
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 26 December 2022
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.1032626
 Shusuke Ohshima1
Shusuke Ohshima1 Yushi Ueki1*
Yushi Ueki1* Yusuke Yokoyama1
Yusuke Yokoyama1 Takeshi Takahashi1
Takeshi Takahashi1 Ryusuke Shodo1
Ryusuke Shodo1 Keisuke Yamazaki1
Keisuke Yamazaki1 Ryuichi Okabe2
Ryuichi Okabe2 Hiroshi Matsuyama3
Hiroshi Matsuyama3 Takafumi Togashi4
Takafumi Togashi4 Sumiko Takatsuka5
Sumiko Takatsuka5 Tatsuya Takenouchi5
Tatsuya Takenouchi5 Arata Horii1
Arata Horii1
Background: Head and neck mucosal melanoma (HNMM) is a rare and aggressive subtype of melanoma. HNMM often develops as a recurrent or metastatic disease, and its prognosis is worse than that of cutaneous melanoma. Recent large-scale clinical studies have reported favorable outcomes with immune checkpoint inhibitors (ICIs) for melanoma. However, these clinical trials included only a small number of HNMM cases. This study aimed to estimate treatment outcomes and prognostic predictors of ICIs for advanced HNMM.
Methods: Cases of advanced HNMM, defined as unresectable or metastatic HNMM at the initial diagnosis (five patients) or development of recurrent/metastatic HNMM after initial treatment (27 patients), were included in this study. Survival analysis and a search for prognostic factors were performed for these 32 patients. Furthermore, the detailed clinical course of patients who received ICI treatment was investigated.
Results: The median overall survival (OS) of 32 patients with advanced HNMM was 25.3 months. The estimated 1-, 3-, and 5-year OS rates were 68.4%, 42.8%, and 34.3%, respectively. Fourteen patients (43.7%) received ICIs, whereas 18 (56.3%) did not. Univariate analysis showed that ICI treatment was the only factor associated with a better 1-year OS. Patients who received ICI treatment had significantly longer OS (median OS: not reached, 1-year OS: 85.7%) than those who did not (median OS: 11.3 months, 1-year OS: 54.5%). The overall response and disease control rates of patients who received ICI treatment were 50% and 64.3%, respectively. Patients who achieved complete response (CR) or partial response (PR) to ICI treatment survived significantly longer (1-year OS: 100%) than those who did not (1-year OS: 71.4%). Among the five patients who discontinued ICI treatment due to severe immune-related adverse events (irAEs), four did not receive salvage treatments but showed durable treatment effects and survived for 9.8–54.2 months at the end of the follow-up period.
Conclusions: ICI treatment achieved a favorable OS for advanced HNMM. CR/PR to ICI treatment and discontinuation owing to severe irAEs were favorable predictors of OS.
Mucosal melanoma (MM) is a rare melanoma subtype that accounts for only 1.3% of all melanoma cases in the United States (1). MM is relatively more common in Asian countries (8%–22.6% of all melanomas) (2, 3), possibly because of the low incidence of cutaneous melanoma (CM). Among MM, 34%–40% arise from the head and neck regions (4, 5). The 5-year-overall survival (OS) of head and neck MM (HNMM) is less than 30% (6, 7), while that for CM is more than 80% (8), indicating that HNMM has a poorer prognosis than CM. Based on the aggressive nature of MM, all HNMM cases are classified as stage III or higher according to the TNM classification (9). The sinonasal and oral cavities are the two most common primary sites of HNMM. Since HNMM shows only non-specific symptoms in the sinonasal and oral cavities, such as epistaxis, nasal obstruction, or discomfort of the throat (10) at the initial stage, most patients are diagnosed only at the advanced stage, making disease management difficult. Furthermore, dermoscopy and confocal microscopy could be used for diagnosing oral MM as for CM and genitourinary MM (11–14), whereas it would be difficult to diagnose sinonasal MM by these devices. Given that early diagnosis of HNMM is often difficult, the development of effective treatment strategies for advanced melanoma is desirable.
Recent advances in immune checkpoint inhibitors (ICIs) have dramatically changed the treatment strategies for melanoma (15–18). In particular, anti-programmed death-1 antibodies, such as nivolumab and pembrolizumab, have significantly prolonged the survival time of patients with advanced melanoma in comparison with dacarbazine, the standard cytotoxic agent for advanced melanoma, and ipilimumab, a fully human monoclonal antibody against CTL antigen 4 (15, 17). However, patients enrolled in this phase III trial mostly had CM, and the proportion of MM patients was very small: 29 MM (3.2%) and 766 CM (84.5%) patients out of 906 melanoma patients (18). Moreover, no report to date has exclusively focused on the treatment outcome of ICI therapy for HNMM; previous reports have included subtypes other than HNMM (19–21). Therefore, this study aimed to examine the treatment outcomes of ICIs in patients with advanced HNMM who had unresectable and/or metastatic disease at the initial diagnosis or who developed recurrent and/or metastatic disease after the initial treatment.
