- Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Zhejiang Provincial Clinical Research Center for Oral Diseases, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer center of Zhejiang University, Hangzhou, China
Background: Osteoblastoma is quite rare in the oromaxillo-facial region, while the mandible is always the predilection. However, in our case, the lesion was located in the left temporal articular tubercle, involving the adjacent skull base, which is extremely rare in the literature.
Case reports: It had been diagnosed as the most common temporomandibular joint disorder in the local hospital before the patient came to our department, mainly due to the primary symptom, that was, the patient got pain in the left temporomandibular joint area while opening the mouth. However, we found a mass of bone lesions at the left temporal articular tubercle in MRI and cone beam CT, and it turned out to be an osteoblastoma after surgery. The patient's primary symptom disappeared after recovering from the surgery, and there have been no indications of complication or recurrence up to now.
Conclusion: Osteoblastoma is very rare in the temporomandibular joint region. It could easily miss the possibility of a benign tumor due to its unusual location and confusing chief complaint in this case. Our report provides experience in the identification of osteoblastoma in rare sites.
Introduction
Osteoblastoma is a benign bone-forming tumor with a peak incidence in the second decade of life (1, 2), and its most common locations are the spine and long bones. It is quite rare in the stomatology department, and it tends to occur in the posterior mandible (3–6). In this case, the lesion was located in the left temporal bone, involving the temporomandibular joint (TMJ) and adjacent skull base, which is extremely rare in the literature. Osteoblastoma has two subtypes: the conventional/benign one and the osteoblastic/aggressive one (7). They look almost the same radiologically, but there are subtle differences in histopathology between them; that is, large epithelioid osteoblasts can be found in the latter one. Most importantly, the latter one is more aggressive and has a higher recurrence rate of about 15%–20% (8–10). Luckily, the lesion in this case was classified as a conventional osteoblastoma. However, it was reported that osteoblastoma located near important tissues has a poorer outcome after surgery, probably due to the difficulty associated with complete resection (1, 2, 6, 11), which indicates that the case we presented may have a higher recurrence.
The patient's clinical symptoms were quite similar to those of the most common temporomandibular joint disorders (TMD), and they may easily miss the possibility of a benign tumor. We therefore hope that this case report will provide experience in the identification of osteoblastoma in rare sites.
Clinical presentation
A 26-year-old male patient came to our department with the chief complaint of pain in the left preauricular area while opening his mouth for the last 3 years. It was diagnosed as TMD after a physical and radiological examination at the local hospital over a year ago. Besides giving instructions about protecting the TMJ area, “glucosamine hydrochloride capsule” was also given at that time to reduce intra-articular inflammation and prevent damage to the articular cartilage. However, the symptoms did not receive effective relief. Physical examination showed that there was swelling and pain with palpation in the left TMJ area. The largest mouth opening range was normal but with pain and deviation to the left, while without joint noise or limitation during protrusive and lateral sliding movements. There were no ear complaints, such as ear pain or hearing impairment. Then further radiological examinations were performed, including cone beam CT (CBCT) and magnetic resonance imaging (MRI), which showed anterior displacement of the left disc, and local swelling was detected on the anterior and lateral sides of the left temporal articular tubercle, with interrupted continuity of the cortical bone (Figure 1A). CBCT showed a heterogeneous mass sized approximately 14.5 mm * 10 mm * 8 mm at the left temporal articular tubercle (Figures 1B,C).
