
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 10 November 2022
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1035840
 Zhipeng Ye
Zhipeng Ye Keren Wu*
Keren Wu* Zhao Hu
Zhao Hu Fa Jin
Fa Jin
Background: Damage to the parathyroid glands remains a frequent complication after thyroidectomy, often resulting in hypoparathyroidism. Accordingly, identifying the parathyroid glands during thyroid surgical procedures is indispensable to prevent accidental surgical removal.
Methods: The participants were randomly divided into three groups (indocyanine green [ICG], nanocarbon [NC], and control group). To identify and protect parathyroid glands during neck lymph node dissection in patients with thyroid cancer, IG was intravenously administered to the ICG group, whereas the NC group received an intra-thyroid injection of the NC suspension before dissection. IG was intravenously administered to each group after dissection. Subsequently, we analyzed surgical outcomes, including operative time, number of lymph nodes, serum calcium, and number of parathyroid glands.
Results: We included 30 patients who underwent gasless transaxillary endoscopic thyroidectomy for thyroid cancer. Based on our findings, a greater number of parathyroid glands (P < 0.01) and higher postoperative parathyroid hormone (PTH) levels were detected in the NC and ICG groups than those in the control group (P < 0.01). The number of parathyroid glands and postoperative PTH levels in the NC group were higher than those in the ICG group (P < 0.01).
Conclusions: Gasless transaxillary endoscopic thyroidectomy with NC and ICG for thyroid cancer could effectively protect the parathyroid gland and afford satisfactory clinical efficacy. NC could offer an advantage over ICG for protecting the parathyroid gland.
The number of patients undergoing thyroid surgery has steadily grown in recent years (1, 2), with the rate of new thyroid cancer cases increasing by an average of 4.5% annually (3). Iatrogenic injury of the parathyroid gland post-thyroidectomy remains a common complaint. Damage to the parathyroid glands often leads to hypoparathyroidism, accounting for up to 11% of cases and necessitating calcium and vitamin D supplementation (4). Among patients who underwent permanent hypoparathyroidism, the mortality rate is 2.2%, which is significantly higher than that in patients without permanent hypoparathyroidism (5). Identifying the parathyroid glands during thyroid-related surgical procedures remains crucial to prevent accidental surgical removal and iatrogenic devascularization.
Several intraoperative procedural techniques, such as indocyanine green (ICG) fluorescence and nanocarbon (NC), have been explored to distinguish the parathyroid gland and were found to be safe and feasible (6, 7). Carbon nanoparticles or NCs exhibit a characteristically high degree of lymphatic system tropism, tracing speed, rate of black staining, and high color contrast with the surrounding tissue (8) and can be used to identify the parathyroid during thyroid surgery (9). Angiography using ICG has been used to identify sentinel lymph nodes and parathyroid glands, as well as to evaluate the vascular blood flow of the parathyroid glands (10, 11).
Transaxillary endoscopic thyroidectomy (ET) affords a concealed incision and clear vision and has emerged as an alternative to conventional open thyroidectomy, with superior cosmetic results (12–14). Previous studies assessing ET combined with ICG or NC have compared surgical and oncologic outcomes, reporting similar or superior outcomes to those without ICG or NC (6, 7). However, few studies have compared parathyroid gland protection and hypoparathyroidism or hypocalcemia between transaxillary ET using ICG or NC.
Therefore, we aimed to compare the feasibility of near-infrared fluorescence (NIRF) imaging with ICG and contrast development with NC to identify the parathyroid gland and assess its correlation with parathyroid gland NIRF imaging with ICG and postoperative complications, including hypoparathyroidism or hypocalcemia.
This study was conducted at the Department of Surgery, the First Affiliated Hospital of Zhejiang Chinese Medicine University (ZCMU), between December 2021 and December 2022.
The study protocol was approved by the ethics committee of the First Affiliated Hospital of ZCMU. The reference number for study approval was 2021-KL-046-01. The protocol was registered at Chictr.org.cn (registration number ChiCTR2100049512).
Inclusion criteria for study participation were as follows: consecutive male and female patients, aged ≥18 years, scheduled for hemithyroidectomy and central cervical lymph node dissection, with normal liver and renal function, and who provided written informed consent. Selected participants with a pathological diagnosis of papillary thyroid carcinoma (tumor size <1.0 cm) were randomly divided into three groups. Preoperative ultrasonography and computed tomography revealed no lateral lymph node metastases or papillary thyroid carcinoma. The flowchart of the study procedure is shown in Figure 1.