This study was retrospective, multicenter, non-randomized study to evaluate the treatment outcomes of patients with advanced HNMM. This study was approved by the Institutional Review Board of Niigata University Medical and Dental Hospital (IRB No. 2019-0398).
The flow diagram of the study participants is shown in Figure 1. The inclusion criteria were as follows: previously untreated patients presenting with histologically proven MM between January 2001 and August 2021 at our institutions and showing advanced HNMM, defined as unresectable and/or metastatic disease at initial diagnosis or recurrent/metastatic disease after definitive treatments (19–21). A total of 41 patients were diagnosed with HNMM. Of the 36 patients who received definitive treatment, four who survived without recurrent/metastatic disease did not meet the criteria for advanced disease and were excluded from the analyses. Thus, a total of 32 patients were analyzed: five patients had an unresectable disease and/or distant metastasis at the initial diagnosis, while 27 developed local recurrence and/or distant metastasis after the initial treatment. Regarding disease status, 18 patients (56.3%) had unresectable primary tumors or local recurrence, whereas 14 (43.7%) showed distant metastasis alone (Table 1).
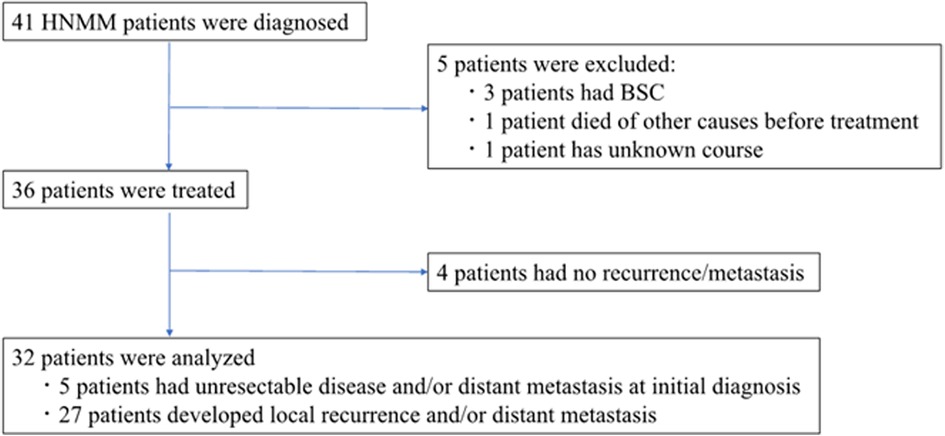
Figure 1. Flow diagram of study participants. Of the 36 HNMM patients who received any treatment, we excluded four patients who survived without recurrent/metastatic disease. The remaining 32 patients were analyzed: 5 patients had an unresectable disease and/or distant metastasis at the initial diagnosis, while 27 developed local recurrence and/or distant metastasis after the initial treatment.
Data on the following baseline characteristics were collected: age, sex, primary site, T classification, N classification, M classification, stage, treatment history, v-Raf murine sarcoma viral oncogene homolog B1 (BRAF)/neuroblastoma RAS viral oncogene homolog (NRAS) mutational status, and programmed death-ligand 1(PD-L1) expression.
The treatment strategy for HNMM at our institution has changed since the medical insurance approval of ICI in Japan in 2014. Before 2013, radical resection plus postoperative radiotherapy (RT) or heavy ion beams was performed as an initial treatment, and chemotherapy with dacarbazine was applied to recurrent or metastatic disease. Since 2014, ICI has been the key-drug in the treatment for recurrent/metastatic disease (advanced HNMM).
OS was defined as the time from the initiation of treatment for advanced disease to the date of death from any cause. The survival time was estimated from the start of the initial treatment for five patients who were diagnosed with advanced HNMM at the initial diagnosis, while the survival time of patients who were diagnosed with advanced HNMM due to development of recurrence/metastasis after initial treatments was estimated from the start of the treatment for recurrent/metastasis. The overall response to ICI treatment was evaluated 8 weeks after the start of ICI treatment for advanced disease using the Response Evaluation Criteria in Solid Tumors criteria version 1.1 (22). Tumor response was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The overall response rate (ORR) was defined as the proportion of CR plus PR, whereas the disease control rate was defined as the proportion of CR, PR, and SD. Immune-related adverse events (irAEs) were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
PD-L1 expression in surgical and biopsy specimens was evaluated by immunohistochemical testing (The Dako 28-8 pharmDx, Agilent Technologies/Dako, Carpinteria, CA) and grouped by expression levels, comprising levels of <5% and ≥5% in a minimum of 100 evaluated tumor cells, based on data from the Checkmate 067 trial (16).