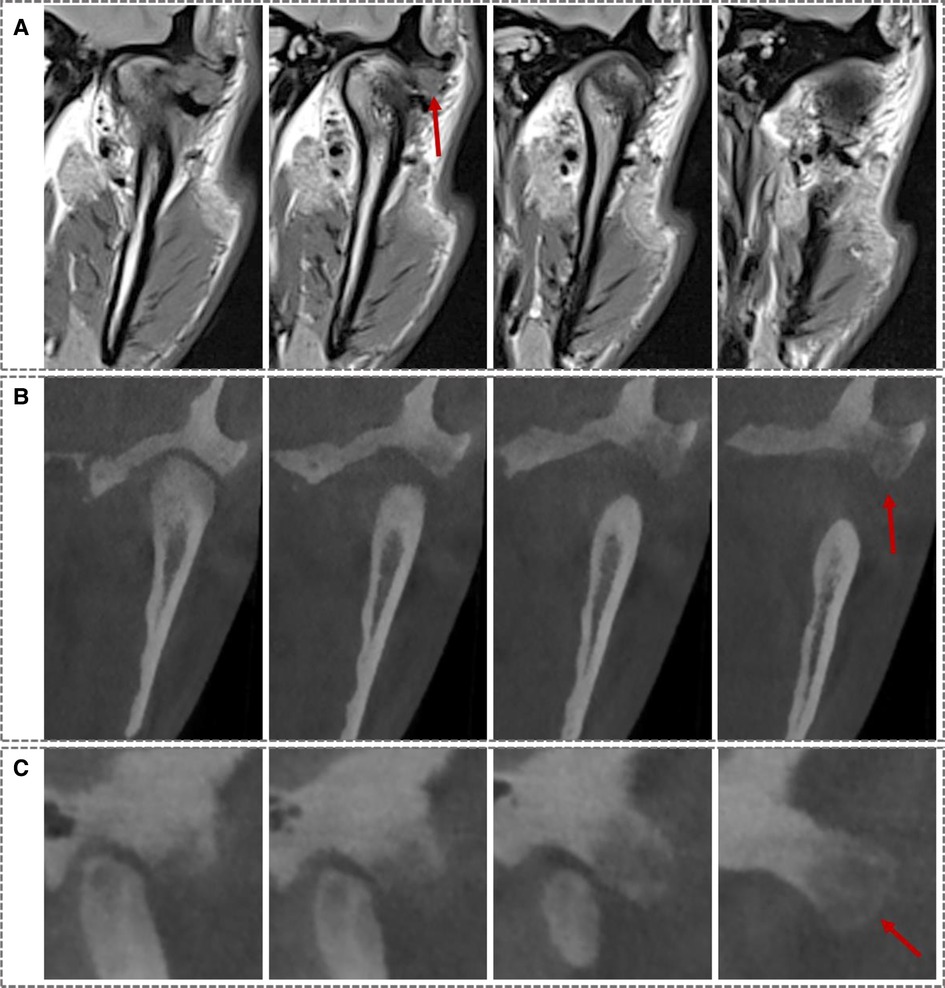
Figure 1. Preoperative MRI and CT images: (A) coronal MRI images showed a mass at the left articular tubercle. (B) Coronal CT images showed a heterogeneous mass at the left articular tubercle. (C) Sagittal CT images showed the same. (Red arrows indicates the lesion).
Treatment and diagnosis
During the operation, a pre-auricular incision was made, the tumor was resected (Figure 2A), followed by curetting to the area with relatively normal bone, reshaping the articular eminence, disc manipulation was not performed, and finally, suturing the incision carefully and placing a high vacuum drainage device (Figure 2B). The intraoperative frozen biopsy could not be performed because the tissue was too hard to slice up immediately. Then all three masses resected or curetted (sized 1.1 cm × 0.8 cm × 0.5 cm, 0.3 cm × 0.3 cm × 0.2 cm, and 0.2 cm × 0.1 cm × 0.1 cm, respectively) were sent for histopathological examination. Immediately post-operative CBCT (Figure 2D) revealed the resection range compared with that pre-operatively (Figure 2C).
