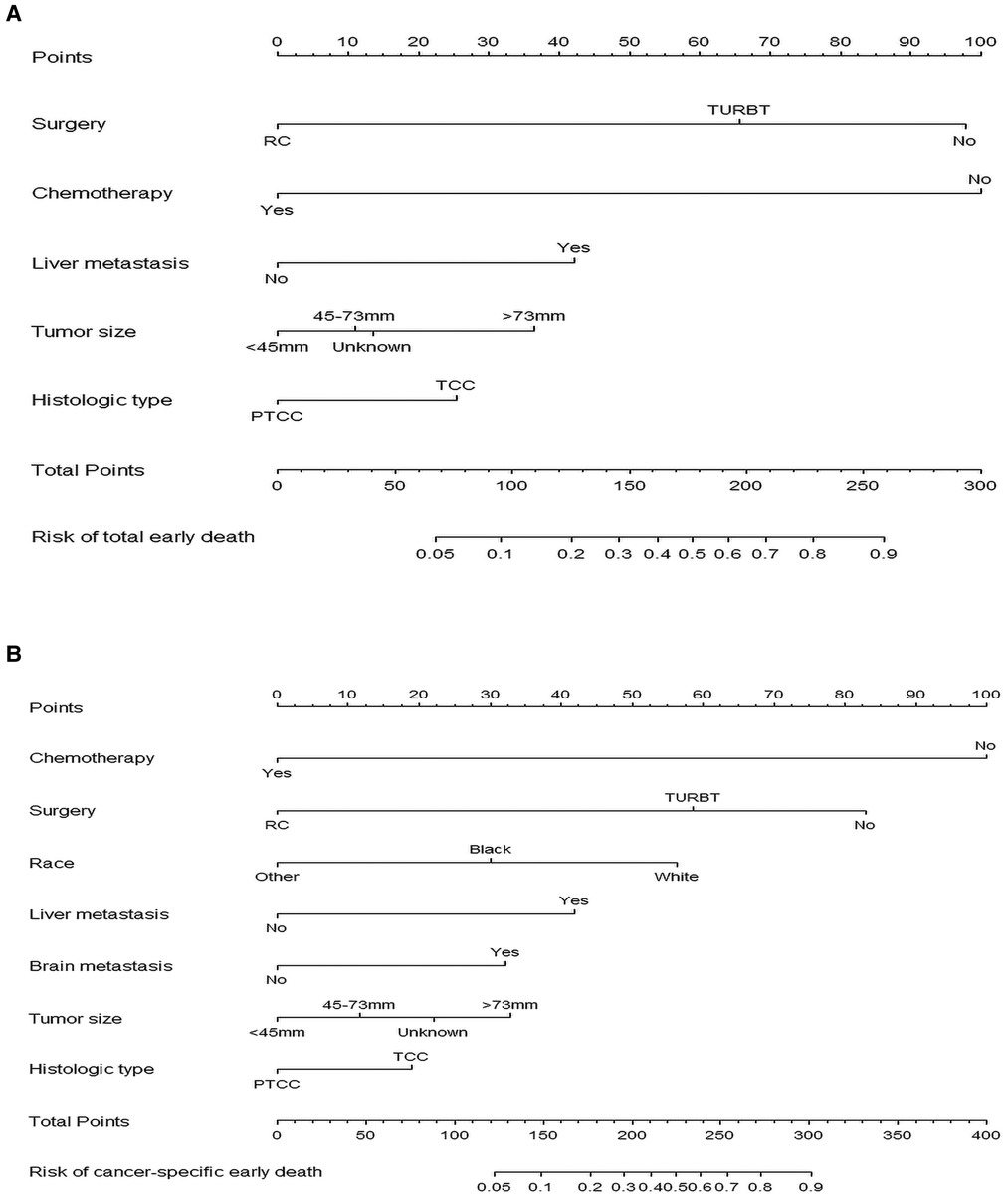
Figure 3. The nomograms of early death in patients with metastatic bladder cancer. (A) The total early death; (B) The cancer-specific early death.