BRAF/NRAS mutation analysis was performed using the THxID BRAF kit (bioMérieux Japan Ltd., Tokyo, Japan) and the MEBGEN RASKET™-B kit (Medical & Biological Laboratories Co. Ltd., Tokyo, Japan). We evaluated two types of BRAF exon 15 mutations (V600 E and V600K), 12 types of NRAS exon 2 (G12, G12C, G12R, G12D, G12V, G12A, G13S, G13C, G13R, G13D, G13V, and G13A), nine types of NRAS exon 3 (A59T, A59G, Q61K, Q61E, Q61L, Q61P, Q61R, Q61Ht, and Q61Hc), and five types of NRAS exon 4 (K117Nc, K117Nt, A146T, A146P, and A146V).
Continuous variables were expressed as medians and ranges. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. All statistical analyses were two-sided, and the significance level was set at p < 0.05. All statistical analyses were performed using EZR (23).
The characteristics at the diagnosis of advanced HNMM in Table 2. The median age was 72.5 years (range, 25–88 years), and 18 patients (56.3%) were male. The primary sites were the sinonasal cavity in 26 patients (81.3%) and other sites in six patients (18.7%). Eighteen patients (56.3%) had unresectable/local recurrence, whereas 14 patients (43.7%) had distant metastasis. As treatment for advanced disease, 14 patients (43.7%) received ICIs, whereas 18 patients (56.3%) did not receive ICI therapy. Among the patients who received ICI therapy, 12 were treated with nivolumab and one each with pembrolizumab and combination therapy of nivolumab and ipilimumab. Among the patients who did not receive ICI treatment, two patients underwent surgery, four received RT, one received chemotherapy, and 11 received the best supportive care.
Gene mutation status was evaluated in 14 patients. The incidences of BRAF and NRAS mutations were observed in no patient (0%) and 4 patients (12.5%), respectively. PD-L1 evaluation was performed on 12 patients. Among them, three patients (9.4%) showed PD-L1 positive, while 9 patients (28.1%) were PD-L1 negative.
The survival analysis of all 32 patients is shown in Figure 2. The median OS of patients with advanced HNMM was 25.3 months (95% CI, 11.3–112.9), and the estimated 1-, 3-, and 5-year OS rates were 68.4%, 42.8%, and 34.3%, respectively.
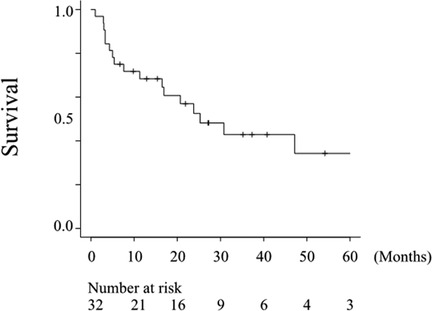
Figure 2. Overall survival of all patients. The median OS after treatment for the advanced disease was 25.3 months (95% CI, 11.3–112.9), and the estimated 5-year OS rate was 34.3%.
Fourteen of the 32 patients received ICI therapy, and CR, PR, SD, and PD were achieved in four, three, two, and five of these patients, respectively (data not shown). Therefore, the ORR and disease control rates were 50% and 64.3%, respectively. The median survival times of patients with or without ICI therapy were not reached (95% CI, 30.8-NA) and 11.3 months (95% CI, 4.7–25.3), respectively (Figure 3). Patients who received ICI therapy showed a significantly better OS than those who did not (p = 0.007, Figure 3A).
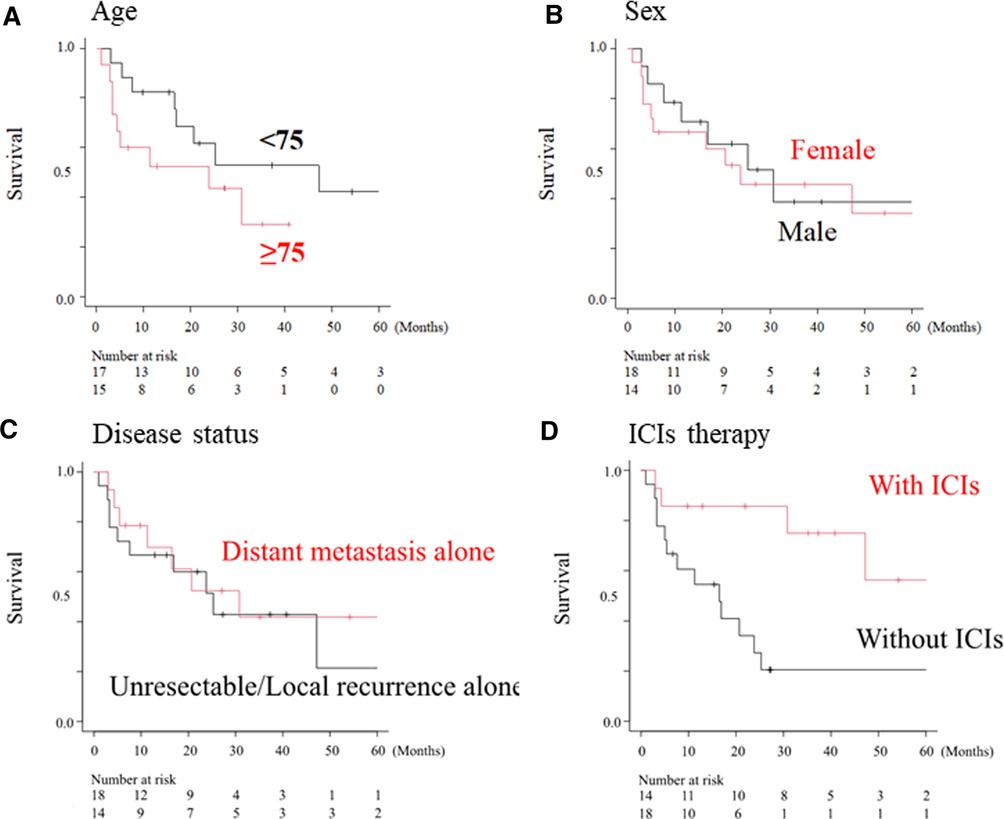
Figure 3. Overall survival curve according to clinical factors. Overall survival according to sex (A), age (B), disease status (C) and ICI treatment (D). The median overall survival in patients who received ICI therapy (with ICI) was significantly longer than that in patients without ICIs (without ICI group) (not reached vs. 11.3 months, p < 0.01).