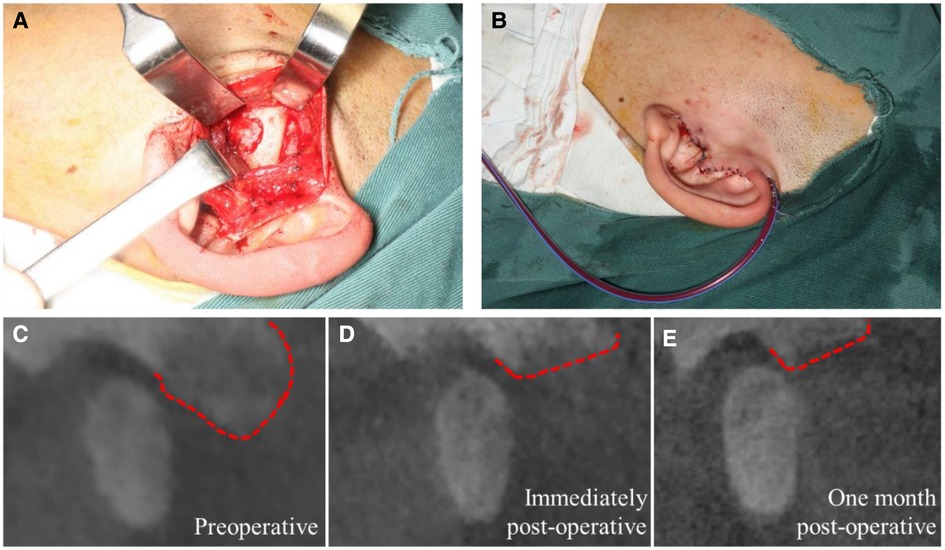
Figure 2. Surgical intervention and radiological change in CBCT images: (A) lesion resection at the left articular tubercle; (B) stitches of the incision and high vacuum drainage device placement; (C–E) CBCT images of the lesion area preoperatively, immediately postoperatively and one month postoperatively, respectively. (Red dotted lines show the border the articular tubercle region).
The histopathological examination was conducted after 7 days of decalcification in a 10% EDTA solution. It showed that the tumor was composed of haphazardly mineralized trabeculae of bone, osteoid rimmed by osteoblasts, and acellular vascular fibrous stroma (Figure 3A). There were giant cells with multiple dense nuclei, and cellular atypia can also be seen (Figure 3B), but no epithelioid osteoblast was seen within sight. Besides, the boundary of the tumor was not clear. The immunohistochemical results showed that both Ki-67 (Figure 4A) and PCNA (Figure 4B) labeling index were low (<5%), indicating that the proliferation capacity is relatively normal (11, 12). Positive expression of SOX2 (Figure 4C) and MDM2 (Figure 4D) indicated that the lesion was osteogenic (13–15). Scattered positive expression of CD68 (Figure 3E) proved the presence of multinucleated osteoclasts (16). Thus, a final diagnosis of conventional osteoblastoma was made with the aid of the immunohistochemical technique.
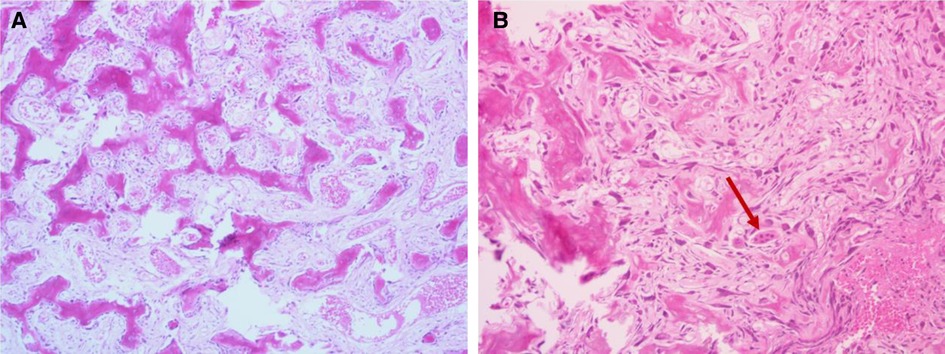
Figure 3. Histopathological features (H&E staining): (A) immature trabeculae rimmed by osteoblasts; (B) giant cells with multiple dense nuclei and cellular atypia can be found. H&E, Hematoxylin and eosin.
