
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 28 October 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1035635
This article is part of the Research Topic Rare and Misdiagnosed Neurosurgical Conditions Volume II View all 3 articles
Hemangiopericytomas (HPCs) invading the cavernous sinus (CS) are extremely rare invasive tumors that have a great propensity for local recurrence. To date, only eight cases have been reported in the literature. Owing to the abundant vascular supply of HPCs, intracavernous bleeding and important blood vessels and nerves passing through the CS, it is very difficult and challenging for neurosurgeons to completely resect HPCs. Here, we report two cases of HPCs invading the CS and introduce their clinical manifestations, imaging findings, surgical approaches and histopathological features in detail. We have implemented the surgery by the endoscopic transpterygoid transcavernous approach (ETPTCa) for the two patients, and one patient has undergone gross total resection (GTR) and another has undergone subtotal resection (STR) and postoperative stereotactic radiosurgery (SRS). The ETPTCa may serve as a viable option to facilitate HPCs resection. Radiotherapy is helpful in prolonging progression-free survival (PFS) following STR of the tumor.
Intracranial HPCs are rare mesenchymal tumors that originate from pericytes around the capillaries and constitute less than 1% of intracranial tumors (1, 2), while intracavernous HPCs are more rare. Although there are similar clinical manifestations and imaging features between HPCs and meningiomas (3), HPCs show more invasive behavior and are more prone to local recurrence and distant metastasis than meningiomas (4). HPCs were classified into HPC (WHO grade II) and anaplastic HPCs (WHO grade III) based on the 2016 WHO histopathological grading system (5). Additionally, HPCs and solitary fibrous tumors (SFTs) have the same STAT6 nuclear expression and NAB2-STAT6 fusion in the 2016 WHO classification system (6). Here, we report our experience in treating two patients with HPCs invading the CS, and we also conducted a literature review.
A 63-year-old female complaining of ptosis of the right eye was admitted. Positive neurological signs included partial inferior visual defects and limited inward, upward and downward ocular movement. A preoperative cranial CT scan showed a slightly hyperintense lesion in the right CS (Figure 1A). Cranial MRI showed a fusiform long T1 and long T2 signal shadow in the right CS with a clear boundary that measured 1.7 cm × 2.5 cm (Figure). After enhancement, the lesion exhibited homogeneous enhancement (Figures 1B–D). Postoperative CT (Figure 1E) and MRI (Figures 1F–H) showed GTR of the tumor and the patient had no tumor progression during the 4.2-year follow-up period.
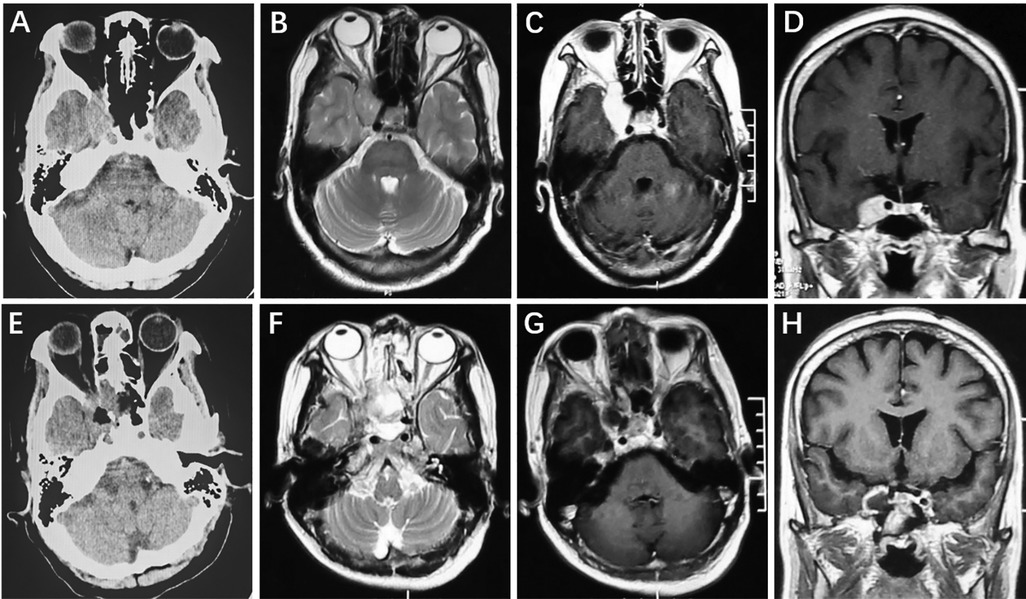
Figure 1. Right intracavernous HPC. Preoperative axial CT showed a slightly hyperintense lesion in the right cavernous sinus (A). Cranial axial MRI showed slight hyperintensity on T2-weighted imaging (B). After enhancement, the axial and coronal MRI showed homogeneous enhancement (C,D). Postoperative CT (E) and MRI (F–H) revealed that the tumor was completely removed.