Nomogram validation
We used ROC curve, calibration plot, DCA and CIC to validate the nomograms. According to the ROC curves, the AUC value for total premature death in the training cohort was 0.859 and for cancer-specific premature death was 0.869; the AUC value for total premature death in the validation cohort was 0.827 and for cancer-specific premature death was 0.816 Figure 4). In the calibration curves for both total premature death and cancer-specific premature death, the solid lines were all close to 45° for both the training and validation cohorts (Figure 5), showing the reliability of the models. Finally, compared with other factors, the DCA and CIC of both the training and validation cohorts yielded excellent net clinical benefit (Figures 6, 7).
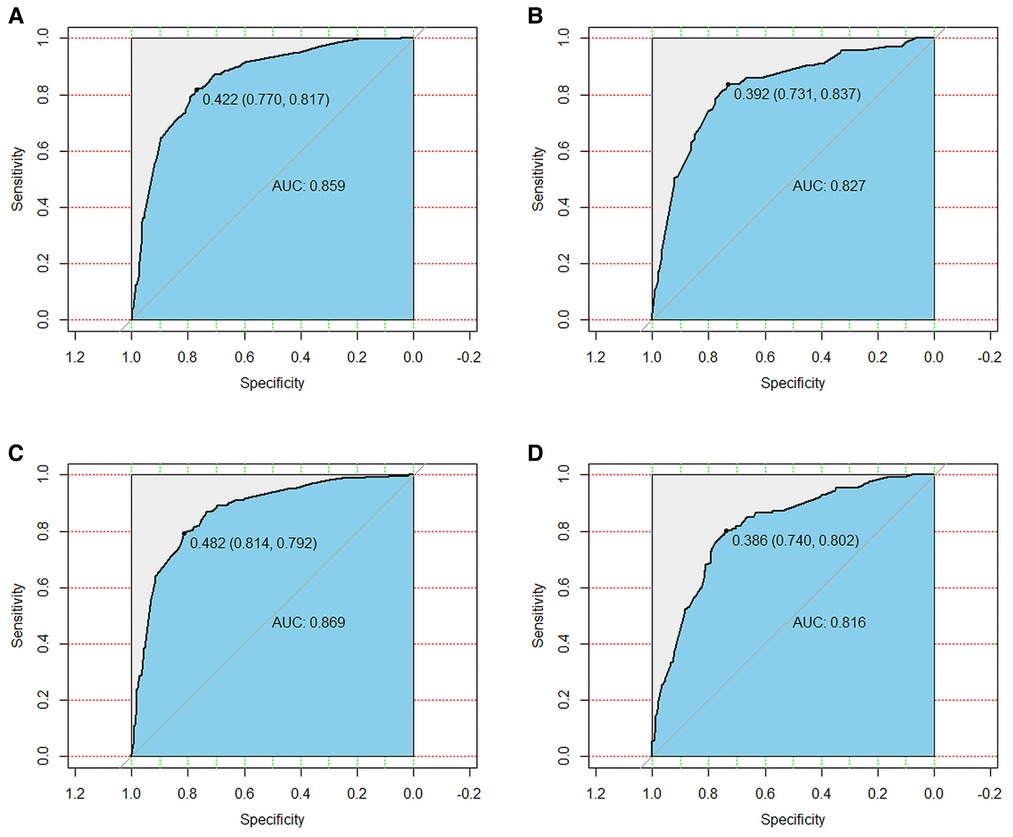
Figure 4. The receiver operating characteristic (ROC) curve for nomogram. (A) The training cohort of total early death; (B) The validation cohort of total early death; (C) The training cohort of cancer-specific early death; (D) The validation cohort of cancer-specific early death. AUC, area under the curve; ROC, receiver operating characteristic.