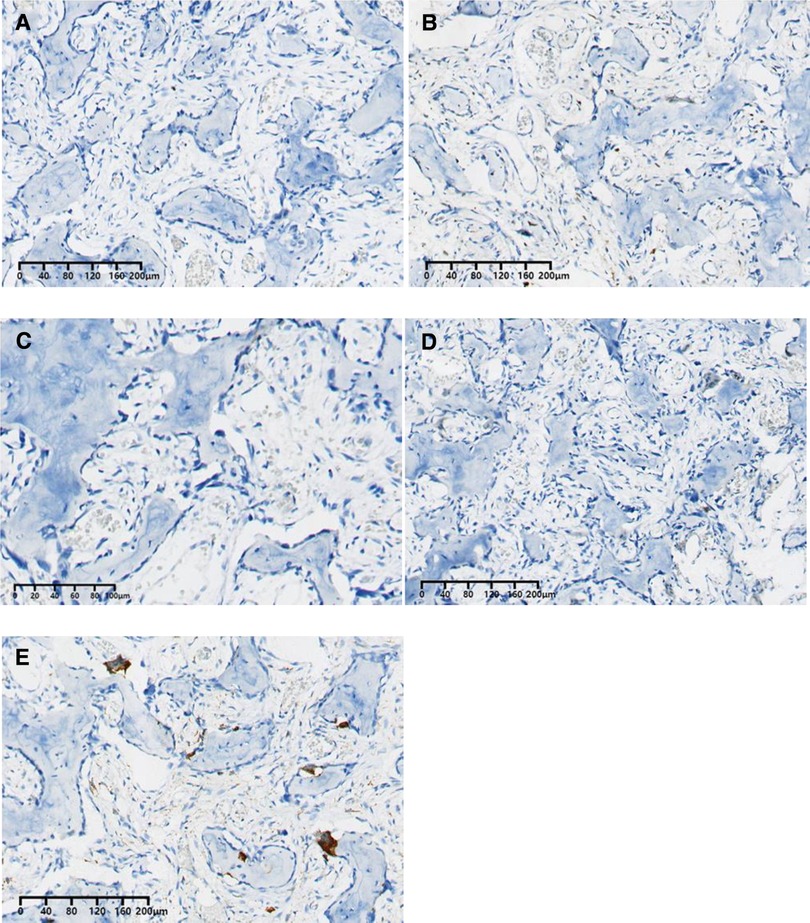
Figure 4. Immunohistopathological results: (A) Ki-67 (<5%); (B) PCNA (<5%); (C) SOX2 (+); (D) MDM2 (+); (E) CD68 scatteredly expression (+).
After one-month follow-up, postoperatively showed that the mouth opening range was normal without any pain or limitation, and the incision was quite concealed. Radiological examination (Figure 2E) also showed no difference compared with the immediate CBCT postoperatively (Figure 2D), which indicated that there were no signs of recurrence. Considering the huge trauma a second surgery will cause and the complete relief of his symptoms after recovering from the surgery, the patient chose to keep a regular follow-up to detect any recurrence instead of an immediate second surgery.
Differentiation diagnosis
Due to the identical clinical and radiological features, it is hard to differentiate these bone lesions, including ossifying fibroma, osteoid osteoma, osteoblastoma, giant cell lesions of the jaws, and low-grade osteosarcoma.
Ossifying fibroma is composed of hyper-cellular fibroblastic stroma containing variable amounts of calcified structures. It is usually less vascular, more fibrous, and has less osteoblastic activity compared with osteoblastoma (17). Besides, the presence of abundant osteoblasts around the trabeculae also supports the diagnosis of osteoblastoma rather than ossifying fibroma (18). Osteoid osteoma is histologically identical to osteoblastoma. They are usually distinguished on the basis of size: osteoid osteoma is typically <1 cm and lesions >2 cm will be considered osteoblastoma (19–21). Osteoblastoma, on the other hand, can be locally aggressive, whereas osteoid osteoma lacks growth and invasiveness potential (1, 19). In this case, the main lesion was over 1 cm, and it affected the neighboring skull base radiologically, so it was more likely to be diagnosed as an osteoblastoma. Giant cell lesions of the jaws also show similar radiographic and clinical features to osteoblastoma (22). However, giant cell lesions of the jaws are always composed of fibrous connective tissue, irregularly distributed multinucleated giant cells, abundant blood vessels, a little bone-like tissue, and a large amount of hemosiderin in histopathological slices (23), so they can be distinguished accordingly.
A more significant differentiation to be made is between osteoblastoma and low-grade osteosarcoma. First, the latter one can be more aggressive and may have more severe symptoms clinically. Second, they can be distinguished by histopathological methods that show that hyperchromatic nuclei, mitosis, pleomorphic cells, and poorly circumscribed margins with infiltration of the host bone are more common in osteosarcoma (24, 25). Immunohistochemistry can also help to distinguish them; positive results for CDK4 and MDM2 support the diagnosis of osteosarcoma (26–29), for instance. Besides, osteoblastoma shows nuclear staining, whereas osteosarcoma shows cytoplasmic or membranous staining for β-catenin (30). Furthermore, osteoblastoma always shows strong and diffuse nuclear expression of FOS immunohistochemically, but it is usually negative in low-grade osteosarcoma (2, 31, 32). The expression of microRNA-210 is elevated in osteosarcoma when compared with osteoblastoma (33).