We performed the surgery via an endoscopic endonasal transpterygoid approach to resect the intracavernous lesion in a four-handed and binostril manner under general anesthesia (Figures 2A–H). After disinfecting the nasal cavity with diluted iodophor, the nasal mucosa was covered with epinephrine and lidocaine cotton pieces for 20 min. First, a nasal septum-base mucosa flap was created, and the olfactory mucosa needed to be preserved to prevent postoperative anosmia. The right middle turbinate was removed to create a wide surgical corridor to ensure that the endoscope and instrument could easily enter the surgical area. The right maxillary sinus and mucosa were exposed after the ethmoid bulla was removed, and the anterior wall of the maxillary sinus was opened to gain access to its posterior wall.
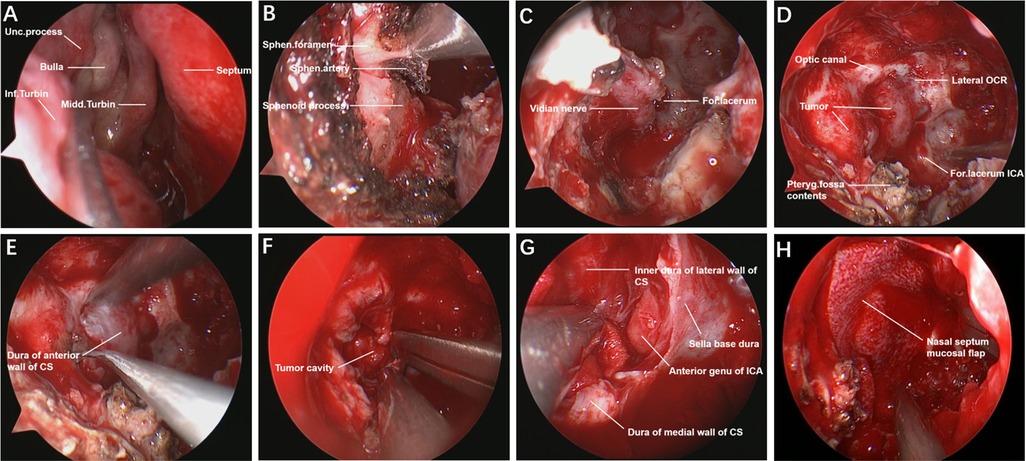
Figure 2. Endoscopic endonasal transpterygoid approach to resect intracavernous HPC. The normal structures of the nasal cavity in the endoscopic view (A). The sapopalatine artery from the sphenopalatine foramen was exposed and cut off (B). Drilling the vidian canal and exposing the vidian nerve, ICA from foramen lacerum was identified in the lateral-superior of vidian nerve (C). The main anatomical landmarks and the tumor were exposed by stepwise bone resection (D). Incision of dura mater of the anterior wall of the CS (E). The tumor was removed piece by piece under the endoscope (F). Overall view of the surgical area after total tumor resection (G). The operation area was covered by a nasal septum mucosal flap to prevent cerebrospinal fluid leakage (H). Unc., uncinate; Inf., inferior; Midd., middle; Turbin., turbinate; Sphen., sphenopalatine; For., foramen; OCR., Opticocarotid recess; Pteryg., pterygopalatine; CS, cavernous sinus; ICA, internal carotid artery.