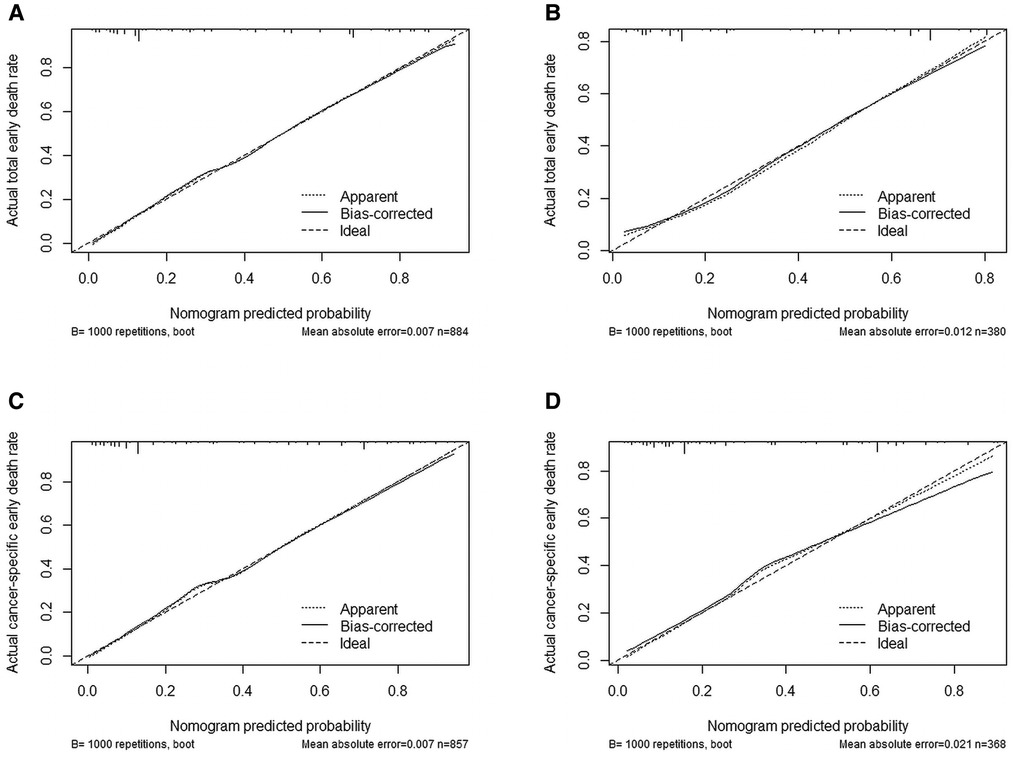
Figure 5. Internal verification plots of nomogram calibration curves by bootstrapping with 1,000 resamples. (A) The training cohort of total early death; (B) The validation cohort of total early death; (C) The training cohort of cancer-specific early death; (D) The validation cohort of cancer-specific early death.