Discussion
To our knowledge, there is only one case report of osteoblastoma at the temporal articular tubercle (34). And in our case, the lesion even affected the neighboring skull base, which showed bone swelling radiologically. The primary symptom of pain with mouth opening easily led to a diagnosis of the most common TMD and neglected the possibility of a benign tumor. This case reminds us to be careful with TMD diagnoses. Radiological examinations can sometimes be crucial in analyzing the location and features of the lesion. Moreover, histopathological examination is of great importance for the accurate diagnosis of osteoblastoma, and immunohistochemistry can additionally help with an auxiliary diagnosis, of which we used five antibodies, including Ki-67, PCNA, CD68, SOX2, and MDM2 in this case. Besides, according to recent findings, recurrent rearrangements of FOS and FOSB can be detected in osteoblastoma (2). Among them, FOS immunohistochemistry can be exploited as an auxiliary diagnostic tool for short-decalcified osteoblastoma tissue within 3 days (31).
As a matter of fact, gross total tumor resection is the standard treatment for osteoblastoma (35). Hence, a further surgery combined with the cooperation of the neurosurgery department is proposed for this patient. However, if the lesion was proximal to important structures, curettage would be the only feasible alternative to resection. Besides, carbon ion radiotherapy may also be a choice, which was reported to have good outcomes after 10 years of follow-up (36). However, because radiotherapy may cause malignant transformation of tumors (37, 38), it should be used prudently.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective case report, formal consent is not required. The tumor tissue and radiological data included in the manuscript were obtained as part of the standard of care for the patient and retrospectively collected for the case report.
Author contributions
Conceptualization and supervision, HW, ZL and MY; writing—original draft preparation, FZ, XZ, QP; writing—review and editing, FZ and XY. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. (2011) 19(11):678–89. doi: 10.5435/00124635-201111000-00004
2. Fittall MW, Mifsud W, Pillay N, Ye H, Strobl AC, Verfaillie A, et al. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. (2018) 9(1):2150. doi: 10.1038/s41467-018-04530-z
3. Manjunatha BS, Sunit P, Amit M, Sanjiv S. Osteoblastoma of the jaws: report of a case and review of literature. Clin Pract. (2011) 1(4):e118. doi: 10.4081/cp.2011.e118
4. Low Y, Foo CL, Seow WT. Childhood temporal bone osteoblastoma: a case report. J Pediatr Surg. (2000) 35(7):1127–9. doi: 10.1053/jpsu.2000.7843
5. Lucas DR, Unni KK, McLeod RA, O'Connor MI, Sim FH. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. (1994) 25(2):117–34. doi: 10.1016/0046-8177(94)90267-4
7. Sharma R, Mahajan S, Gupta D. Aggressive cranial osteoblastoma of the parietotemporo-occipital bone: a case report and review of literature with special emphasis on recurrence/residue. World Neurosurg. (2020) 142:255–67. doi: 10.1016/j.wneu.2020.06.093
8. Al-Ibraheem A, Yacoub B, Barakat A, Dergham MY, Maroun G, Haddad H, et al. Case report of epithelioid osteoblastoma of the mandible: findings on positron emission tomography/computed tomography and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. (2019) 128(1):e16–20. doi: 10.1016/j.oooo.2018.12.021