Then, the anterior wall of the sphenoid sinus and the posterior part of the septum were removed, and the sphenoid sinus mucosa and lateral nasal mucosa were resected with a circle excision. When the sphenopalatine artery from the sphenopalatine foramen was identified, it was removed after electrocoagulation, and the sphenopalatine foramen was expanded to expose the root of the pterygoid process. The root of the pterygoid process was removed to expose the vidian canal and pterygopalatine fossa, and then the medial, upper and lower bones of the vidian canal were ground to completely expose the vidian nerve. ICA from the foramen lacerum was recognized in the lateral-superior portion of the vidian nerve. The anterior wall of the pterygopalatine fossa was drilled, which made it easy to displace pterygopalatine fossa contents laterally. After adequate exposure of the surgical field, the tumor boundaries were located by intraoperative navigation, and the Doppler located the ICA position. The dura mater of the anterior wall of the CS was opened by a hook knife in the safe area of the CS, and grayish yellow fish-like tumor tissue was seen. After the tumor was totally resected, piece by piece, the inner dura mater of the lateral wall of the CS, the medial wall of the CS and the anterior genu of the ICA were observed. Finally, the operation area was covered with artificial meninges and a nasal septum-base mucosal flap, which was fixed with biological glue. The intraoperative blood loss was 600 ml, and blood transfusion was not performed in the patient. Oculomotor nerve paralysis was obviously improved at three months after the operation.
Histopathological examination (H&E) showed that the tumor cells of HPCs with round or egg-shaped nuclei and inconspicuous nucleoli had consistent sizes, and the boundary between the tumor cells was unclear (Figures 3A,B). The mitotic index was 3 mitoses per 10 high-power fields. Immunohistochemistry staining (magnification, ×400) showed that the tumor cells were positive for STAT6, CD34, Ki67 (10%), CD99, Bcl2, and Vimentin (Figures 3C–H).
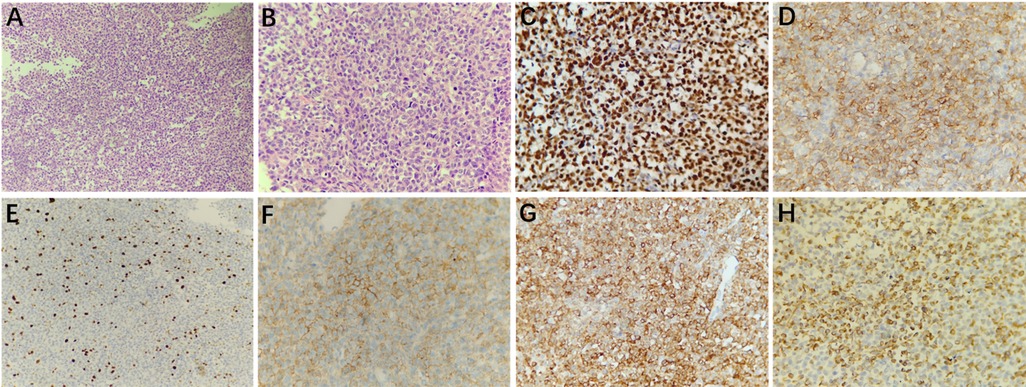
Figure 3. Microscopically, the tumor cells had a consistent size with less cytoplasm, and the boundary between the cells was unclear; the nuclei were round or egg-shaped and had inconspicuous nucleoli (A, 200× magnification and B, 400× magnification; hematoxylin and eosin [H&E] staining). Immunohistochemistry staining at 400× magnification showed that the tumor cells were positive for STAT6 (C), CD34 (D), Ki67 (10%, E), CD99 (F), Bcl2 (G), and Vimentin (H).
A 23-year-old female complaining of intermittent headache and numbness of the left face was admitted. Physical examination showed that the left masseter muscle was slightly atrophied. Preoperative head CT revealed a slightly hyperintense lesion in the left CS and middle cranial fossa (Figure 4A). Cranial MRI showed a mass with isointensity on T1 and T2 and a clear boundary in the left middle cranial fossa (Figure 4B); the lesion showed an uneven signal with a small round of long T1 and long T2 signal shadows inside. The boundary between the trigeminal nerve and the lesion that compressed the adjacent temporal lobe and pons was unclear. After enhancement, the lesions showed uneven enhancement with a size of 2.9 cm × 3.8 cm × 4.7 cm, and obvious linear enhancement of the local dura mater and dural tail sign were found (Figures 4C,D). Postoperative CT (Figure 4E) and MRI (Figures 4F–H) showed STR of the tumor and there was no tumor recurrence during the 5.1-year follow-up period.