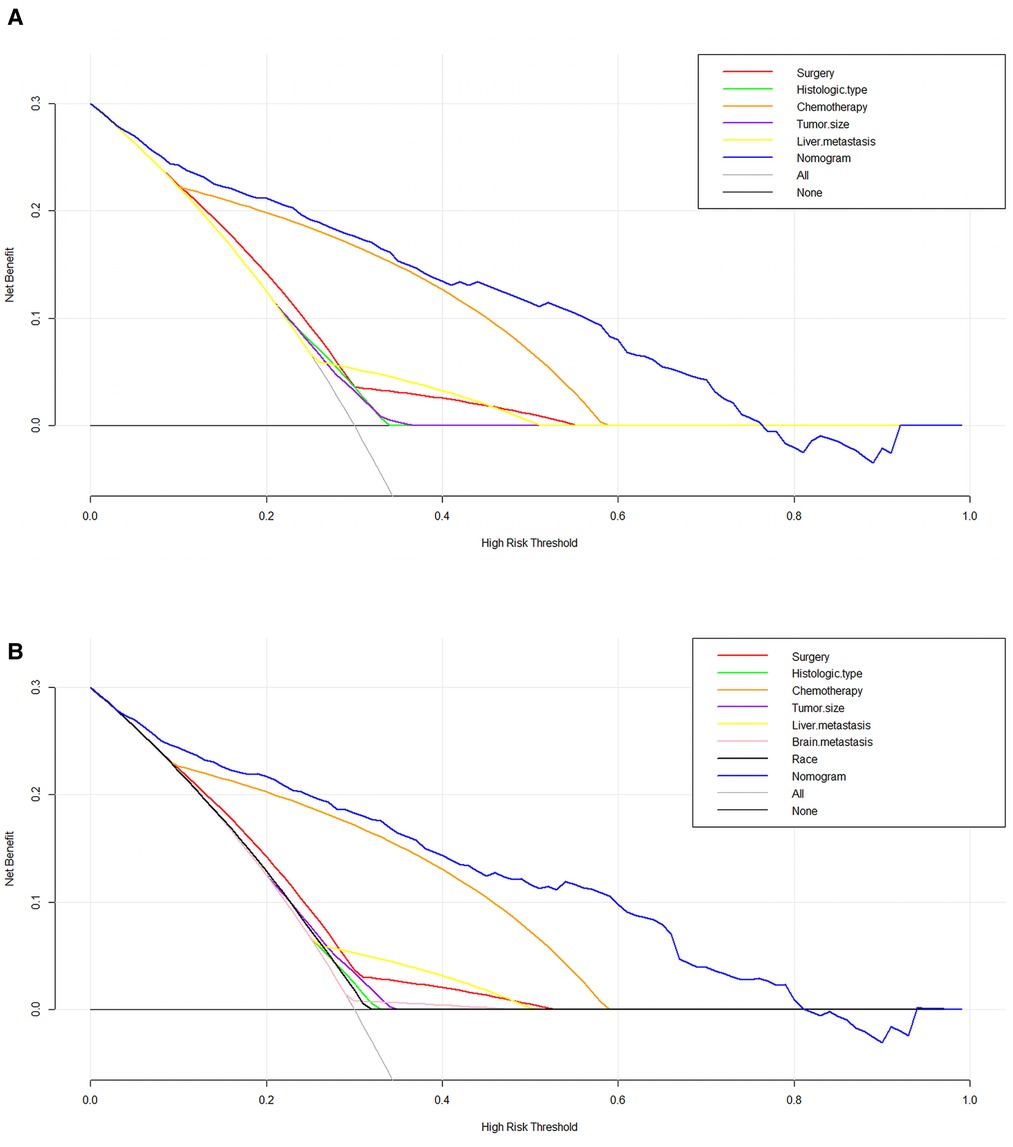
Figure 6. The decision curve analysis (DCA) curve for nomogram. (A) The total early death; (B) The cancer-specific early death.