9. Lypka MA, Goos RR, Yamashita DD, Melrose R. Aggressive osteoblastoma of the mandible. Int J Oral Maxillofac Surg. (2008) 37(7):675–8. doi: 10.1016/j.ijom.2008.01.013
10. Garvayo M, Cossu G, Broome M, Maeder P, Renella R, Maduri R, et al. Pediatric cranial osteoblastoma: technical note of surgical treatment and review of the literature. Neurochirurgie. (2021) 67(4):383–90. doi: 10.1016/j.neuchi.2020.05.010
11. Sobecki M, Mrouj K, Camasses A, Parisis N, Nicolas E, Llères D, et al. The cell proliferation antigen Ki-67 organises heterochromatin. Elife. (2016) 5: e13722. doi: 10.7554/eLife.13722
12. Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal. (2013) 18(2):e174–9. doi: 10.4317/medoral.18573
13. Olivos DJ 3rd, Perrien DS, Hooker A, Cheng YH, Fuchs RK, Hong JM, et al. The proto-oncogene function of Mdm2 in bone. J Cell Biochem. (2018) 119(11):8830–40. doi: 10.1002/jcb.27133
14. Seo E, Basu-Roy U, Zavadil J, Basilico C, Mansukhani A. Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol Cell Biol. (2011) 31(22):4593–608. doi: 10.1128/MCB.05798-11
15. Akkouch A, Eliason S, Sweat ME, Romero-Bustillos M, Zhu M, Qian F, et al. Enhancement of MicroRNA-20°c on osteogenic differentiation and bone regeneration by targeting Sox2-mediated Wnt signaling and Klf4. Hum Gene Ther. (2019) 30(11):1405–18. doi: 10.1089/hum.2019.019
16. Hou JM, Lin JL, Wen JP, Jin L, Tang FQ. Immunohistochemical identification of osteoclasts and multinucleated macrophages. Cell Immunol. (2014) 292(1-2):53–6. doi: 10.1016/j.cellimm.2014.09.002
17. Mohanty S, Rani A, Urs AB, Dabas J. A rare case of an aggressive osteoblastoma of the squamous temporal bone: a unique presentation with literature review. J Craniomaxillofac Surg. (2014) 42(7):1207–10. doi: 10.1016/j.jcms.2014.02.010
18. Prabhu S, Adyanthaya S, Jose M, Sripathi Rao BH. Epithelioid osteoblastoma: a histopathological dilemma between juvenile ossifying fibroma and low-grade osteosarcoma. J Oral Maxillofac Pathol. (2017) 21(1):165–7. doi: 10.4103/jomfp.JOMFP_199_16
19. Hidaka H, Yamauchi D, Fujishima F, Watanabe M, Kato Y, Nomura K, et al. Osteoid osteoma of the temporal bone manifesting as first bite syndrome and a meta-analysis combined with osteoblastoma. Eur Arch Otorhinolaryngol. (2017) 274(2):607–16. doi: 10.1007/s00405-016-4050-1
20. Dugert E, Lagleyre S, Brouchet A, Deguine O, Cognard C, Bonneville F. Osteoid osteoma invading the posterior labyrinth of the petrous bone. AJNR Am J Neuroradiol. (2010) 31(9):1764–6. doi: 10.3174/ajnr.A1910
21. Doshi SV, Frantz TD, Korol HW. Benign osteoblastoma of the temporal bone: case report and literature review. Am J Otolaryngol. (2001) 22(3):211–4. doi: 10.1053/ajot.2001.23432
22. Alsufyani NA, Aldosary RM, Alrasheed RS, Alsaif RF. A systematic review of the clinical and radiographic features of hybrid central giant cell granuloma lesions of the jaws. Acta Odontol Scand. (2021) 79(2):124–31. doi: 10.1080/00016357.2020.1797160
23. Jadu FM, Pharoah MJ, Lee L, Baker GI, Allidina A. Central giant cell granuloma of the mandibular condyle: a case report and review of the literature. Dentomaxillofac Radiol. (2011) 40(1):60–4. doi: 10.1259/dmfr/85668294
24. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. (2010) 21(Suppl 7):vii320–25. doi: 10.1093/annonc/mdq276
25. Gambarotti M, Dei Tos AP, Vanel D, Picci P, Gibertoni D, Klein MJ, et al. Osteoblastoma-like osteosarcoma: high-grade or lowgrade osteosarcoma? Histopathology. (2019) 74(3):494–503. doi: 10.1111/his.13746