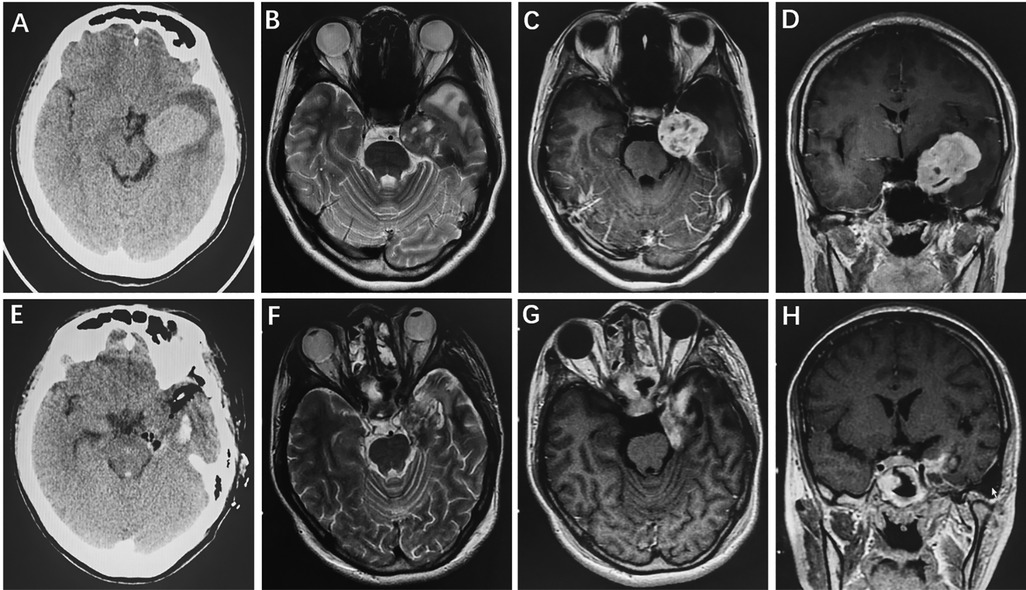
Figure 4. HPC invading the left CS and middle cranial fossa. Preoperative axial CT showed a slightly hyperintense lesion in the left CS and middle cranial fossa (A). Cranial axial MRI demonstrated isointensity on T2-weighted imaging (B). After enhancement, the axial and coronal MRI showed heterogeneous enhancement (C,D). Postoperative CT (E) and MRI (F–H) revealed that the tumor was subtotally removed.
We performed the surgery by an endoscopic endonasal transpterygoid combined with a pterional approach to resect a lesion invading the cavernous and middle cranial fossa. When the optical canal, lateral opticocarotid recess, vidian nerve, foramen lacerum, pterygopalatine fossa contents, anterior wall of the CS and dura mater of the middle cranial fossa were fully exposed based on the procedures of case 1, the dura mater of the middle cranial fossa and the capsule of the tumor were sectioned. The tumor was solid and hard and showed abundant blood supply and obvious bleeding, which invaded into the CS and adhered firmly to the ICA and trigeminal nerve. The intracavernous tumor was subtotally resected because of severe bleeding of the CS and the tumor. After successful hemostasis, the dural defect was repaired with artificial meninges, and the skull base was covered by a nasal septum-base mucosal flap. Then, we performed the craniotomy via the left pterional interfascial approach, made an arc incision with a length of 20 cm, cut the skin, subcutaneous tissue and galea aponeurotica layer by layer, freed the temporal muscle and removed the bone flap. The dura tension was high, and the temporal encephalocele was apparent, so we carefully released cerebrospinal fluid from the sylvian fissure to create a surgical corridor. After lifting the temporal lobe, the tumor was found in the middle cranial fossa base, which was grayish white, hard, and tightly attached to the trigeminal ganglion; the tumor was resected piece by piece. After subtotal tumor resection, complete hemostasis was achieved, and the dura defect was repaired with artificial meninges. Intraoperative blood loss was 6,000 ml, and twelve red blood cell units, 1,600 ml fresh plasma and twenty cryoprecipitate units were infused into the patient. The patients' headache disappeared, and numbness of the left face was not relieved. The patient showed no new symptoms of neurological deficits after the operation and underwent SRS forty days after the operation.