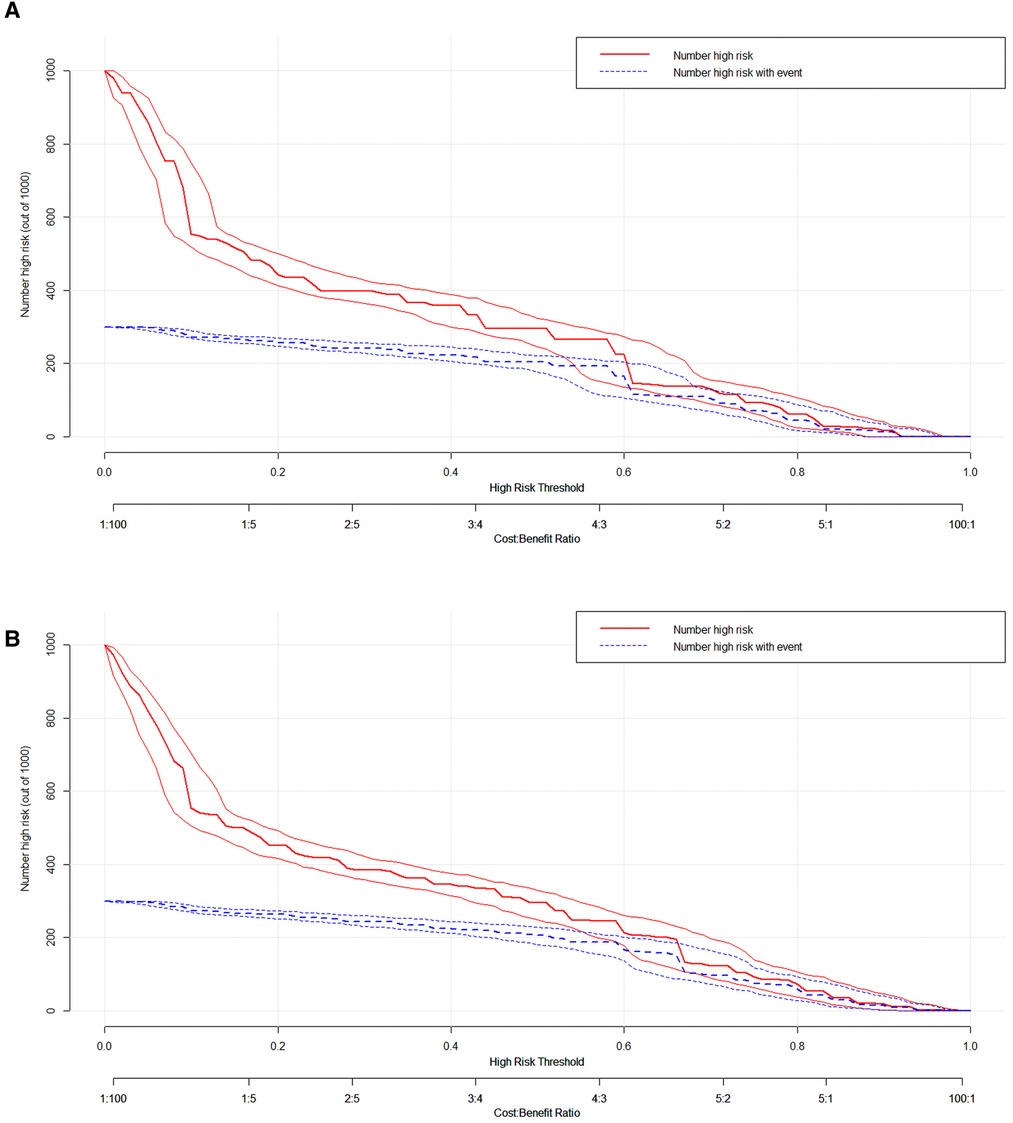
Figure 7. The clinical impact curve (CIC) curve for nomogram. (A) The total early death; (B) The cancer-specific early death.
Discussion
Bladder cancer is a highly heterogeneous disease, which shows a difference in prognosis. The prognosis of non-muscle invasive bladder cancer is generally favorable, with a 5-year recurrence-free survival rate of 23% and a 5-year progression-free survival rate of 54% (11). In contrast, the prognosis is poorer for muscle-invasive bladder cancer, with an overall 5-year survival rate about 36%–48% (12). Due to patients' adherence and treatment limitations, surgical treatment of patients with muscle-invasive bladder cancer is often not as effective as it could be. As previous studies have shown that more than half of patients with muscle-invasive bladder cancer do not receive the radical treatment (13). Even in patients who have undergone radical treatment, 50% of patients will progress to metastatic bladder cancer (14, 15), In addition, previous predictive models have indicated that patients with non-muscle invasive bladder cancer with lymphovascular infiltration (16) and micropapillary tissue (17) are also susceptible to progression to metastatic disease, bringing a poor prognosis. Patients with MBC have a poor prognosis and are often prone to early death. Therefore, to predict early death in MBC patients is a crucial issue that need to be addressed. As far as we know, few people have explored the risk factors of early death in metastatic bladder cancer. In 2014, Aziz et al. established a nomogram predicting 90-day mortality in patients with bladder cancer after radical cystectomy. Their model included age, American Society of Anesthesiologists score, hospital volume, clinically lymphatic metastases and clinically distant metastases (18). Unlike their research, the target population of our study was metastatic bladder cancer patients, which were more prone to early death. The early mortality of metastatic bladder cancer was higher (37% vs. 9.0%). In addition, our research was based on a larger sample size (1,264 vs. 597). The prediction accuracy of our model was higher (0.859 vs. 0.788). Ultimately, our study showed that larger tumor size, transitional cell carcinoma, liver metastases, lung metastases, and patients who did not undergo surgery and chemotherapy had an increased probability of early death.