Detailed clinical courses of all 32 patients are shown as swimmer plots in Figure 4. Ten and twenty-two patients were alive and died of the disease, respectively. Among the patients who received ICIs therapy (shown by black bars), the median duration of ICI therapy as the first-line treatment for advanced HNMM was 12.95 months (0.5–47.4 months) (data not shown). Nine patients (64.3%) experienced irAEs (data not shown). During the follow-up period, two patients continued ICI therapy, whereas 12 patients discontinued it due to disease progression (n = 7) or ≥grade 3 irAEs (n = 5). All five patients who discontinued treatment due to irAEs achieved long survival (9.8–54.2 months) at the end of the follow-up period. Furthermore, four survived after discontinuation without salvage treatment.
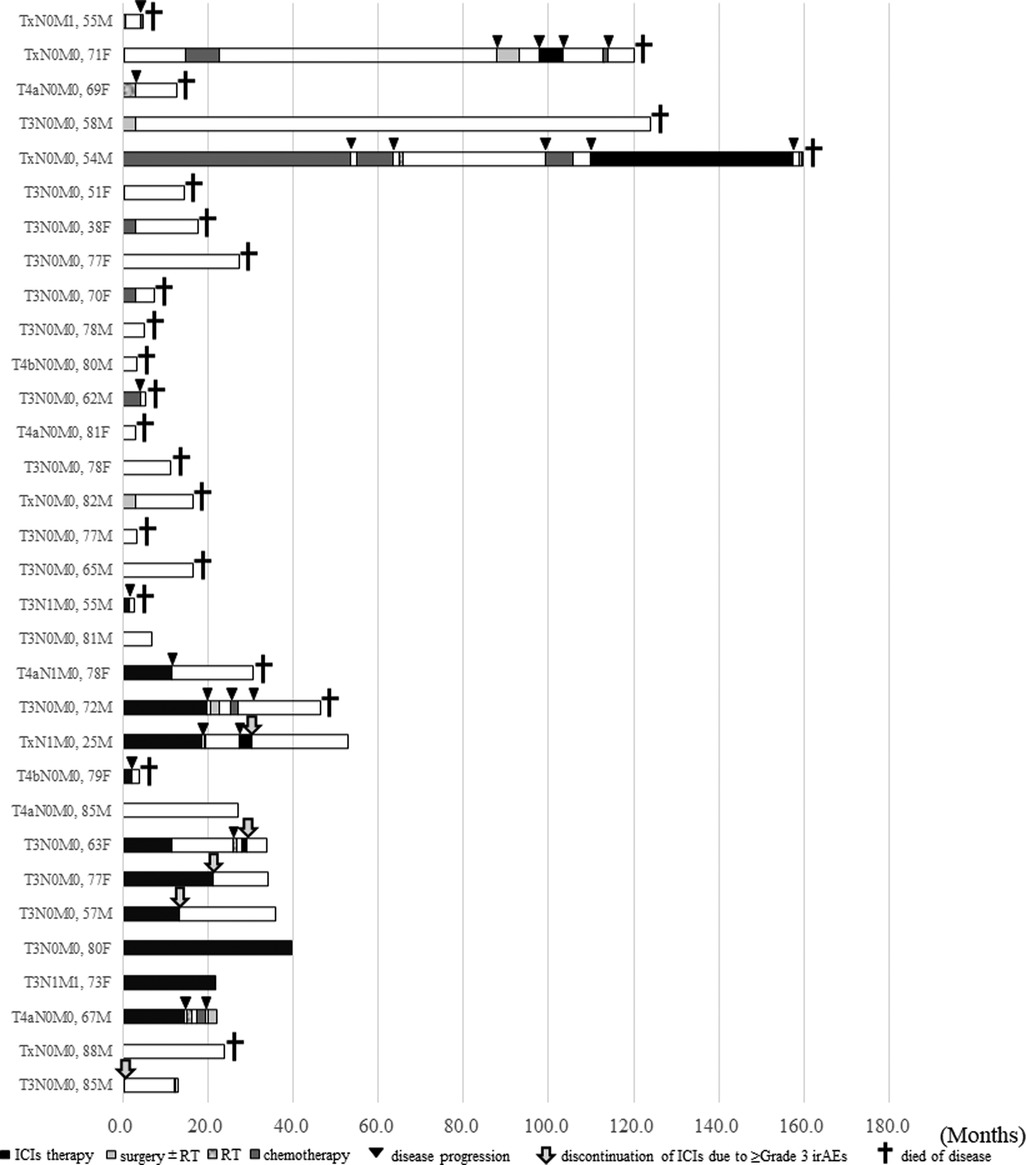
Figure 4.. Swimmer plot showing the detailed clinical courses of patients with advanced HNMM (n = 32). The data are shown in chronological order of treatment initiation for advanced disease. Black bars; the period of ICIs therapy. Light gray bars; the period of salvage surgery ± following radiotherapy. Striped gray bars; the period of radiotherapy. Dark gray bars; the period of chemotherapy. White bars; off treatment, Black triangles; disease progression. Gray arrow; the onset of irAEs (≥Grade 3) Black cross; Died of disease.