Histopathological examination (H&E) showed that HPCs were composed of closely packed cells with thin-wall fissure-like vessels, and staghorn-like vessel lacunes were observed; the nuclei were ovoid or long-spindle-shaped with inconspicuous nucleoli (Figure 5A). The mitotic index was 4 mitoses per 10 high-power fields. Immunohistochemistry staining (magnification, ×400) showed that the tumor cells were positive for CD34, STAT6, Ki67 (8%), CD99, and Vimentin (Figures 5B–F).
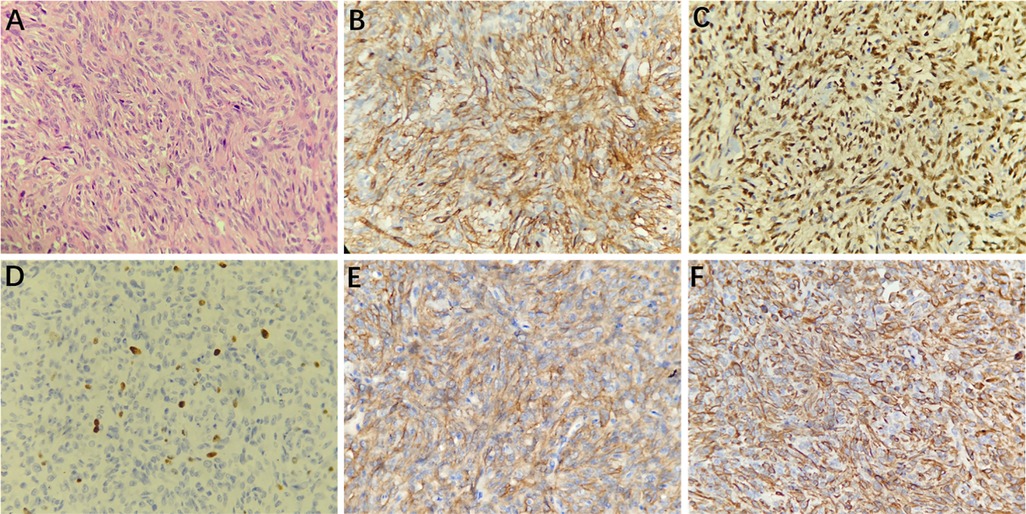
Figure 5. Microscopically, the tumor cells were arranged densely with fissure-like vessels, and staghorn-like vessel lacunes were observed; the nuclei were ovoid or long-spindle-shaped with inconspicuous nucleoli (A, hematoxylin and eosin [H&E] staining, 400× magnification). Immunohistochemistry staining at 400× magnification showed that the tumor cells were positive for CD34 (B), STAT6 (C), Ki67 (8%, D), CD99 (E), and Vimentin (F).
HPCs of the central nervous system are rare malignancies that have a high propensity for local recurrence and distant metastasis (7–9). They can grow in any intracranial site, including the convexity, parasagittal or parafalx region, brain parenchyma, ventricle, CS, foramen magnum and sellar region (8, 10–13). The patient's clinical symptoms can vary depending on the tumor invasion site. For patients with HPCs invading the CS, whose symptoms are caused by tumors compressing the oculomotor, trochlear, trigeminal, and abducens nerves, the predominant presentation is ptosis, limited ocular movement, diplopia, and facial numbness or pain. When the tumor grows to a large volume and extends into the middle cranial fossa, intracranial pressure can increase. To date, there have been 8 cases of intracavernous HPCs reported in the relevant literature, in addition to our two cases (Table 1) (12, 14–19). In our study, one patient presented with ptosis and limited ocular movement and showed obvious improvement at three months after undergoing surgery. Another patient manifested headache and facial numbness; although the headache disappeared, facial numbness was not significantly improved.
A definite preoperative diagnosis of HPCs is important for surgeons to select the optimal surgical method to carry out the operation. Nevertheless, it is difficult to distinguish HPCs from meningiomas, especially an angiomatous meningioma, because of their similar radiological features. The HPCs are generally irregular or lobulated with obvious intratumoural flow voids and no calcification and may be related to osteolytic destruction of adjacent bone based on MRI. Cystic changes and necrosis are common in HPCs. In the present two cases, the tumors were all irregular on MRI; the tumor of case 2 showed obvious intratumoral flow voids, cystic changes and necrosis. Some studies have shown that susceptibility-weighted imaging (SWI) and diffusion-weighted imaging (DWI) are helpful for the differential diagnosis of HPC and AM; HPCs have higher normalized apparent diffusion coefficients (nADC) and degrees of intratumoral susceptibility signal intensity (ITSS) than meningiomas (20, 21). Furthermore, combining nADC and ITSS can achieve good discriminative ability with a specificity of 78.12% and sensitivity of 81.25% (20). In the two cases, the nADC values were 0.94 and 1.02, respectively.