All thyroid surgeries were performed by the same surgeon using a modified operative technique and under general anesthesia. The standard operation was performed as reported by Kwak et al. (15). The recurrent laryngeal nerve (RLN) was systematically examined and identified by intraoperative neuromonitoring.
To identify and protect the parathyroid glands during neck lymph node dissection in patients with thyroid cancer, ICG angiography (7.5 mg/3 ml, intravenously) was performed intraoperatively (ICG group), whereas the NC suspension (0.2 ml) was injected into the thyroid gland over 2 min (NC group). After neck lymph node dissection, each group underwent ICG angiography to assess the vascularization of the parathyroid gland. ICG vascularization was scored using a previously established methodology: 0 (no vascularization), 1 (moderate vascularization), and 2 (excellent vascularization).
Intraoperative changes in serum parathyroid hormone (PTH) levels were measured at the start and end of the surgery, and blood loss, length of stay, vocal cord injury, and clinical manifestation of hypocalcemia were also examined to assess postoperative outcomes. Additional data were collected to establish a database and record the surgical approach, patient sex and age, largest tumor diameter, operative time, postoperative drainage volume, the total number of central lymph nodes, and the number of metastatic central lymph nodes.
All statistical calculations were conducted using SPSS (version 22.0; IBM Corp., Armonk, NY, USA). Statistical analyses were performed using the chi-square test and analysis of variance test, where appropriate, with significance set at P < 0.05. Measurement data are expressed as the sample mean ± standard deviation .
From January 2022 to June 2022, 30 patients who underwent transaxillary ET were selected randomly for inclusion in the present study. Table 1 summarizes the demographic characteristics of all patients included in the analysis. We detected no significant differences in average age (P = 0.405), sex (P = 0.787), body mass index (P = 0.833), tumor size (P = 0.476), and tumor location (P = 0.873).
Operative time, blood loss, drainage content, and hospitalization days did not significantly differ between groups (P = 0.826, P = 0.805, P = 0.797, and P = 0.160, respectively). Five cases of transient hoarseness were documented in all groups, which resolved within 3 months. Permanent RLN palsy was not observed in any patient. One patient in the control group, who underwent right hemithyroidectomy with central neck dissection, experienced acroagnosis. The overall complication rate did not significantly differ between groups (P = 0.535). There were no significant differences in the number of lymph node metastases and the total number of lymph node dissections among examined groups (P = 0.354 and P = 0.305) (Table 2).
As shown in Table 3, parathyroid glands were present in all patients. The NC and ICG groups displayed a higher number of parathyroid glands and total ICG scores, with a lower incidence of accidental parathyroid excision and temporary hypoparathyroidism than the control group (P < 0.05). The number of parathyroid glands and the total ICG score were higher in the NC group than those in the ICG group (P < 0.05). Permanent hypoparathyroidism did not occur in any group. There were no significant differences in preoperative serum calcium and PTH levels across examined groups. In the NC group, postoperative PTH and serum calcium levels in all patients returned to normal levels within three days (P = 0.923 and P = 0.931, respectively); in the control group, normal levels were restored within two weeks (Figures 2A,B).