According to the latest European Association of Urology guidelines, cisplatin-based chemotherapy is till the first choice for appropriate patients with metastatic bladder cancer. For patients who do not meet the requirements of cisplatin, immunotherapy is recommended for patients with positive programmed death ligand 1 (PD-L1), or carboplatin therapy is recommended for patients with negative PD-L1 (19). Though the long-term results have not been so satisfactory (20), it has been demonstrated in many phase II/III clinical trials that chemotherapy regimens can provide a considerable benefit to patients. In our study, 80.7% of patients did not experience chemotherapy, and in the final nomograms, patients without chemotherapy received the highest risk scores. Our results demonstrated there were still many patients with MBC who neglect the importance of chemotherapy. In the future, clinicians need to strengthen the management of MBC patients with adjuvant chemotherapy. A systematic search of English language literature displayed that the surgical resection of metastatic tumors is technically feasible and can be carried out safely. It may help improve cancer control and ultimately improve the survival rate of specific patients with limited metastatic burden (21). Similar to this, we also found that surgery can improve the prognosis of patients with metastatic bladder cancer to a certain extent, but clinicians still need to strictly set the indications for surgery. In an analysis of metastatic patterns in MBC patients, Shou J, et al. found that patients with bone metastasis accounted for the majority, liver metastasis had the worst prognosis, and it has been demonstrated that there is no significant difference in the overall survival of patients with multiple metastatic sites compared to single metastatic site (22). In our study, bone metastasis and liver metastasis were significant risk factors of early death in MBC patients, which shows good agreement with the above findings.
In recent years, several studies have reported that bladder cancer is associated with the expression of certain genes and proteins, which can be used to determine metastasis, prognosis and treatment of bladder cancer (23), Currently, Smith, et al. has already established a 20 genetic models for identifying bladder cancer patients with high risk of lymph node metastasis (24), in addition, Karyopherin-a2 (KPNA2) interaction with Chromobox 8 (CBX8) has been suggested as an indicator that patients are at high risk of metastasis (25). Due to the SEER database lack the information of molecular markers, our study did not establish a model containing molecular markers. However, it is difficult to popularize the clinical application of molecular markers.
Our nomograms were built on the SEER database and had a large sample size, which ensures the reliability of our results. Through internal validation, our models perform perfect in terms of predictiveness and clinical benefits. As a practical tool, DCA and CIC can be used to check the validity of model-based clinical decisions (26), Our study showed that the net benefit of our nomograms was better than the other two conditions (all screening or no screening). The scores obtained from the analysis of the nomogram can be used to derive a patient's probability of early death, which can be used by clinicians for end-of-life care and by oncologists to design clinical trials. In addition, identifying patients at risk of early death is essential to reduce the burden on patients, because the side effects of treatment and the inconvenience of going to the hospital may outweigh the benefits of treatment.