GTR of intracranial HPCs is difficult and pose challenges for neurosurgeons because of severe intraoperative bleeding. Many case reports show that for large or giant HPCs, preoperative embolization can remarkably reduce blood loss during surgery and achieve GTR of these tumors (16, 22, 23). Muto et al. (16) reported that a 51-year-old male patient with a giant HPC, with a maximum diameter of 8 cm, presented with a toothache and underwent preoperative embolization and craniotomy using an anterior petrosal approach. Then, the patient underwent STR and SRS.
In recent years, with the rapid development of endoscopic endonasal techniques, lesions in the parasellar CS region can be removed by ETPTCa. However, owing to the high bleeding risk in CS, the rich blood supply of HPCs and the surrounding important blood vessels and nerves, it is still very difficult to resect intracavernous HPCs because doing so requires sufficient preoperative preparation, skilled endoscopic techniques and considerable experience. ETPTCa may be more advantageous than the transcranial approach in resecting intracavernous tumors because the internal carotid artery (ICA) can be observed at 360° under endoscopy. In previous literature reports, seven of the eight patients with intracavernous HPCs underwent craniotomy, and one underwent an endoscopic endonasal approach; GTR was achieved in 3 patients, STR in 3, near subtotal resection in 1 and partial resection in 1. In our two cases, GTR was achieved in one case by ETPTCa, and STR was achieved in one case via ETPTCa combined with the pterional approach. The GTR of HPCs is highly important for patients to improve OS and PFS, which is supported by many studies (4, 7, 9, 24–26). Rutkowski MJ et al. reviewed a sample of 563 patients with intracranial hemangiopericytoma and the overall median survival was 13 years, with 1-, 5-, 10-, and 20-year survival rates of 95%, 82%, 60%, and 23%, respectively (27). Fritchie K et al. also reported that the outcomes of HPCs in which the median OS was 12.7 years, with 5-year and 10-year OS rates of 73.7% and 55.3%, respectively, and the median PFS was 7.8 years, with 5-year and 10-year PFS rates of 81.1% and 41.7%, respectively (28).
Of the 8 cases, 7 were confirmed as HPCs (WHO grade II) by histopathology and immunohistochemistry. There was only one case diagnosed as anaplastic HPCs (WHO grade III) in which an 11-year-old male complaining of headache and diplopia died due to septicemia two days after craniotomy. The diagnostic criteria of anaplastic HPCs included 5 or more mitoses per 10 high power fields, necrosis and at least 2 of the following features: hemorrhage, high cellularity and moderate to high nuclear atypia (29).
For giant HPCs invading the CS and middle and posterior cranial fossa, even though combined approaches were used for tumor excision, it is still difficult to achieve complete tumor resection. Adjuvant radiation therapy (RT) to prevent tumor recurrence is beneficial for patients with residual tumors. Some studies indicated that adjuvant RT could significantly prolong PFS after the initial operation (3, 9, 26, 30) and improve OS (9, 31) irrespective of the extent of resection, while others showed that RT did not have a significant effect on OS (7, 9). Furthermore, reoperation, repeat GKS or RT was effective in increasing OS or PFS in all patients with recurrent HPCs (32). Nakajo K et al. (12) described a 67-year-old male experiencing numbness of the left face who underwent one procedure and four GKSs to inhibit tumor relapse.