We investigated the clinical parameters associated with 1-year OS in patients with advanced HNMM. As shown in Table 3, univariate analysis revealed that ICI therapy was the only significant factor associated with a better OS. Because all BRAF mutations were negative in all patients, BRAF status was omitted from the univariate analysis.
For the clinical parameters associated with 1-year OS after ICI therapy, univariate analysis revealed that the 1-year OS of patients who achieved CR or PR after ICIs was 100%, which was significantly better than that of patients who showed SD or PD (71.4%, p = 0.036) (Table 4).
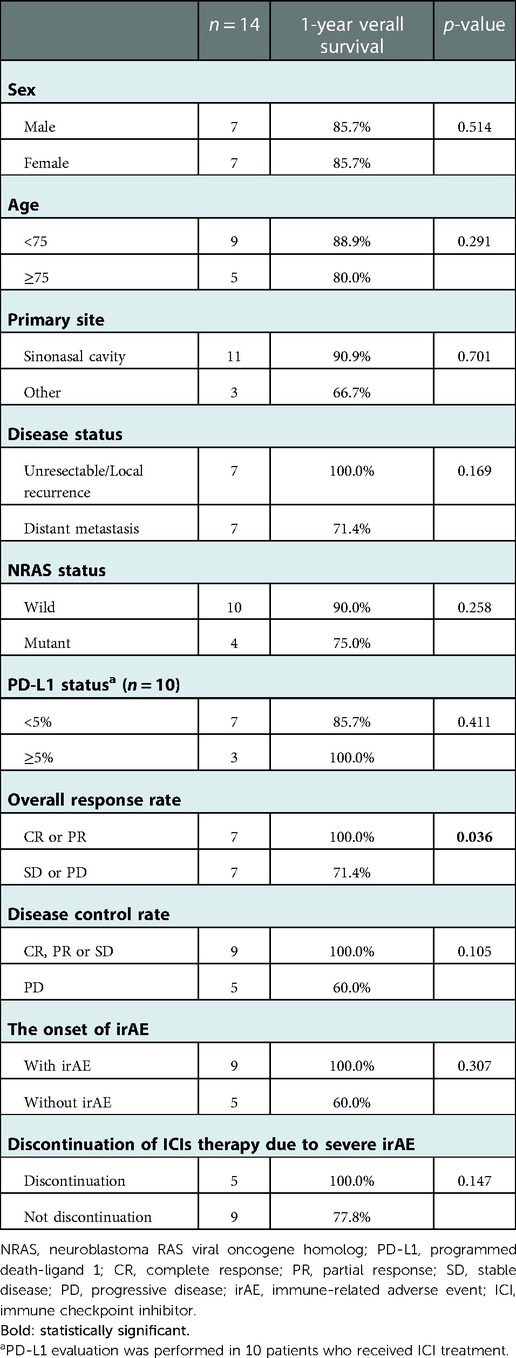
Table 4. Univariate analysis of overall survival on patients who received immune checkpoint inhibitors therapy.
Locoregional recurrence and distant metastasis often occur even after intensive treatments, such as surgery in combination with postoperative radiotherapy (24) and carbon-ion radiation (25), suggesting that the development of more effective treatment strategies for recurrent/metastatic HNMM is desirable. Indeed, only four of the 36 patients in this study achieved no recurrence or metastasis after the initial definitive treatment (Figure 1). Therefore, we focused on the treatment outcomes of HNMM patients with advanced disease, including five patients with unresectable or distant metastasis at the initial diagnosis and 27 patients with recurrence/metastasis after initial treatments including surgery plus postoperative radiotherapy and carbon-ion radiotherapy (Figure 1).