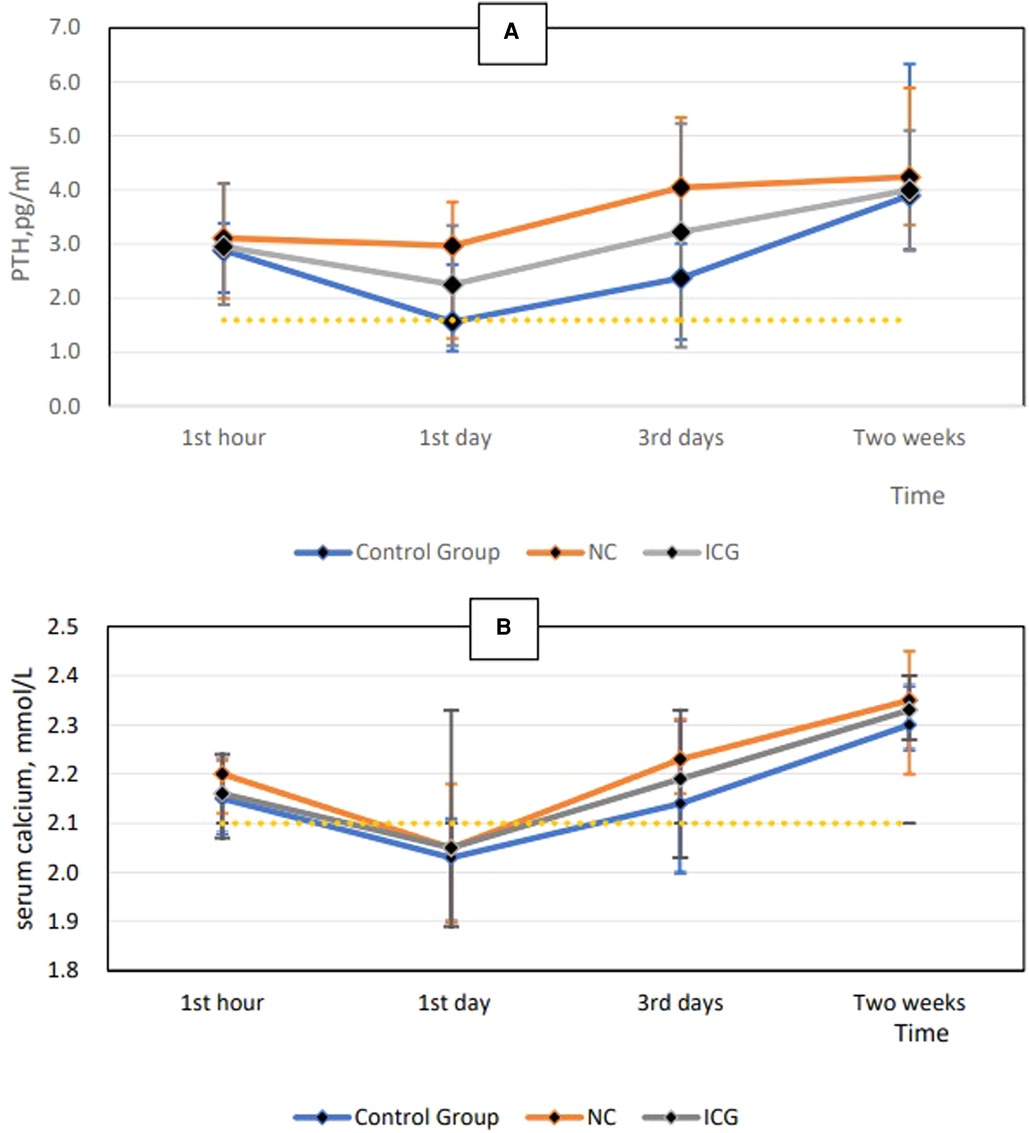
Figure 2. Postoperative PTH level at each time point (A) and postoperative serum calcium level at each time point (B). PTH, parathyroid hormone.
As shown in Table 4, the postoperative biochemical profile, including levels of serum calcium and PTH alterations, was measured at 1 h postoperatively in all patients. No significant differences in PTH alterations or symptomatic postoperative hypocalcemia were detected for different ICG vascularization scores. Furthermore, we noted no significant difference in serum calcium levels.
Postoperative hypoparathyroidism is the most common complication associated with total thyroidectomy (16). In previous reports assessing thyroid surgery, the incidence of temporary hypoparathyroidism ranged between 14% and 60% (17, 18). Accordingly, developing a reliable tool to protect the parathyroid gland and prevent hypocalcemia remains crucial. Staining with NC or ICG angiography could reduce the possibility of accidental parathyroid excision, thereby decreasing the occurrence of hypoparathyroidism, while facilitating the rapid restoration of PTH and calcium levels (19, 20). However, which of these two modalities can be deemed superior for protecting the parathyroid gland?
Herein, we detected no significant differences between groups in terms of intraoperative and postoperative blood loss, operative time, hospital stay, postoperative complications, and other examined parameters. Therefore, gasless insufflation transaxillary ET combined could be feasibly and safely performed with NC and ICG imaging.
Establishing the anatomical position and vascular supply of parathyroid glands is essential to avoid hypoparathyroidism after thyroid surgery (21). During lymph node dissection, intravenous ICG can aid in identifying the parathyroid gland through immediate parathyroid gland angiography and green staining of the parathyroid gland. Removing the unstained adipose lymphatic tissue can protect the parathyroid gland from damage and prevent the occurrence of postoperative hypocalcemia (Figure 3B). However, ectopic parathyroids embedded in adipose tissue or thyroid tissue cannot be stained green or identified by ICG; these are often removed or damaged accidentally, leading to hypocalcemia after thyroidectomy.