HPCs invading the CS are extremely rare and there have been eight cases reported in the literature thus far. Surgical resection was the most effective and optimal way to treat HPCs, and ETPTCa was feasible and more promising for tumors in the sellar and parasellar regions. RT or GKS significantly improved PFS after STR of the tumor.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QY and ZT: chief surgeon, writing—review and editing. WYX, GL, and ZY: writing—original draft preparation. ZM, LJT and XYF: data collection and analysis. All authors contributed to the article and approved the submitted version
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HPCs: hemangiopericytomas, CS: cavernous sinus, ICA: internal carotid artery, MRI: magnetic resonance imaging, SFT: solitary fibrous tumor, GTR: gross total resection, STR: subtotal resection, ETPTCa: endoscopic transpterygoid transcavernous approach, RT: radiotherapy, SRS: stereotactic radiosurgery, OS: overall survival, PFS: progress-free survival
1. Bassiouni H, Asgari S, Hubschen U, Konig HJ, Stolke D. Intracranial hemangiopericytoma: treatment outcomes in a consecutive series. Zentralbl Neurochir. (2007) 68(3):111–8. doi: 10.1055/s-2007-981674
2. Ghose A, Guha G, Kundu R, Tew J, Chaudhary R. CNS hemangiopericytoma: a systematic review of 523 patients. Am J Clin Oncol. (2017) 40(3):223–7. doi: 10.1097/COC.0000000000000146
3. Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G. Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. (2014) 21(8):1310–4. doi: 10.1016/j.jocn.2013.11.026
4. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg. (2011) 114(3):747–55. doi: 10.3171/2010.6.JNS091660
5. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. (2007) 114(2):97–109. doi: 10.1007/s00401-007-0243-4
6. Fritchie KJ, Jin L, Rubin BP, Burger PC, Jenkins SM, Barthelmess S, et al. NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol. (2016) 75(3):263–71. doi: 10.1093/jnen/nlv026
7. Zhu H, Duran D, Hua L, Tang H, Chen H, Zhong P, et al. Prognostic factors in patients with primary hemangiopericytomas of the central nervous system: a series of 103 cases at a single institution. World Neurosurg. (2016) 90:414–9. doi: 10.1016/j.wneu.2016.02.103
8. Kinslow CJ, Bruce SS, Rae AI, Sheth SA, McKhann GM, Sisti MB, et al. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: a population-based study. J Neurooncol. (2018) 138(1):173–82. doi: 10.1007/s11060-018-2787-7
9. Sung KS, Moon JH, Kim EH, Kang SG, Kim SH, Suh CO, et al. Solitary fibrous tumor/hemangiopericytoma: treatment results based on the 2016 WHO classification. J Neurosurg. (2018) 130(2):418–25. doi: 10.3171/2017.9.JNS171057
10. Arai N, Takahashi S, Mami H, Tokuda Y, Yoshida K. A case report of surgical management of hemangiopericytoma at the foramen magnum. Surg Neurol Int. (2017) 8:151. doi: 10.4103/sni.sni_484_16
11. Yang H, Zhang Y, Zheng T, Li C, Tang G, Chen G. A solitary fibrous tumor/hemangiopericytoma of the fourth ventricle: case report and literature review. J Int Med Res. (2019) 47(12):6349–55. doi: 10.1177/0300060519885567
12. Nakajo K, Iwai Y, Yoshimura M, Watanabe Y, Yamanaka K. Intracavernous hemangiopericytoma: case report and review of the literature. NMC Case Rep J. (2019) 6(4):111–5. doi: 10.2176/nmccrj.cr.2018-0300
13. Lin Z, Wang Y, Zhao M, Li Z, Chen X, Jiang Z. Unusual presentation of an intracranial hemangiopericytoma as a cystic intraparenchymal mass lesion closely mimicking a glioma. Neurol India. (2017) 65(1):208–9. doi: 10.4103/0028-3886.198222
14. Ganesan K, Masoumi H, Dietrich R, Hesselink J. Intracavernous sinus solid-cystic haemangiopericytoma: a rare entity with radiology-pathology correlation. Clin Radiol. (2010) 65(4):339–42. doi: 10.1016/j.crad.2009.08.014
15. Bonde VR, Goel A. Two patients with intracavernous haemangiopericytoma. J Clin Neurosci. (2009) 16(2):330–3. doi: 10.1016/j.jocn.2008.01.019