26. Pereira TDSF, Andrade BAB, Romañach MJ, Pereira NB, Gomes CC, Mariz BALA, et al. Clinicopathologic study of 6 cases of epithelioid osteoblastoma of the jaws with immunoexpression analysis of FOS and FOSB. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 130(2):191–9. doi: 10.1016/j.oooo.2020.03.001
27. Hirose K, Okura M, Sato S, Murakami S, Ikeda JI, Noda Y, et al. Gnathic giant-cell-rich conventional osteosarcoma with MDM2 and CDK4 gene amplification. Histopathology. (2017) 70(7):1171–3. doi: 10.1111/his.13141
28. Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, Larousserie F, Muret AD, et al. MDM2 And CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. (2011) 24(5):624–37. doi: 10.1038/modpathol.2010.229
29. Chen CY, Zhang HZ, Jiang ZM, Zhou J, Chen J, Liu L. Value of MDM2, CDK4 and SATB2 immunohistochemistry in histologic diagnosis of low-grade osteosarcoma. Zhonghua Bing Li Xue Za Zhi. (2016) 45(6):387–92. doi: 10.3760/cma.j.issn.0529-5807.2016.06.007
30. Wan Y, Zhao W, Jiang Y, Liu D, Meng G, Cai Y. β-catenin is a valuable marker for differential diagnosis of osteoblastoma and osteosarcoma. Hum Pathol. (2014) 45(7):1459–65. doi: 10.1016/j.humpath.2014.02.022
31. Lam SW, Cleven AHG, Kroon HM, Briaire-de Bruijn IH, Szuhai K, Bovée JVMG. Utility of FOS as diagnostic marker for osteoid osteoma and osteoblastoma. Virchows Arch. (2020) 476(3):455–63. doi: 10.1007/s00428-019-02684-9
32. Amary F, Markert E, Berisha F, Ye H, Gerrand C, Cool P, et al. FOS expression in osteoid osteoma and osteoblastoma: a valuable ancillary diagnostic tool. Am J Surg Pathol. (2019) 43(12):1661–7. doi: 10.1097/PAS.0000000000001355
33. Riester SM, Torres-Mora J, Dudakovic A, Camilleri ET, Wang W, Xu F, et al. Hypoxia-related microRNA-210 is a diagnostic marker for discriminating osteoblastoma and osteosarcoma. J Orthop Res. (2017) 35(5):1137–46. doi: 10.1002/jor.23344
34. Emanuelsson J, Allen CM, Rydin K, Sjöström M. Osteoblastoma of the temporal articular tubercle misdiagnosed as a temporomandibular joint disorder. Int J Oral Max Surg. (2017) 46(5):610–3. doi: 10.1016/j.ijom.2016.12.005
35. Gibson M, Michalowicz M, Chrisinger JSA, Bell D, Shah K, Demonte F, et al. Osteoblastoma of the temporal bone in a child. Otol Neurotol. (2022) 43(2):e276–8. doi: 10.1097/MAO.0000000000003381
36. Honda A, Iizuka Y, Imai R, Nishinome M, Hirato J, Koshi H, et al. Recurrent lumbar-origin osteoblastoma treated with multiple surgery and carbon ion radiotherapy: a case report. BMC Musculoskelet Disord. (2020) 21(1):321. doi: 10.1186/s12891-020-03349-4
37. Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive “benign” primary spine neoplasms: osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine. (2009) 34(22 Suppl):S39–47. doi: 10.1097/BRS.0b013e3181ba0024
Keywords: osteoblastoma, temporomandibular joint, ossifying fibroma, temporomandibular joint disorder, osteoid osteoma, osteosarcoma
Citation: Zhao F, Zhang X, Pan Q, Ye X, Yu M, Li Z and Wang H (2023) Temporal bone osteoblastoma involving temporomandibular joint diagnosed as simple disc disorders: A case report. Front. Surg. 9:1033342. doi: 10.3389/fsurg.2022.1033342
Received: 31 August 2022; Accepted: 26 October 2022;
Published: 6 January 2023.
Edited by:
Alessandro Di Rienzo, Marche Polytechnic University, Italy© 2023 Zhao, Zhang, Pan, Ye, Yu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Li aHhsenkyMDAyQHpqdS5lZHUuY24= Huiming Wang d2htd2htQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
 Feiya Zhao
Feiya Zhao Xinyue Zhang
Xinyue Zhang Qiaoting Pan
Qiaoting Pan Mengfei Yu
Mengfei Yu Huiming Wang
Huiming Wang