However, there are some unavoidable limitations of this study. Firstly, a number of known correlated factors were not considered in the nomograms. For example, Karnofsky performance status, functional comorbidities, the aforementioned biomarkers, and some emerging biomarkers such as TNFRSF14AS1, AL354919.2, OCIAD1-AS1 (32) were thought to be associated with MBC prognosis, but SEER database does not include these data. Secondly, our study was developed retrospectively and potential selection bias could have adversely affected the conclusions. Thirdly, although our models were validated internally, our models have not been validated with external clinical data. Future researches could validate it with clinical data to assess its plausibility.
Conclusion
In summary, two comprehensive nomograms established in our study can accurately predict early death from MBC, which enable surgeons to develop targeted treatment, follow-up strategies and improve survival outcomes for patients with MBC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
CT proposed research idea and collected the data. SSB, LYH and ZJ wrote the manuscript writing. ZXP and ZP completed statistical analysis and graph drawing. LDS, CLY and FB reviewed the research framework. All authors contributed to the article and approved the submitted version.
Funding
This study were supported by the National Science Foundation of Jiangxi Province (Grant Nos. 20202BAB216006), Research and Cultivation Foundation for Young Teacher of Medical Department of Nanchang University (Grant Nos. PY201913) and Science and Technology Research Project of Education Department of Jiangxi Province (Grant Nos. GJJ170011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Facchini G, Cavaliere C, Romis L, Mordente S, Facchini S, Iovane G, et al. Advanced/metastatic bladder cancer: current status and future directions. Eur Rev Med Pharmacol Sci. (2020) 24:11536–52. doi: 10.26355/eurrev_202011_23795
2. Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. (2020) 38:1895–904. doi: 10.1007/s00345-019-02984-4
3. Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. (2019) 76:10–21. doi: 10.1016/j.ctrv.2019.04.002
4. Zou Y, Han H, Ruan S, Jian Z, Jin L, Zhang Y, et al. Development of a nomogram to predict disease-specific survival for patients after resection of a non-metastatic adenocarcinoma of the pancreatic body and tail. Front Oncol. (2020) 10:526602. doi: 10.3389/fonc.2020.526602
5. Hu CY, Pan ZY, Yang J, Chu XH, Zhang J, Tao XJ, et al. Nomograms for predicting long-term overall survival and cancer-specific survival in lip squamous cell carcinoma: a population-based study. Cancer Med. (2019) 8:4032–42. doi: 10.1002/cam4.2260
6. Zhu Y, Fang X, Wang L, Zhang T, Yu D. A predictive nomogram for early death of metastatic gastric cancer: a retrospective study in the SEER database and China. J Cancer. (2020) 11:5527–35. doi: 10.7150/jca.46563
7. Zhang Z, Pu J, Zhang H. Development and validation of a simple-to-use nomogram to predict early death in metastatic pancreatic adenocarcinoma. Front Oncol. (2021) 11:729175. doi: 10.3389/fonc.2021.729175
8. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the hosmer-lemeshow test revisited. Crit Care Med. (2007) 35:2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0
9. Janssens A, Martens FK. Reflection on modern methods: revisiting the area under the ROC curve. Int J Epidemiol. (2020) 49:1397–403. doi: 10.1093/ije/dyz274
10. Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. (2018) 74:796–804. doi: 10.1016/j.eururo.2018.08.038
11. Ritch CR, Velasquez MC, Kwon D, Becerra MF, Soodana-Prakash N, Atluri VS, et al. Use and validation of the AUA/SUO risk grouping for nonmuscle invasive bladder cancer in a contemporary cohort. J Urol. (2020) 203:505–11. doi: 10.1097/JU.0000000000000593
12. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
13. Westergren DO, Gårdmark T, Lindhagen L, Chau A, Malmström PU, Nationwide A. Population based analysis of patients with organ confined, muscle invasive bladder cancer not receiving curative intent therapy in Sweden from 1997 to 2014. J Urol. (2019) 202:905–12. doi: 10.1097/JU.0000000000000350
14. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. (2001) 19:666–75. doi: 10.1200/JCO.2001.19.3.666
15. Ghoneim MA, el-Mekresh MM, el-Baz MA, el-Attar IA, Ashamallah A. Radical cystectomy for carcinoma of the bladder: critical evaluation of the results in 1,026 cases. J Urol. (1997) 158:393–9. doi: 10.1016/S0022-5347(01)64487-2
16. Werntz RP, Smith ZL, Packiam VT, Smith N, Steinberg GD. The impact of lymphovascular invasion on risk of upstaging and lymph node metastasis at the time of radical cystectomy. Eur Urol Focus. (2020) 6:292–7. doi: 10.1016/j.euf.2018.09.019
17. Kamat AM, Dinney CP, Gee JR, Grossman HB, Siefker-Radtke AO, Tamboli P, et al. Micropapillary bladder cancer: a review of the university of Texas M. D. Anderson cancer center experience with 100 consecutive patients. Cancer. (2007) 110:62–7. doi: 10.1002/cncr.22756
18. Aziz A, May M, Burger M, Palisaar RJ, Trinh QD, Fritsche HM, et al. Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort. Eur Urol. (2014) 66:156–63. doi: 10.1016/j.eururo.2013.12.018
19. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2021) 79:82–104. doi: 10.1016/j.eururo.2020.03.055
20. Bellmunt J, Albanell J, Paz-Ares L, Climent MA, González-Larriba JL, Carles J, et al. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. (2002) 95:751–7. doi: 10.1002/cncr.10762
21. Abufaraj M, Dalbagni G, Daneshmand S, Horenblas S, Kamat AM, Kanzaki R, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. (2018) 73:543–57. doi: 10.1016/j.eururo.2017.09.030
22. Shou J, Zhang Q, Zhang D. The prognostic effect of metastasis patterns on overall survival in patients with distant metastatic bladder cancer: a SEER population-based analysis. World J Urol. (2021) 39:4151–8. doi: 10.1007/s00345-021-03721-6
23. Ru Y, Dancik GM, Theodorescu D. Biomarkers for prognosis and treatment selection in advanced bladder cancer patients. Curr Opin Urol. (2011) 21:420–7. doi: 10.1097/MOU.0b013e32834956d6
24. Smith SC, Baras AS, Dancik G, Ru Y, Ding KF, Moskaluk CA, et al. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. (2011) 12:137–43. doi: 10.1016/S1470-2045(10)70296-5
25. Zeng F, Luo L, Li D, Guo J, Guo M. KPNA2 Interaction with CBX8 contributes to the development and progression of bladder cancer by mediating the PRDM1/c-FOS pathway. J Transl Med. (2021) 19:112. doi: 10.1186/s12967-021-02709-5
Keywords: metastatic bladder cancer, SEER database, early death, prognosis, nomograms
Citation: Chen T, Shi S, Zheng P, Zhan X, Zhang J, Li Y, Li D, Fu B and Chen L (2023) Predictive nomograms for early death in metastatic bladder cancer. Front. Surg. 9:1037203. doi: 10.3389/fsurg.2022.1037203
Received: 5 September 2022; Accepted: 11 October 2022;
Published: 13 January 2023.
Edited by:
Yongbao Wei, Fujian Provincial Hospital, ChinaReviewed by:
Chengliang Yin, Chinese PLA General Hospital, ChinaXiande Cao, Affiliated Hospital of Jining Medical University, China
Jinzhuo Ning, Renmin Hospital of Wuhan University, China
© 2023 Chen, Shi, Zheng, Zhan, Zhang, Li, Li, Fu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DongShui Li bGRzaGxsbEAxMjYuY29t Luyao Chen Y2hlbmx1eWFvMzAxQDE2My5jb20= Bin Fu dXJvZmJpbkAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Tao Chen
Tao Chen Shuibo Shi1,†
Shuibo Shi1,† Xiangpeng Zhan
Xiangpeng Zhan Bin Fu
Bin Fu Luyao Chen
Luyao Chen