16. Muto J, Kawase T, Yoshida K. Meckel's cave tumors: relation to the meninges and minimally invasive approaches for surgery: anatomic and clinical studies. Neurosurgery. (2010) 67(3 Suppl Operative):ons291–8; discussion ons8–9. doi: 10.1227/01.NEU.0000382967.84940.52
17. Agarwal A, Sankhe S, Goel N, Mahore A. Juvenile anaplastic hemangiopericytoma of cavernous sinus. J Pediatr Neurosci. (2012) 7(3):237–8. doi: 10.4103/1817-1745.106492
18. Patrona A, Patel KS, Bander ED, Mehta A, Tsiouris AJ, Anand VK, et al. Endoscopic endonasal surgery for nonadenomatous, nonmeningeal pathology involving the cavernous sinus. J Neurosurg. (2017) 126(3):880–8. doi: 10.3171/2015.8.JNS15275
19. Wanibuchi M, Akiyama Y, Mikami T, Iihoshi S, Miyata K, Horita Y, et al. Radical removal of recurrent malignant meningeal tumors of the cavernous sinus in combination with high-flow bypass. World Neurosurg. (2015) 83(4):424–30. doi: 10.1016/j.wneu.2015.01.019
20. Chen T, Jiang B, Zheng Y, She D, Zhang H, Xing Z, et al. Differentiating intracranial solitary fibrous tumor/hemangiopericytoma from meningioma using diffusion-weighted imaging and susceptibility-weighted imaging. Neuroradiology. (2020) 62(2):175–84. doi: 10.1007/s00234-019-02307-9
21. Shankar JJS, Hodgson L, Sinha N. Diffusion weighted imaging may help differentiate intracranial hemangiopericytoma from meningioma. J Neuroradiol. (2019) 46(4):263–7. doi: 10.1016/j.neurad.2018.11.002
22. Matsushige T, Nakaoka M, Yahara K, Shinagawa K, Ohnuma H, Shibukawa M, et al. Single-stage operation for a giant haemangiopericytoma following intracranial feeder embolization. J Clin Neurosci. (2007) 14(2):162–7. doi: 10.1016/j.jocn.2005.09.019
23. Ding D, Kreitel D, Liu KC. Onyx embolization of an intracranial hemangiopericytoma by direct transcranial puncture. Interv Neuroradiol. (2013) 19(4):466–70. doi: 10.1177/159101991301900410
24. Espat NJ, Lewis JJ, Leung D, Woodruff JM, Antonescu CR, Shia J, et al. Conventional hemangiopericytoma: modern analysis of outcome. Cancer. (2002) 95(8):1746–51. doi: 10.1002/cncr.10867
25. Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG. Management of recurrent meningeal hemangiopericytoma. Cancer. (1998) 82(10):1915–20. doi: 10.1002/(SICI)1097-0142(19980515)82:10%3C1915::AID-CNCR15%3E3.0.CO;2-W
26. Melone AG, D'Elia A, Santoro F, Salvati M, Delfini R, Cantore G, et al. Intracranial hemangiopericytoma–our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. (2014) 81(3-4):556–62. doi: 10.1016/j.wneu.2013.11.009
27. Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. (2010) 113(2):333–9. doi: 10.3171/2010.3.JNS091882
28. Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M, et al. The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol. (2019) 137(2):307–19. doi: 10.1007/s00401-018-1952-6
29. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
30. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. (2012) 118(6):1628–36. doi: 10.1002/cncr.26411
31. Zhang GJ, Wu Z, Zhang LW, Li D, Zhang JT. Surgical management and adverse factors for recurrence and long-term survival in patients with hemangiopericytoma. World Neurosurg. (2017) 104:95–103. doi: 10.1016/j.wneu.2017.05.010
Keywords: hemangiopericytomas, cavernous sinus, endoscopic endonasal approach, neuroimaging, histopathology, radiotherapy, overall survival, progress-free survival
Citation: Wu Y, Gong L, Zhang Y, Zheng M, Li J, Xue Y, Qu Y and Zhao T (2022) Endoscopic endonasal resection of two rare cases of hemangiopericytomas invading the cavernous sinus and literature review. Front. Surg. 9:1035635. doi: 10.3389/fsurg.2022.1035635
Received: 3 September 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Ehab Shiban, Augsburg University Hospital, GermanyReviewed by:
Thorsteinn Gunnarsson, Linköping University Hospital, Sweden© 2022 Wu, Gong, Zhang, Zheng, Li, Xue, Qu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianzhi Zhao emhhb3RpYW56aGkxOTgxQDE2My5jb20= Yan Qu eWFucXUwMTIzQGZtbXUuZWR1LmNu
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.