Among the 32 HNMM patients with advanced disease, 14 were treated with ICI. The remaining 18 patients received treatments other than ICI: two patients underwent surgery, four received RT, one received chemotherapy, and 11 received the best supportive care. The median OS of the 32 patients was 25.3 months and the estimated rates of 1-, 3-, and 5-year OS were 68.4%, 42.8%, and 34.3%, respectively (Figure 2). López et al. reported that the 5-year OS of HNMM was less than 30% in most of the cited articles that included non-advanced diseases (10). As Although the present study enrolled only patients with advanced HNMM, the survival rate was comparable to or better than that in López's review (10). This could be partly because 14 patients who received ICI treatment were enrolled in our study, which was approved in 2014 in Japan. Moreover, the univariate analysis demonstrated that ICI therapy was the only factor that significantly improved OS in patients with advanced HNMM (Table 3 and Figure 3). According to a systematic review collecting 52 papers that reported the significance of ICI for MM, immunotherapy improved overall survivals (26). These findings may suggest two key-points for the establishment of optimal treatment strategy for HNMM. First, recurrent-free control of HNMM only with high-intensity initial treatment is extremely difficult. Second, ICI is an essential treatment option for recurrent/metastatic HNMM (advanced HNMM).
Regarding the response of 14 patients who received ICI therapy, CR, PR, SD, and PD were achieved in four, three, two, and five patients, respectively. Thus, the ORR and disease control rates were 50% and 64.3%, respectively. The median survival time of patients who received ICI therapy was not reached (Figure 3), and the 1-year OS rate of patients who received ICI was 85.7% (Table 3). Although no reports focusing on HNMM are available, a few studies have reported the efficacy of ICI therapy for MM of mixed primary sites, including the gastrointestinal and urogenital tracts. The ORR of anti- programmed death-1 monotherapy was 15%–30%, and the ORR of combined administration of anti-CTL antigen 4 and anti-programmed death-1 agents was 28%–37% (19–21, 27, 28). Since the ORR exclusive to HNMM in the present study was 50%, MM derived from the head and neck regions may be suitable targets for ICI therapy among MM of various primary sites.
Regarding the clinical parameters associated with OS after ICI therapy, CR/PR to ICI therapy was associated with a better 1-year OS (Table 4). Moreover, analysis of detailed clinical courses in patients who received ICI therapy revealed that patients who discontinued ICI therapy due to severe irAEs achieved long-term survival (Figure 4). Surprisingly, four of the five patients achieved a durable response for more than 1 year without salvage therapy (Figure 4). The presence of irAEs has been reported to be a favorable prognostic factor for various types of cancer (29–31). Patients with severe irAEs that resulted in discontinuation of ICI therapy could achieve a durable response without additional treatment (32, 33). Our results did not reach a significant difference; however, all of these findings suggest that the CR/PR to ICI therapy and the discontinuation of ICIs due to severe irAEs may be favorable prognostic factors in the treatment of advanced HNMM.
BRAF mutation is the most common mutation in CM (35%–50%) (34). The efficacy of the combination of BRAF and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase inhibitors has been reported in patients with BRAF-mutated metastatic melanoma (35). However, BRAF mutations have been observed in only 6% of MM cases (34). Therefore, to search for therapeutic targets for MM, MM-specific genetic features different from those of CM should be explored. These include the NF1, NRAS, and C-kit mutations observed in 14%, 8%, and 13% of MM cases, respectively, all of which are more common than BRAF (34). NRAS and BRAF are oncogenes that constitute the mitogen-activated protein kinase pathways. The efficacy of extracellular signal-regulated kinase kinase inhibitors in NRAS-mutated advanced melanoma has been reported recently (36, 37), and a favorable response to ICI therapy in NRAS-mutated melanoma (38) has also been reported, suggesting the potential role of NRAS mutations as a novel therapeutic target in HNMM. In the present study, no BRAF mutation was observed, and NRAS mutations were confirmed in four of the 14 patients (29%). The NRAS-positive rate seemed to be higher than that in previous reports (34); however, treatment outcomes were not different between patients with and without NRAS mutations (Table 1), suggesting that NRAS mutations play only a minor role in predicting favorable outcomes of ICI therapy for advanced HNMM. However, because we analyzed BRAF/NRAS mutations in only a small number of patients, the results would not be conclusive.
This study had several limitations. First, the study was retrospective in nature, and the sample size was small because of the rarity of HNMM. Second, we analyzed BRAF/NRAS mutations in a small number of patients who received ICI therapy. Future multicenter, large-cohort studies are needed to establish the optimal treatment strategy for HNMM, including ICI therapy, and to detect novel therapeutic targets through genomic analysis.