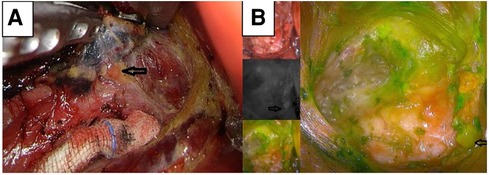
Figure 3. NC-stained black lymph nodes and high color contrast with the surrounding tissue for locating parathyroid (A). Green-stained parathyroid located by ICG (B). ICG, indocyanine green; NC, nanocarbon.
NCs exhibit a markedly high degree of lymphatic system tropism, tracing speed, rate of black staining, and high color contrast with the surrounding tissue (8). When NCs were used in thyroid surgery, black-stained lymph nodes were dissected. We detected no significant differences in the number of lymph nodes or metastatic lymph nodes between the two groups. Reportedly, the dissection of sentinel lymph nodes with NCs is effective and safe for thyroid cancer surgery (22). Black-stained lymph nodes can indicate the position of adipose tissue and parathyroid glands and prevent the removal of parathyroid glands within the remaining tissue damage (Figure 3A). Likewise, it has been reported that NCs can be used to identify the parathyroid gland during thyroid surgery (9, 19). Careful identification and removal of black-stained lymphatic tissues during thyroidectomy with neck lymph node dissection can ensure complete lymph node dissection and prevent parathyroid damage, thereby effectively reducing the incidence of hypoparathyroidism. Consequently, the findings of the present study suggest that staining with NCs combined with gasless insufflation transaxillary ET can better reduce the possibility of accidental parathyroid gland excision.
In addition to a good safety profile, intravenous ICG is rapidly excreted into the bile. The short half-life of ICG in systemic circulation allows repeated application for identifying the parathyroid gland during thyroid surgery. It has been suggested that NC extravasation can impact the surgical field and the surgery. Herein, we found that the integrity of the thyroid capsule during surgery can prevent the staining of other tissues. Even if NCs spilled over and were removed gradually, the surgical field under the endoscope remained clear.
Accumulated evidence suggests that ICG angiography enables early, direct evaluation of the parathyroid glands and could help the surgeon decide whether to autotransplant a devascularized parathyroid gland. Calcium and/or PTH measurements may no longer be necessary for patients with at least one well-perfused parathyroid gland, as demonstrated using ICG angiography after thyroidectomy (20). However, we found no direct relationship between intraoperative parathyroid imaging grade and postoperative hypocalcemia or decreased PTH levels. Conversely, hypocalcemia was detected at every parathyroid imaging grade. However, the blood supply to the contralateral parathyroid glands remains intact during unilateral thyroid surgery, and hypocalcemia can recur. Calcium and/or PTH measurements are necessary for patients and cannot be replaced by ICG angiography. As a method for evaluating parathyroid gland autotransplantation, ICG remains debatable. Interestingly, high parathyroid imaging scores were found to return to normal shortly after surgery.
This study has certain limitations. First, the sample size of the present study was small, and the results need to be validated in a larger patient population. Second, randomized controlled trials assessing parathyroid autotransplantation should be conducted to clarify the relationship between ICG angiography and autotransplantation. Finally, given that unilateral thyroid surgery was performed and the contralateral parathyroid gland was intact, the accuracy of the present study may be uncertain.
In summary, gasless transaxillary ET with NC and ICG for thyroid cancer can protect the parathyroid gland and provide satisfactory clinical effects. NC was superior to ICG in protecting the parathyroid gland. Intraoperative ICG angiography can be used to assess postoperative parathyroid function and serum calcium recovery; however, it is not recommended as a standard for parathyroid autotransplantation or a substitute for postoperative serum calcium/PTH measurements.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Zhejiang Chinese Medicine University. The patients/participants provided their written informed consent to participate in this study.
ZY contributed to the conception of the study, performed the experiment, and wrote the manuscript. KW performed the experiment. ZH performed the data analyses. FJ helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
This study was supported by the Health Science and Technology Project of Zhejiang Province (No. 2021KY837).
The authors would like to thank Li Ning for excellent technical support and critically reviewing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Myriam L, Ralph PT, Christine GG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. (2013) 123(8):2056–63. doi: 10.1002/lary.23923
2. Brito JP, Davies L. Is there really an increased incidence of thyroid cancer? Curr Opin Endocrinol Diabetes Obes. (2014) 21(5):405–8. doi: 10.1097/MED.0000000000000094
3. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available at: www.seer.cancer.gov (Accessed November 1, 2016).
4. Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR. Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab. (2017) 102(7):2543–51. doi: 10.1210/jc.2017-00255
5. Almquist M, Ivarsson K, Nordenström E, Bergenfelz A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg. (2018) 105:1313–8. doi: 10.1002/bjs.10843
6. Ma JJ, Zhang DB, Zhang WF, Wang X. Application of nanocarbon in breast approach endoscopic thyroidectomy thyroid cancer surgery. J Laparoendosc Adv Surg Tech A. (2020) 30(5):547–52. doi: 10.1089/lap.2019.0794
7. Razavi AC, Ibraheem K, Haddad A, Saparova L, Shalaby H, Abdelgawad M, et al. Efficacy of indocyanine green fluorescence in predicting parathyroid vascularization during thyroid surgery. Head Neck. (2019) 41(9):3276–81. doi: 10.1002/hed.25837
8. Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y, et al. Magnetic functionalized carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer. (2011) 47:1873–82. doi: 10.1016/j.ejca.2011.03.018
9. Zhu J, Tian W, Xu Z, Jiang K, Sun H, Wang P, et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann Transl Med. (2015) 3(16):230. doi: 10.3978/j.issn.2305-5839.2015.08.20
10. Imboden S, Papadia A, Nauwerk M, McKinnon B, Kollmann Z, Mohr S, et al. A comparison of radiocolloid and indocyanine green fluorescence imaging, sentinel lymph node mapping in patients with cervical cancer undergoing laparoscopic surgery. Ann Surg Oncol. (2015) 22:4198–203. doi: 10.1245/s10434-015-4701-2
11. Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg. (2016) 103:537–43. doi: 10.1002/bjs.10101
12. Vidal O, Delgado-Oliver E, Pino V, Vilaça J. Trans-axillary endoscopic thyroidectomy: another approach to offer our patients. Cri Esp (Engl Ed). (2018) 96(9):586. doi: 10.1016/j.ciresp.2018.06.008
13. Kang S-W, Jeong JJ, Yun J-S, Sung TY, Lee SC, Lee YS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach: surgical outcome of 581 patients. Endocr J. (2009) 56:361–9. doi: 10.1507/endocrj.K08E-306
14. Bhargav PR, Kumbhar US, Satyam G, Gayathri KB. Gasless single incision trans-axillary thyroidectomy: the feasibility and safety of hypo-morbid endoscopic thyroidectomy technique. J Minim Access Surg. (2013) 9(3):116–21. doi: 10.4103/0972-9941.115370
15. Kwak HY, Kim SH, Chae BJ, Song BJ, Jung SS, Bae JS. Learning curve for gasless endoscopic thyroidectomy using the trans-axillary approach: cUSUM analysis of a single surgeon's experience. Int J Surg. (2014) 12:1273–7. doi: 10.1016/j.ijsu.2014.10.028
16. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. (2014) 101:307–20. doi: 10.1002/bjs.9384
17. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American thyroid association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
18. Lee YS, Kim SW, Kim SW, Kim SK, Kang HS, Lee ES, et al. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg. (2007) 31:1954–9. doi: 10.1007/s00268-007-9171-7
19. Shi C, Tian B, Li S, Shi T, Qin H, Liu S. Enhanced identification and functional protective role of carbon nanoparticles on parathyroid in thyroid cancer surgery. Medicine (Baltimore). (2016) 95(46):e5148. doi: 10.1097/MD.0000000000005148
20. Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg. (2018) 105:350–7. doi: 10.1002/bjs.10783
21. Arlt W, Fremerey C, Callies F, Reincke M, Schneider P, Timmermann W, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. (2002) 146:215–22. doi: 10.1530/eje.0.1460215
Keywords: ICG angiography, parathyroid, gasless insufflation transaxillary endoscopic thyroidectomy, nano-carbon, thyroid cancer
Citation: Ye Z, Wu K, Hu Z and Jin F (2022) Nanocarbon or indocyanine green: Which is superior for gasless transaxillary endoscopic thyroidectomy to protect the parathyroid gland?. Front. Surg. 9:1035840. doi: 10.3389/fsurg.2022.1035840
Received: 3 September 2022; Accepted: 25 October 2022;
Published: 10 November 2022.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Vasiliy Semikov, I.M. Sechenov First Moscow State Medical University, Russia© 2022 Ye, Wu, Hu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keren Wu d2tyMzAwMEBzaW5hLmNvbQ==
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.