The median OS of the 32 patients with advanced HNMM, which was defined as unresectable/distant metastasis at the initial diagnosis or recurrence/metastasis after initial treatment, was 25.3 months. ICI treatment was the only factor associated with a better OS in patients with advanced HNMM. The ORR of the 14 patients who received ICI therapy was 50%, and the 1-year OS was 85.7%. Among patients who received ICI treatment, the ORR was a favorable predictor of OS. Moreover, patients who discontinued ICI therapy owing to severe irAEs achieved a durable response without salvage treatment, which might be an alternative prognostic factor.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Niigata University Medical and Dental Hospital (IRB No. 2019-0398). The patients/participants provided their written informed consent to participate in this study.
Conception and design: SO, YU, AH. Analysis and interpretation of data: SO, YU, YY, TT, RS, KY, RO, HM, ST and TT. Drafting of the manuscript: SO, YU, AH. Final approval of the version to be published: All authors. YU had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
We would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Many Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. (1998) 83:1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8%3C1664::aid-cncr23%3E3.0.co;2-g
2. Tomizuka T, Namikawa K, Higashi T. Characteristics of melanoma in Japan: a nationwide registry analysis 2011-2013. Melanoma Res. (2017) 27:492–7. doi: 10.1097/CMR.0000000000000375.28609317
3. Chi Z, Li S, Sheng X, Si L, Cui C, Han M, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. (2011) 11:85. doi: 10.1186/1471-2407-11-85.21349197
4. Al-Haseni A, Vrable A, Qureshi MM, Mathews S, Pollock S, Truong MT, et al. Survival outcomes of mucosal melanoma in the USA. Future Oncol. (2019) 15:3977–86. doi: 10.2217/fon-2019-0465.31724885
5. Mallone S, De Vries E, Guzzo M, Midena E, Verne J, Coebergh JW, et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. (2012) 48:1167–75. doi: 10.1016/j.ejca.2011.10.004.22119735
6. Schmidt MQ, David J, Yoshida EJ, Scher K, Mita A, Shiao SL, et al. Predictors of survival in head and neck mucosal melanoma. Oral Oncol. (2017) 73:36–42. doi: 10.1016/j.oraloncology.2017.08.002.28939074
7. Jethanamest D, Vila PM, Sikora AG, Morris LG. Predictors of survival in mucosal melanoma of the head and neck. Ann Surg Oncol. (2011) 18:2748–56. doi: 10.1245/s10434-011-1685-4.21476106
8. Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe - a systematic review of the literature. Clin Epidemiol. (2016) 8:109–22. doi: 10.2147/CLEP.S99021.27307765
9. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. Berlin, Germany: Springer International Publishing: American Joint Commission on Cancer (2017).
10. López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, Mendenhall WM, et al. Update on primary head and neck mucosal melanoma. Head Neck. (2016) 38:147–55. doi: 10.1002/hed.23872.
11. De Giorgi V, Sestini S, Bruscino N, Janowska A, Grazzini M, Rossari S, et al. Prevalence and distribution of solitary oral pigmented lesions: a prospective study. J Eur Acad Dermatol Venereol. (2009) 23:1320–3. doi: 10.1111/j.1468-3083.2009.03186.x.19470066
12. Luppi JB, Pereira de Souza R, Florezi GP, Nico MMS, Lourenço SV. The role of reflectance confocal microscopy in the evaluation of pigmented oral lesions and their relationship with histopathological aspects. Am J Dermatopathol. (2022) 44:658–63. doi: 10.1097/DAD.0000000000002220.35503878
13. Cinotti E, Labeille B, Cambazard F, Perrot JL. Confocal microscopy for special sites and special uses. Dermatol Clin. (2016) 34:477–85. doi: 10.1016/j.det.2016.05.010.27692453
14. De Pascalis A, Perrot JL, Tognetti L, Rubegni P, Cinotti E. Review of dermoscopy and reflectance confocal microscopy features of the mucosal melanoma. Diagnostics (Basel). (2021) 11:91. doi: 10.3390/diagnostics11010091.33429900
15. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. (2015) 372:320–30. doi: 10.1056/NEJMoa1412082.25399552
16. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030.26027431
17. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093.25891173
18. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. (2017) 377:1824–35. doi: 10.1056/NEJMoa1709030.28891423
19. Umeda Y, Yoshikawa S, Kiniwa Y, Maekawa T, Yamasaki O, Isei T, et al. Real-world efficacy of anti-PD-1 antibody or combined anti-PD-1 plus anti-CTLA-4 antibodies, with or without radiotherapy, in advanced mucosal melanoma patients: a retrospective, multicenter study. Eur J Cancer. (2021) 157:361–72. doi: 10.1016/j.ejca.2021.08.034.34563991
20. Ogata D, Haydu LE, Glitza IC, Patel SP, Tawbi HA, McQuade JL, et al. The efficacy of anti-programmed cell death protein 1 therapy among patients with metastatic acral and metastatic mucosal melanoma. Cancer Med. (2021) 10:2293–9. doi: 10.1002/cam4.3781.33686688
21. Otsuka M, Sugihara S, Mori S, Hamada K, Sasaki Y, Yoshikawa S, et al. Immune-related adverse events correlate with improved survival in patients with advanced mucosal melanoma treated with nivolumab: a single-center retrospective study in Japan. J Dermatol. (2020) 47:356–62. doi: 10.1111/1346-8138.15246.31984569
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026.19097774
23. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244.23208313
24. Moya-Plana A, Aupérin A, Obongo R, Baglin A, Ferrand FR, Baujat B, et al. Oncologic outcomes, prognostic factor analysis and therapeutic algorithm evaluation of head and neck mucosal melanomas in France. Eur J Cancer. (2019) 123:1–10. doi: 10.1016/j.ejca.2019.09.007.31670075
25. Koto M, Demizu Y, Saitoh JI, Suefuji H, Tsuji H, Okimoto T, et al. Multicenter study of carbon-ion radiation therapy for mucosal melanoma of the head and neck: subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int J Radiat Oncol Biol Phys. (2017) 97:1054–60. doi: 10.1016/j.ijrobp.2016.12.028.28332989
26. Wehbe J, Jaikaransingh D, Walker A. Immunotherapy as a treatment modality for mucosal melanoma of the head and neck: a systematic review. Medicine (Baltimore). (2022) 101:e29979. doi: 10.1097/MD.0000000000029979.35945708
27. Li J, Kan H, Zhao L, Sun Z, Bai C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: a systematic review. Ther Adv Med Oncol. (2020) 12:1758835920922028. doi: 10.1177/1758835920922028.32489431
28. D'Angelo SP, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. (2017) 35:226–35. doi: 10.1200/JCO.2016.67.9258.
29. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. (2019) 145:479–85. doi: 10.1007/s00432-018-2805-3.30506406
30. Matsuo M, Yasumatsu R, Masuda M, Toh S, Wakasaki T, Hashimoto K, et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. (2020) 101:104525. doi: 10.1016/j.oraloncology.2019.104525.31863963
31. Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. (2019) 37:355.e21–9. doi: 10.1016/j.urolonc.2019.03.003.30935847
32. Komiya K, Nakamura T, Abe T, Ogusu S, Nakashima C, Takahashi K, et al. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. (2019) 10:1798–804. doi: 10.1111/1759-7714.13149.31328416
33. Martini DJ, Hamieh L, McKay RR, Harshman LC, Brandao R, Norton CK, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD-1/PD-L1 therapy for immune-related adverse events. Cancer Immunol Res. (2018) 6:402–8. doi: 10.1158/2326-6066.CIR-17-0220.29437040
34. Nassar KW, Tan AC. The mutational landscape of mucosal melanoma. Semin Cancer Biol. (2020) 61:139–48. doi: 10.1016/j.semcancer.2019.09.013.31655118
35. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. (2015) 386:444–51. doi: 10.1016/S0140-6736(15)60898-4.26037941
36. Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. (2013) 14:249–56. doi: 10.1016/S1470-2045(13)70024-X.23414587
37. Muñoz-Couselo E, Adelantado EZ, Ortiz C, García JS, Perez-Garcia J. NRAS-mutant melanoma: current challenges and future prospect. Onco Targets Ther. (2017) 10:3941–7. doi: 10.2147/OTT.S117121.
Keywords: Head and neck mucosal melanoma, Advanced disease, Immune checkpoint inhibitor, Immune-related adverse events, Overall Response Rate (ORR)
Citation: Ohshima S, Ueki Y, Yokoyama Y, Takahashi T, Shodo R, Yamazaki K, Okabe R, Matsuyama H, Togashi T, Takatsuka S, Takenouchi T and Horii A (2022) Treatment outcomes of mucosal melanoma of head and neck: Efficacy of immune checkpoint inhibitors for advanced disease. Front. Surg. 9:1032626. doi: 10.3389/fsurg.2022.1032626
Received: 31 August 2022; Accepted: 12 December 2022;
Published: 26 December 2022.
Edited by:
Rocco Cappellesso, University Hospital of Padua, ItalyReviewed by:
Agata Janowska, University of Pisa, Italy© 2022 Ohshima, Ueki, Yokoyama, Takahashi, Shodo, Yamazaki, Okabe, Matsuyama, Togashi, Takatsuka, Takenouchi and Horii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yushi Ueki dWN1ZWtpbkBnbWFpbC5jb20=
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
Abbreviations CR, complete response; CM, cutaneous melanoma; HNMM, head and neck mucosal melanoma; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; MM, mucosal melanoma; NRAS, neuroblastoma RAS viral oncogene homolog; ORR, overall response rate; OS, overall survival; PR, partial response; PD-L1, programmed death-ligand 1; PD, progressive disease; BRAF, Raf murine sarcoma viral oncogene homolog B1; SD, stable disease.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.